Synergistic Effect of Hexagonal Boron Nitride-Coated Separators and Multi-Walled Carbon Nanotube Anodes for Thermally Stable Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Details
2.1. Growth of the MWCNTs
2.2. Materials Characterization
2.3. Cell Fabrication and Electrochemical Characterization
2.3.1. MWCNT-Based Anode Preparation
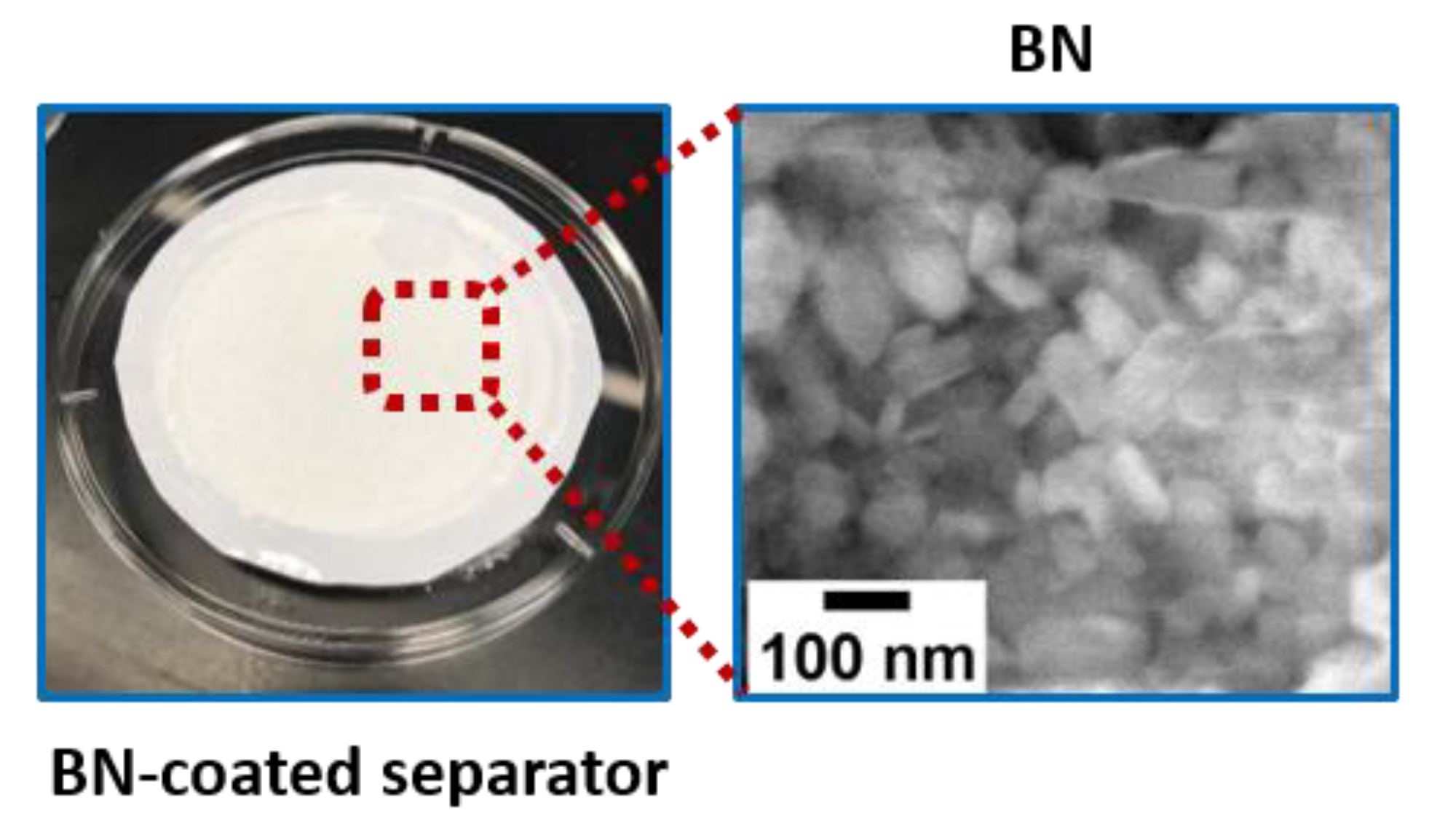
2.3.2. Preparation of the BN-Coated Separator
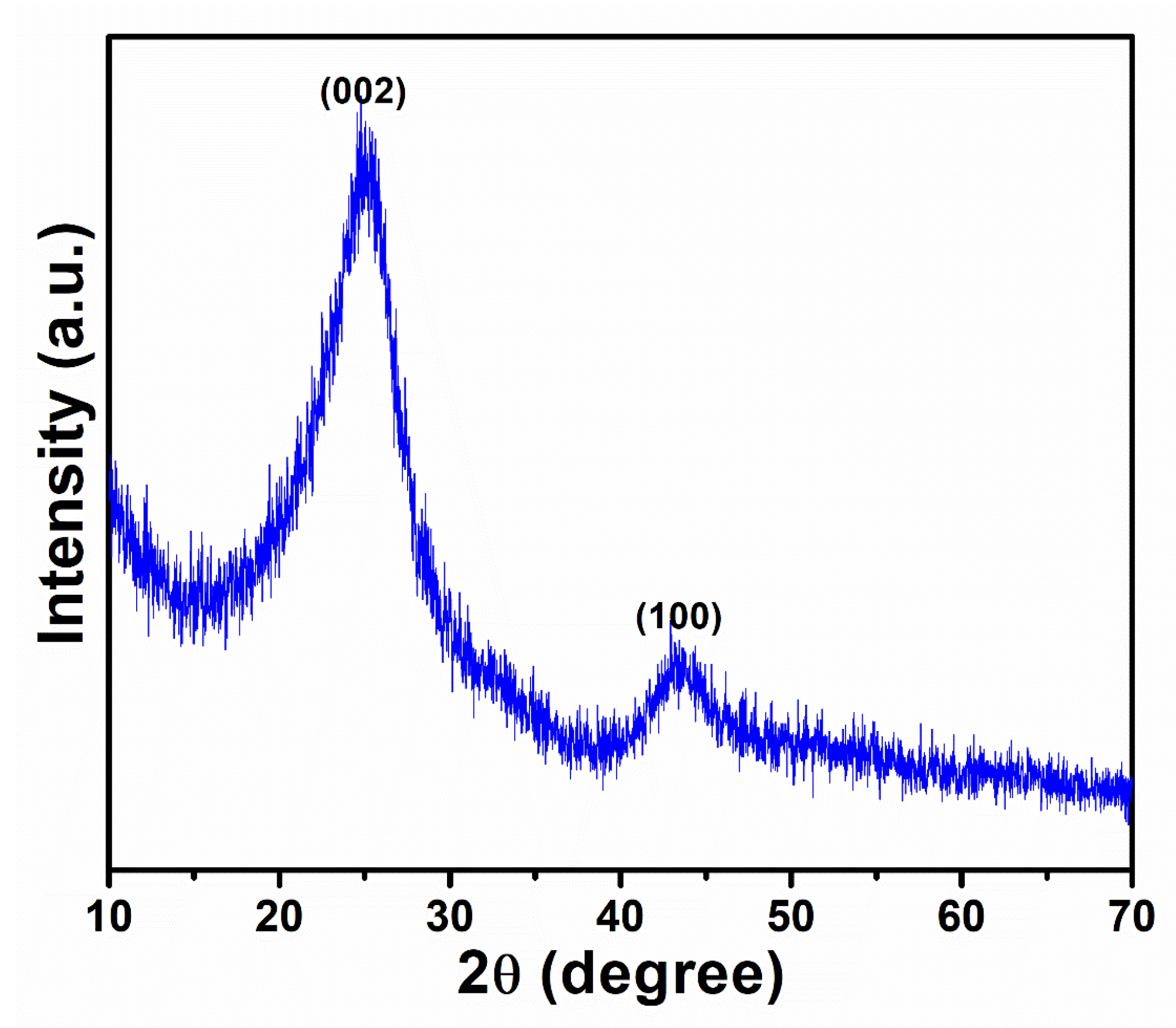
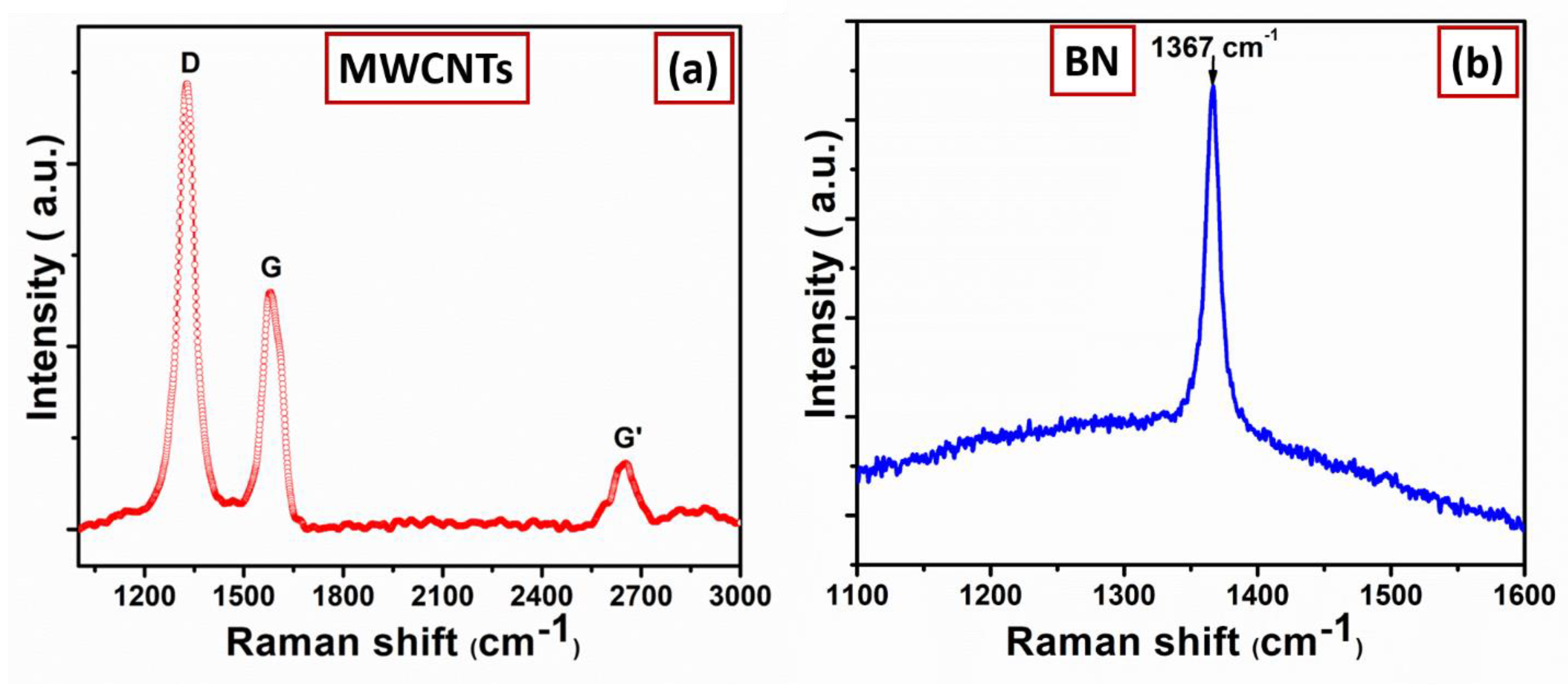
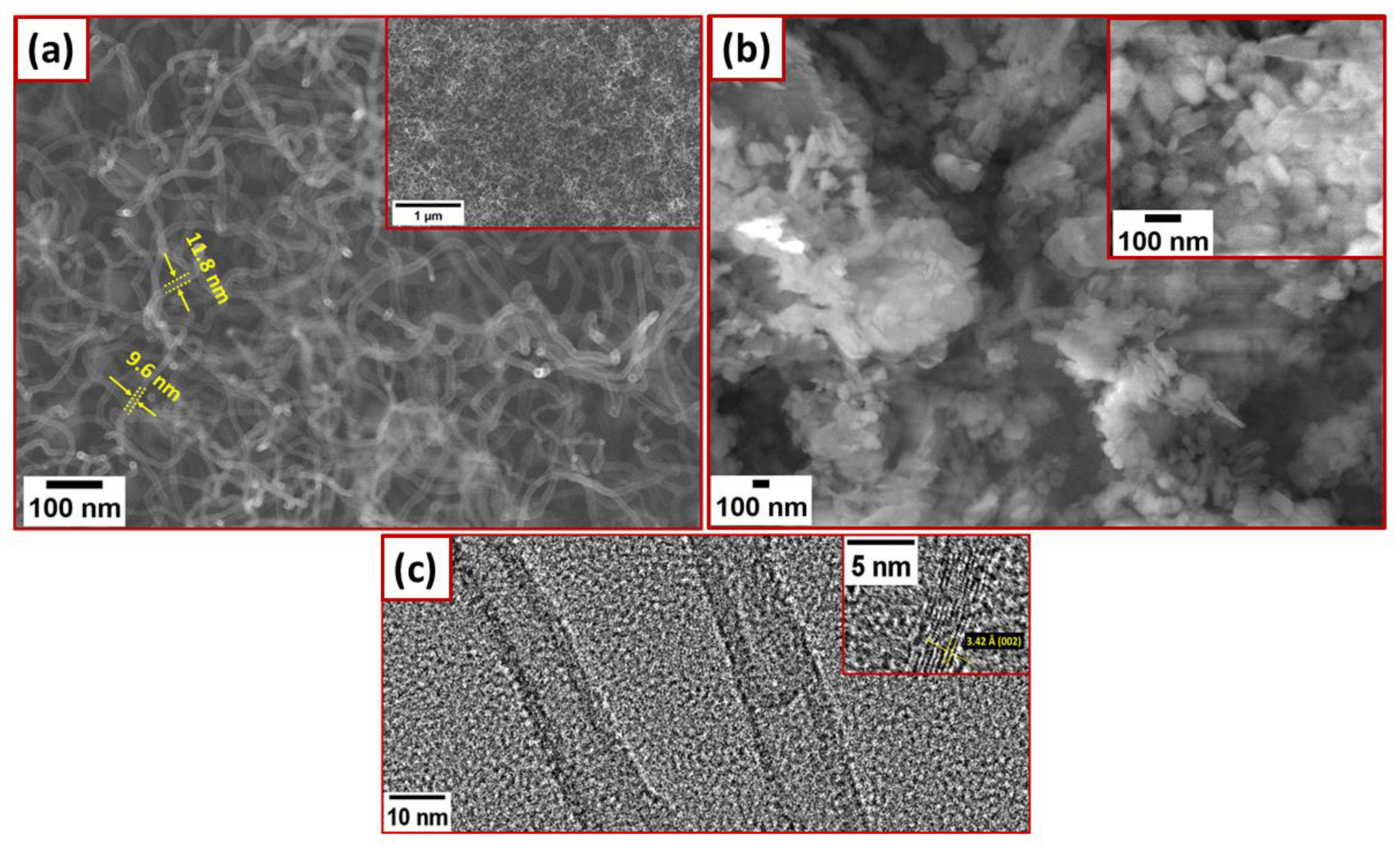
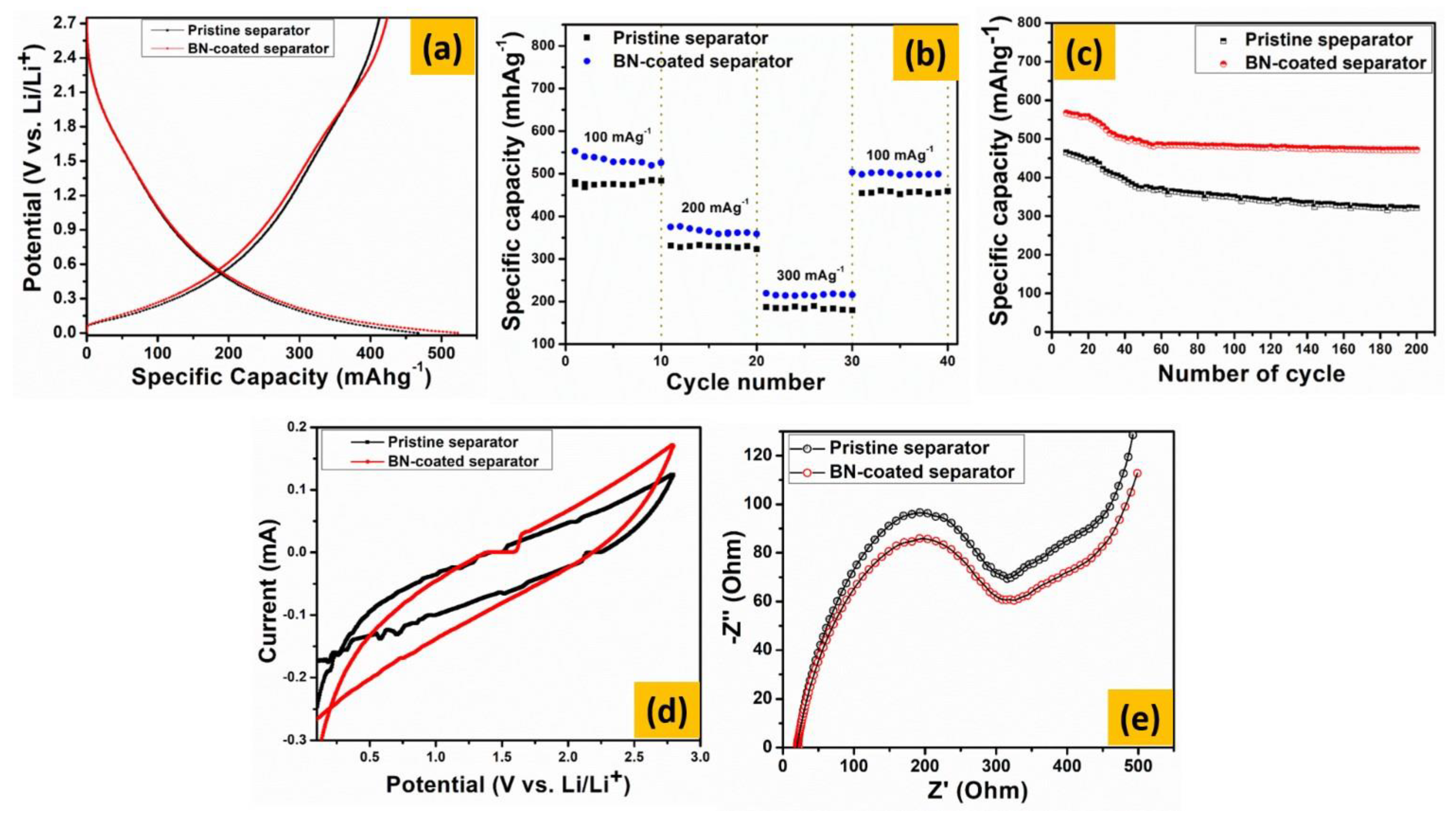
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Osswald, S.; Daniel, R.; Zhu, Y.; Wesel, S.; Ortiz, L.; Sadoway, D.R. Graft copolymer-based lithium-ion battery for high-temperature operation. J. Power Sources 2011, 196, 5604–5610. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Liu, Z.; Kong, Q.; Yao, J.; Zhang, C.; Han, P.; Cui, G. A high temperature operating nanofibrous polyimide separator in Li-ion battery. Solid State Ion. 2013, 232, 44–48. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Shim, J.; Kostecki, R.; Richardson, T.; Song, X.; Striebel, K.A. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef]
