Influence of Culture Medium on Cementation of Coarse Grains Based on Microbially Induced Carbonate Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria
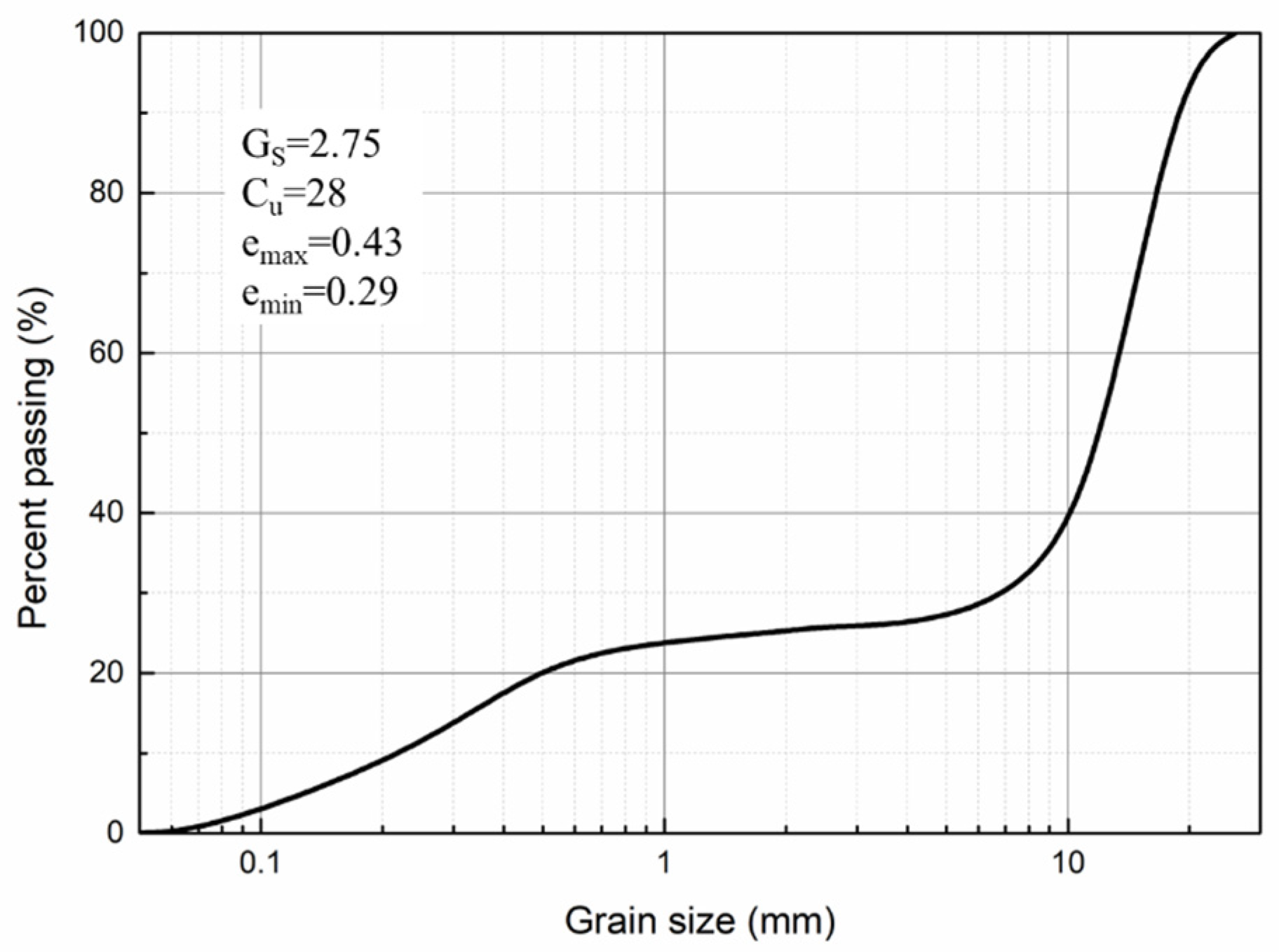
2.2. Granular Column Specimen
2.3. Culture Media
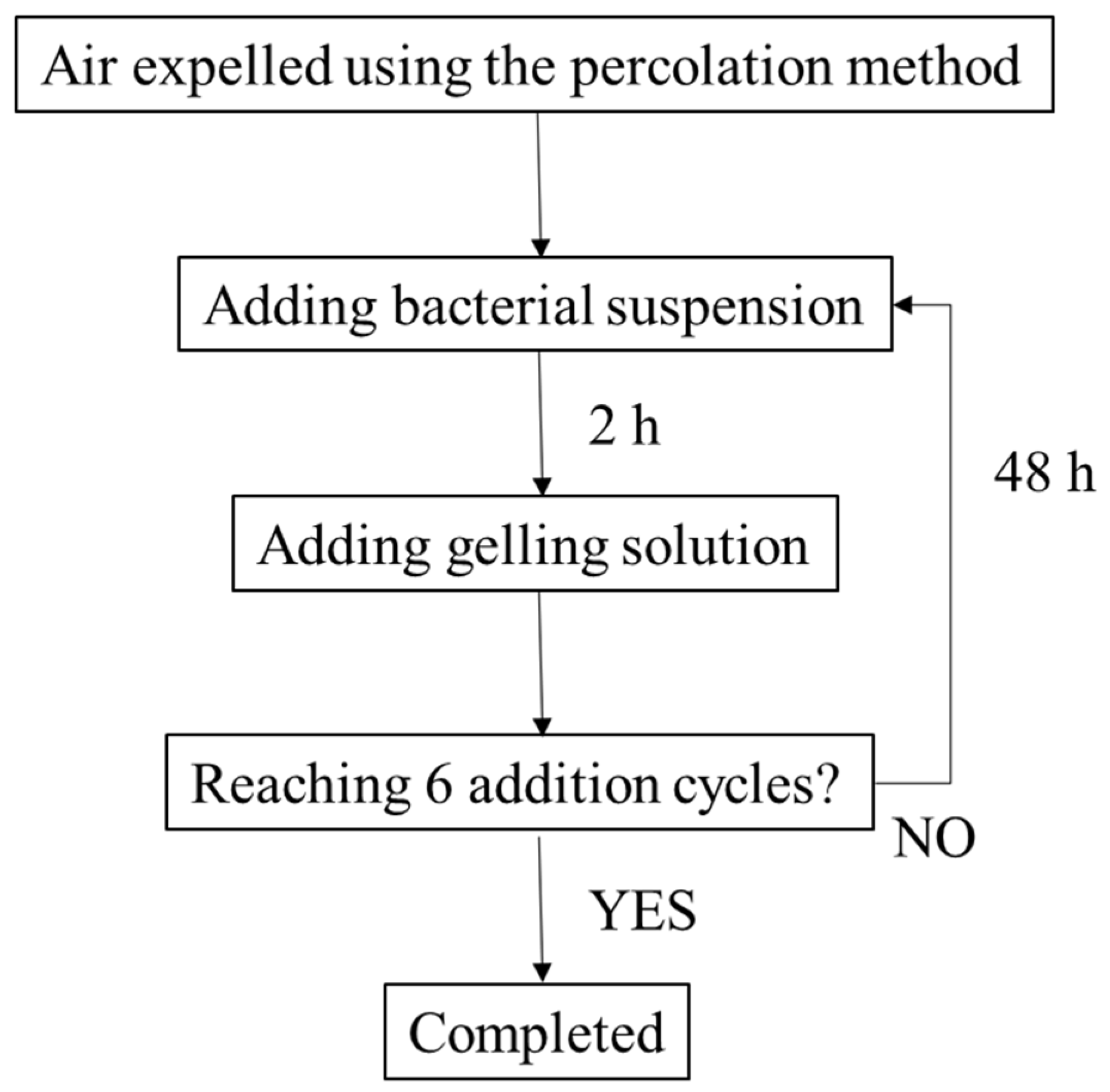
2.4. River Sediments Treatment Procedures
2.5. Evaluation of Treatment Effects
3. Results and Discussion
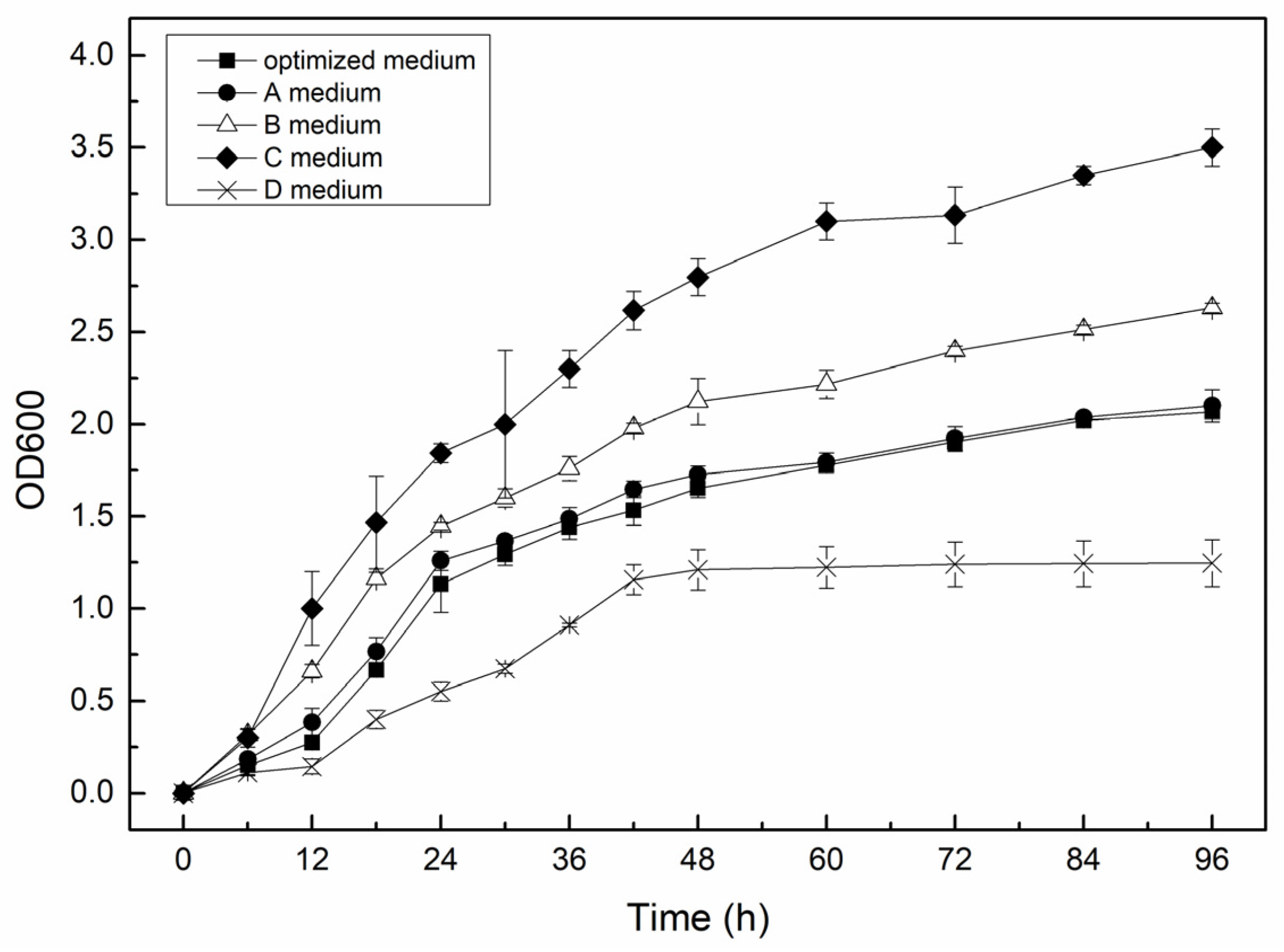
3.1. Preparing Different Culture Media
3.2. Bio-Cementation of Coarse Materials
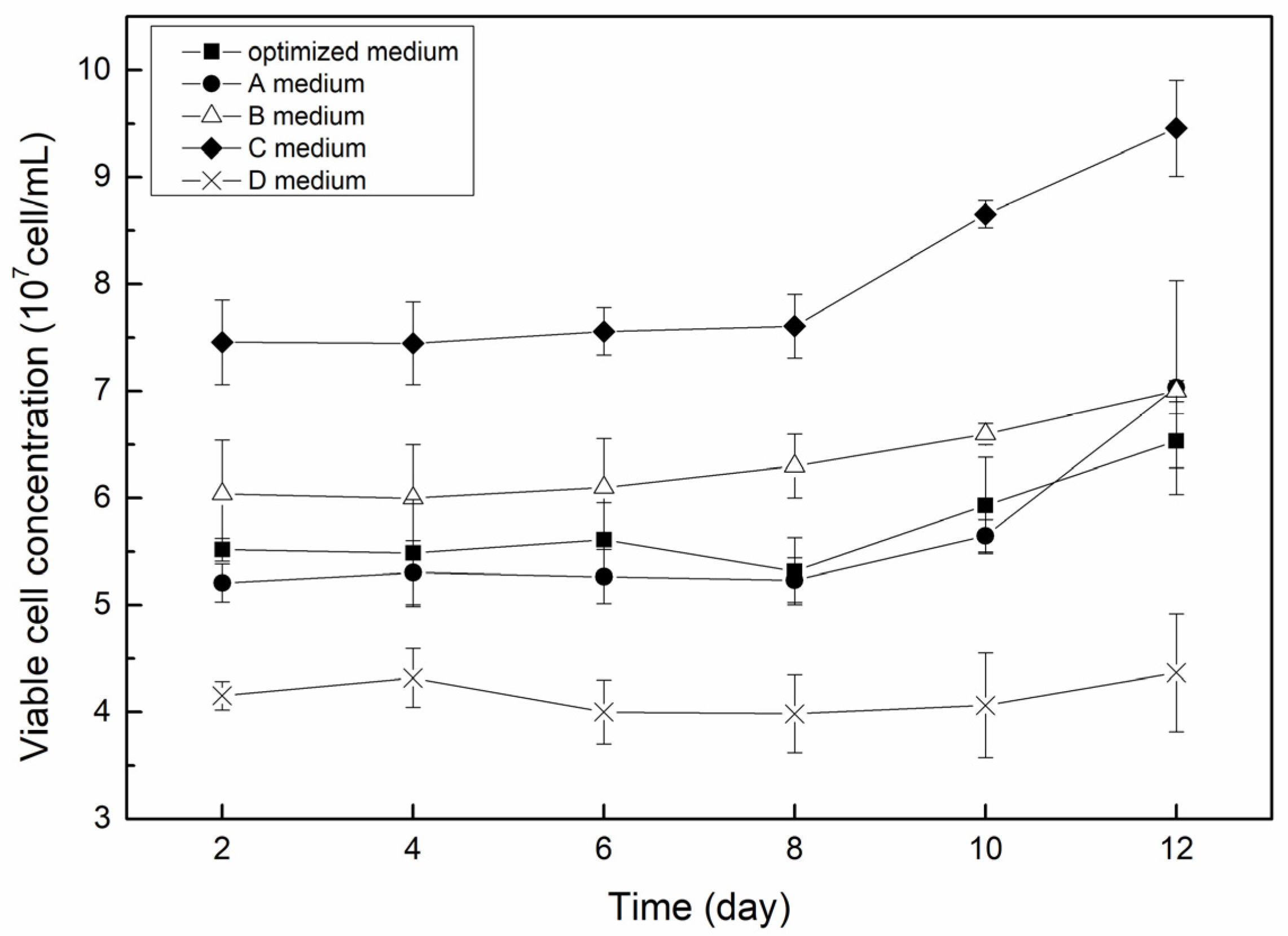
3.2.1. Viable Cell Concentration in Effluent