- Kang, J.; Rizzoni, G. Study of relationship between temperature and thermal energy, operating conditions as well as environmental factors in large-scale lithium-ion batteries. Int. J. Energy Res. 2014, 38, 1994–2002. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. LiBOB-based gel electrolyte Li-ion battery for high temperature operation. J. Power Sources 2006, 154, 276–280. [Google Scholar] [CrossRef]
- Bodenes, L.; Naturel, R.; Martinez, H.; Dedryvère, R.; Menetrier, M.; Croguennec, L.; Pérès, J.-P.; Tessier, C.; Fischer, F. Lithium secondary batteries working at very high temperature: Capacity fade and understanding of aging mechanisms. J. Power Sources 2013, 236, 265–275. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef]
- Ko, D.L.; Hsin, T.; Lai, Y.H.; Ho, S.Z.; Zheng, Y.; Huang, R.; Chu, Y.H. High-stability transparent flexible energy storage based on PbZrO3/muscovite heterostructure. Nano Energy 2021, 87, 106149. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2010, 15, 649–662. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Ramesh, R.; Kumar, P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Anani, A.; Howard, J. Thermal Stability Studies of Li-Ion Cells and Components. J. Electrochem. Soc. 1999, 146, 3224–3229. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; AlOtaibi, B.; Kumar, S.; Arshi, N.; Hussain, S.G.; Umar, A.; Ahmad, N.; Aljaafari, A. Binder-Free Electrode Based on ZnO Nanorods Directly Grown on Aluminum Substrate for High Performance Supercapacitors. Nanomaterials 2020, 10, 1979. [Google Scholar] [CrossRef] [PubMed]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, Y.M.; Park, J.-K.; Choi, J.W. Mussel-Inspired Polydopamine-Treated Polyethylene Separators for High-Power Li-Ion Batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.; Lim, D.Y. Plasma-Modified Polyethylene Membrane as a Separator for Lithium-Ion Polymer Battery. Electrochim. Acta 2009, 54, 3714–3719. [Google Scholar] [CrossRef]
- Ko, J.M.; Min, B.G.; Kim, D.-W.; Ryu, K.S.; Kim, K.M.; Lee, Y.G.; Chang, S.H. Thin-Film Type Li-Ion Battery Using a Polyethylene Separator Grafted with Glycidyl Methacrylate. Electrochim. Acta 2004, 50, 367–370. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A Highly Thermostable Ceramic-Grafted Microporous Polyethylene Separator for Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef]
- Jeon, H.; Yeon, D.; Lee, T.; Park, J.; Ryou, M.-H.; Lee, Y.M. A Water-Based Al2O3 Ceramic Coating for Polyethylene-Based Microporous Separators for Lithium-Ion Batteries. J. Power Sources 2016, 315, 161–168. [Google Scholar] [CrossRef]
- Dai, J.; Shi, C.; Li, C.; Shen, X.; Peng, L.; Wu, D.; Sun, D.; Zhang, P.; Zhao, J. A Rational Design of Separator with Substantially Enhanced Thermal Features for Lithium-Ion Batteries by the Polydopamine–Ceramic Composite Modification of Polyolefin Membranes. Energy Environ. Sci. 2016, 9, 3252–3261. [Google Scholar] [CrossRef] [Green Version]