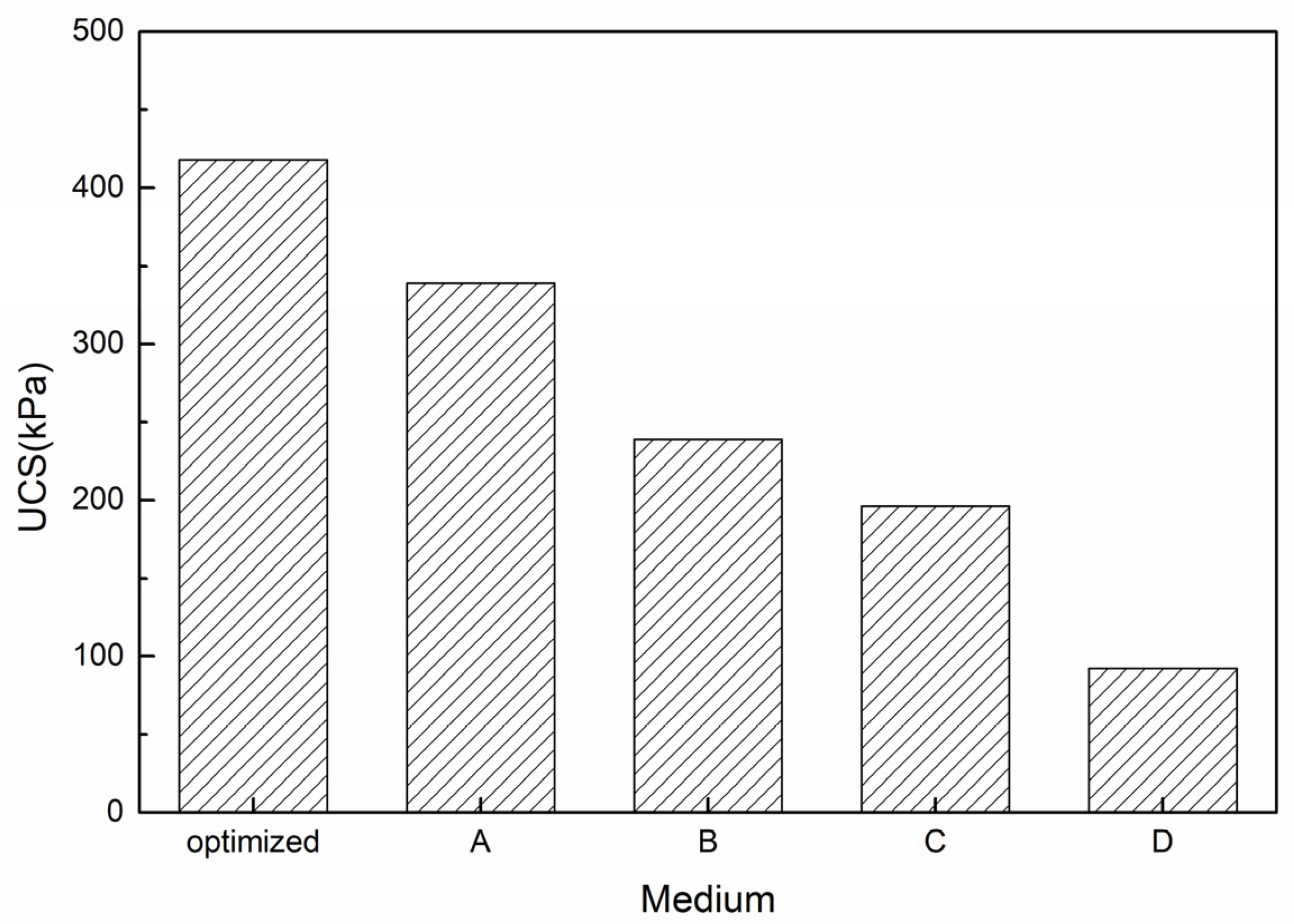
3.2.2. UCS
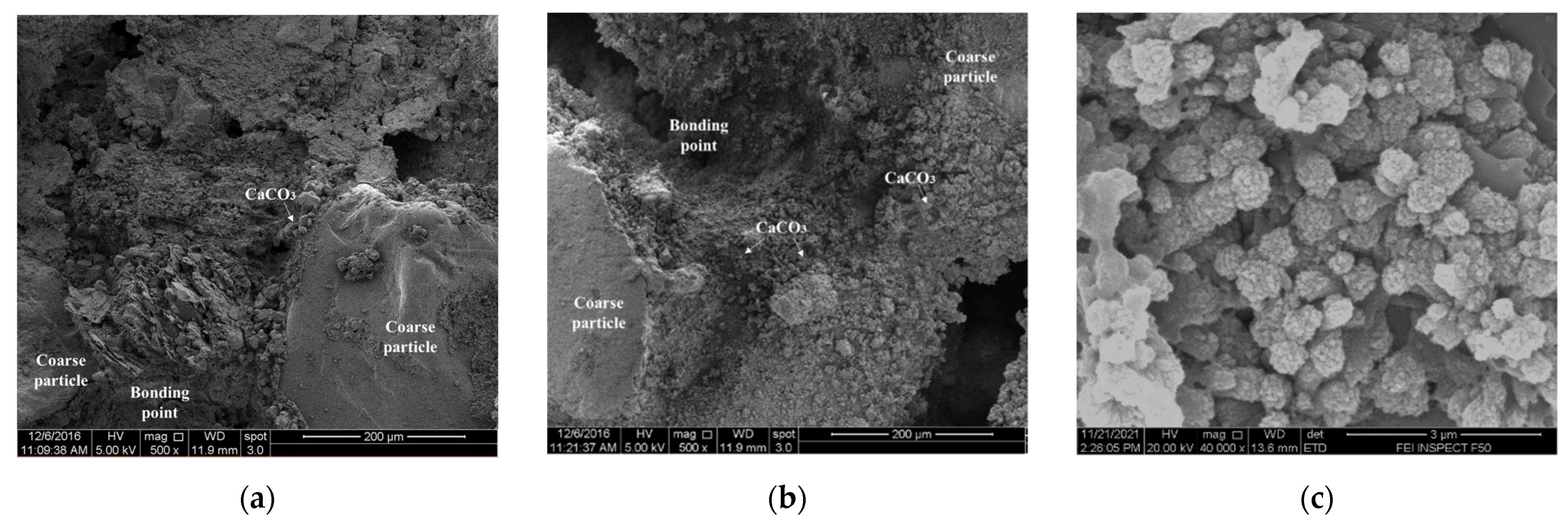
3.2.3. Scanning Electron Microscopy
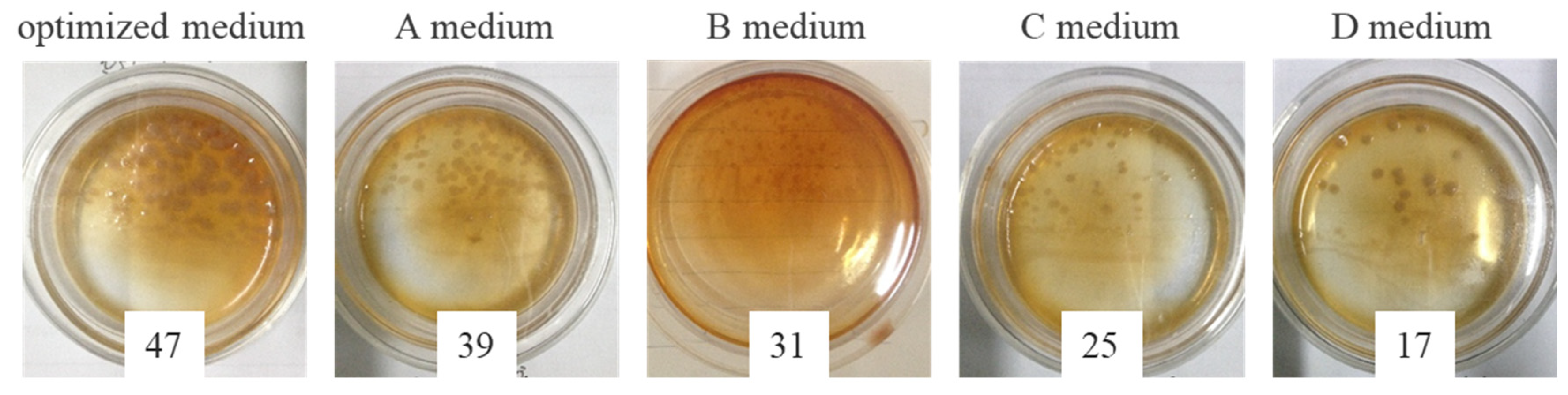
3.2.4. Biofilm Growth
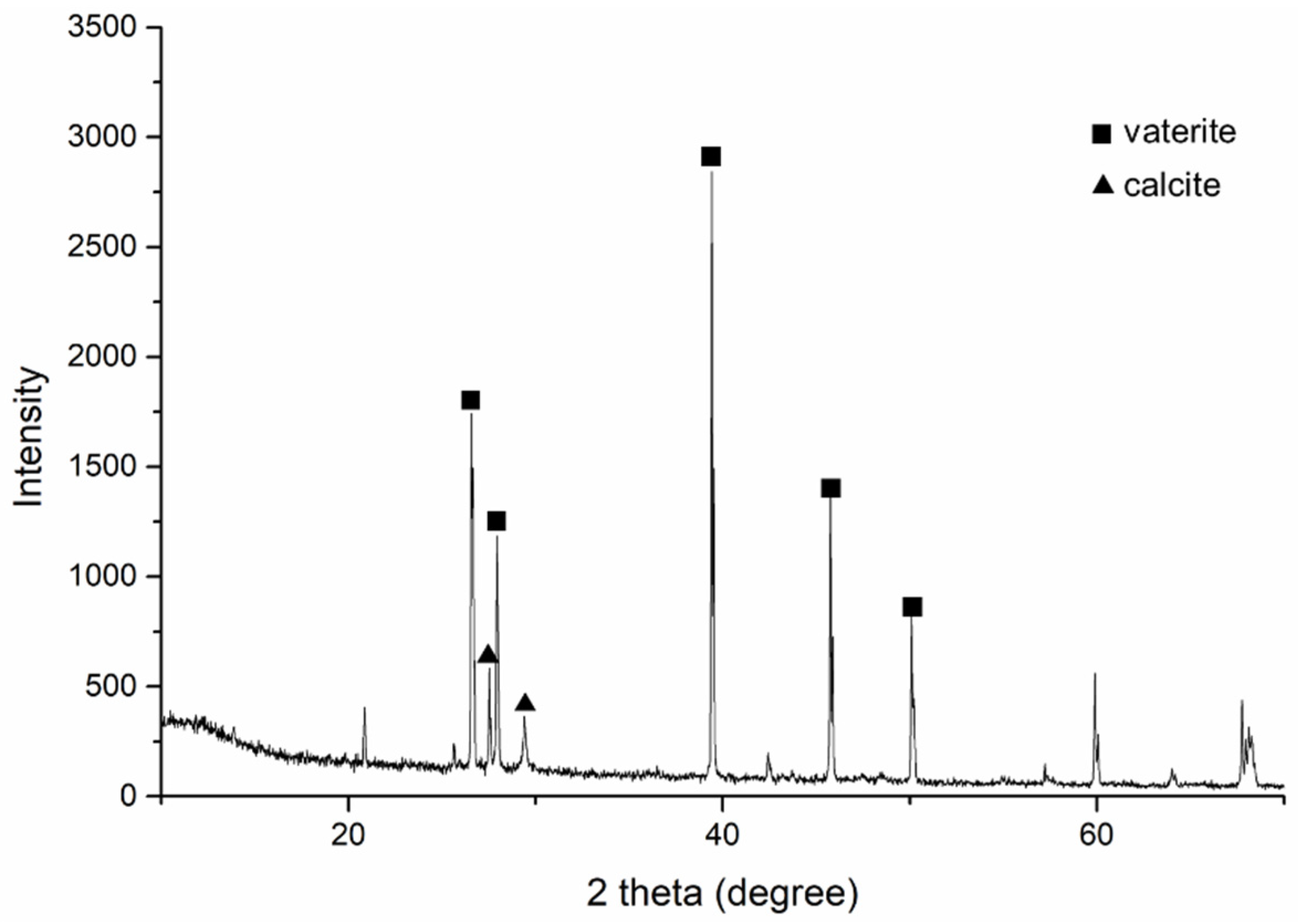
3.2.5. Calcium Carbonate Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuthbert, M.; Mcmillan, L.; Handley-Sidhu, S.; Riley, M.; Tobler, D.; Phoenix, V. A field and modeling study of fractured rock permeability reduction using microbially induced calcite precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643. [Google Scholar] [CrossRef] [PubMed]
- Montoya, B.; DeJong, J.; Boulanger, R. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique 2013, 63, 302–312. [Google Scholar] [CrossRef]
- Soon, N.; Lee, L.; Khun, T.; Ling, H. Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Improvement of Microbial-Induced Calcium Carbonate Precipitation Technology for Sand Solidification. J. Mater. Civ. Eng. 2018, 30, 04018301. [Google Scholar] [CrossRef]
- DeJong, J.; Fritzges, M.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2018, 14, 627–638. [Google Scholar] [CrossRef]
- Pu, Q.; Jiang, L.; Xu, J.; Chu, H.; Xu, Y.; Zhang, Y. Evolution of pH and chemical composition of pore solution in carbonated concrete. Constr. Build. Mater. 2012, 28, 519–524. [Google Scholar] [CrossRef]
- Soon, N.; Lee, L.; Khun, T.; Ling, H. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Wijngaarden, W.; Vermolen, F.; Meurs, G.; Vuik, C. Modelling biogrout: A new ground improvement method based on microbial-induced carbonate precipitation. Transp. Porous Media. 2011, 87, 397–420. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ajo-Franklin, J.B.; Spycher, N.; Hubbard, S.S.; Smith, R. Geophysical monitoring and reactive transport modeling of ureolytically-driven calcium carbonate precipitation. Geochem. Trans. 