- Deepika, D.; Li, L.H.; Glushenkov, A.; Hait, S.K.; Hodgson, P.; Chen, Y. High-Efficient Production of Boron Nitride Nanosheets via an Optimized Ball Milling Process for Lubrication in Oil. Sci. Rep. 2014, 4, 7288. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Zhou, L.; Fu, K.; Yang, Z.; Wan, J.; Manno, M.; Yao, Y.; Zhu, H.; Yang, B.; Hu, L. A Thermally Conductive Separator for Stable Li Metal Anodes. Nano Lett. 2015, 15, 6149–6154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wang, J.N.; Chang, H.; Zhang, Y.F. Preparation of short carbon nanotubes and application as an electrode material in Li-ion batteries. Adv. Funct. Mater. 2007, 17, 3613–3618. [Google Scholar] [CrossRef]
- Xiong, Z.; Yun, Y.; Jin, H.-J. Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials 2013, 6, 1138–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Gao, X.; Yan, J.; Song, D.; Morinaga, M. A first-principles study of lithium absorption in boron- or nitrogen-doped single-walled carbon nanotubes. Carbon 2004, 42, 2677–2682. [Google Scholar] [CrossRef]
- Niu, C.; Sichel, E.K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70, 1480–1482. [Google Scholar] [CrossRef]
- Jung, H.Y.; Hong, S.; Yu, A.; Jung, S.M.; Jeoung, S.K.; Jung, Y.J. Efficient lithium storage from modified vertically aligned carbon nanotubes with open-ends. RSC Adv. 2015, 5, 68875–68880. [Google Scholar] [CrossRef]
- Dubosc, M.; Casimirius, S.; Besland, M.P.; Cardinaud, C.; Granier, A.; Duvail, J.L.; Gohier, A.; Minéa, T.; Arnal, V.; Torres, J. Impact of the Cu-based substrates and catalyst deposition techniques on carbon nanotube growth at low temperature by PECVD. Microelectron. Eng. 2007, 84, 2501–2505. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Melaibari, A.; Hasan, P.M.; Aljaafari, A. Monitoring Food Spoilage Based on a Defect-Induced Multiwall Carbon Nanotube Sensor at Room Temperature: Preventing Food Waste. ACS Omega 2020, 5, 30531–30537. [Google Scholar] [CrossRef]
- Shaalan, N.; Saber, O.; Ahmed, F.; Aljaafari, A.; Kumar, S. Growth of Defect-Induced Carbon Nanotubes for Low-Temperature Fruit Monitoring Sensor. Chemosensors 2021, 9, 131. [Google Scholar] [CrossRef]
- Musso, S.; Giorcelli, M.; Pavese, M.; Bianco, S.; Rovere, M.; Tagliaferro, A. Improving macroscopic physical and mechanical properties of thick layers of aligned multiwall carbon nanotubes by annealing treatment. Diam. Relat. Mater. 2008, 17, 542–547. [Google Scholar] [CrossRef]
- Futaba, D.N.; Yamada, T.; Kobashi, K.; Yumura, M.; Hata, K. Macroscopic Wall Number Analysis of Single-Walled, Double-Walled, and Few-Walled Carbon Nanotubes by X-ray Diffraction. J. Am. Chem. Soc. 2011, 133, 5716–5719. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Holden, J.M.; Jishi, R.A. Vibrational modes of carbon nanotubes; spectroscopy and theory. Carbon 1995, 33, 959–972. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in grapheme. Carbon N. Y. 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Tay, R.Y.; Griep, M.H.; Mallick, G.; Tsang, S.H.; Singh, R.S.; Tumlin, T.; Teo, E.H.T.; Karna, S.P. Growth of Large Single-Crystalline Two-Dimensional Boron Nitride Hexagons on Electropolished Copper. Nano Lett. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Lee, E.-K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.-C.; Choi, Y.-M.; Jung, Y.-G.; Paik, U. Dominant Factors Governing the Rate Capability of a TiO2 Nanotube Anode for High Power Lithium Ion Batteries. ACS Nano 2012, 6, 8308–8315. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Lou, X.W.; Hng, H.H. Titania nanosheets hierarchically assembled on carbon nanotubes as high-rate anodes for lithium-ion batteries. Chem. Eur. J. 2012, 18, 3132. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Lou, X.W. One dimensional hierarchical structures composed of metal oxide nanosheets on CNT backbone and their lithium storage properties. Adv. Funct. Mater. 2011, 21, 4120. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Mao, S.; Cui, S.; He, Z.; Chen, J. CNT@TiO2 nanohybrids for high-performance anode of lithium-ion batteries. Nanoscale Res. Lett. 2013, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Béguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Lee, J.W.; Song, T.; Paik, U. 3D-interconnected nanoporous RGO-CNT structure for supercapacitors application. Electrochim. Acta 2014, 125, 536–542. [Google Scholar] [CrossRef]
- Kang, C.; Patel, M.; Rangasamy, B.; Jung, K. Three-dimensional carbon nanotubes for high capacity lithium-ion batteries. J. Power Sources 2015, 299, 465–471. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Kumar, S.; Shaalan, N.M.; Saber, O.; Rehman, S.; Aljaafari, A.; Abuhimd, H.; Alshahrani, M. Synergistic Effect of Hexagonal Boron Nitride-Coated Separators and Multi-Walled Carbon Nanotube Anodes for Thermally Stable Lithium-Ion Batteries. Crystals 2022, 12, 125. https://doi.org/10.3390/cryst12020125
Ahmed F, Kumar S, Shaalan NM, Saber O, Rehman S, Aljaafari A, Abuhimd H, Alshahrani M. Synergistic Effect of Hexagonal Boron Nitride-Coated Separators and Multi-Walled Carbon Nanotube Anodes for Thermally Stable Lithium-Ion Batteries. Crystals. 2022; 12(2):125. https://doi.org/10.3390/cryst12020125
Chicago/Turabian StyleAhmed, Faheem, Shalendra Kumar, Nagih Mohammed Shaalan, Osama Saber, Sarish Rehman, Abdullah Aljaafari, Hatem Abuhimd, and Mohammad Alshahrani. 2022. "Synergistic Effect of Hexagonal Boron Nitride-Coated Separators and Multi-Walled Carbon Nanotube Anodes for Thermally Stable Lithium-Ion Batteries" Crystals 12, no. 2: 125. https://doi.org/10.3390/cryst12020125