2011, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Elphick, S.; Ngwenya, B.; Dodds, K.; Butler, I. Inhibition of sporosarcina pasteurii under anoxic conditions:implications for subsurface carbonate precipitation and. Environ. Sci. Technol. 2012, 46, 8351–8355. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; DeJong, J.; Ginn, T.; Montoya, B.; Barkouki, T.; Hunt, C.; Tanyu, B.; Major, D. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Cheng, X. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Whiffin, V.; Paassen, L.; Harkes, M. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, C. Glucose addition improves the bio-remediation efficiency for crack repair. Mater. Struct. 2019, 52, 111. [Google Scholar] [CrossRef]
- Khan, M.; Amarakoon, G.; Shimazaki, S.; Kawasaki, S. Coral sand solidification test based on microbially induced carbonate precipitation using ureolytic bacteria. Mater. Trans. 2015, 56, 1725–1732. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Miao, L.; Wu, L.; Wang, H. Theoretical quantification for cracks repair based on microbially induced carbonate precipitation (MICP) method. Cem. Concr. Compos. 2021, 118, 103950. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Zomorodian, S.; Niazi, A.; O’Kelly, B. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng.-Ground Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Salifu, E.; MacLachlan, E.; Iyer, K.; Knapp, C.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, N.; Kavazanjian, E.; Rittmann, B.; Karatas, I. Carbonate mineral precipitation for soil improvement through microbial denitrification. Geomicrobiol. J. 2017, 34, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of Enzymatic Calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 2020, 759, 143468. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Miao, L.; Wang, H.; Yuan, J.; Fan, G. Enhanced rainfall erosion durability of enzymatically induced carbonate precipitation for dust control. Sci. Total Environ. 2021, 791, 148369. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Miao, L.; Wang, H.; Yin, W.; Wu, L. Mineralization crust field experiment for desert sand solidification based on enzymatic calcification. J. Environ. Manag. 2021, 287, 112315. [Google Scholar] [CrossRef] [PubMed]
- Mahawish, A.; Bouazza, A.; Gates, W. Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotechnica. 2018, 13, 1019–1025. [Google Scholar] [CrossRef]
- Khoubani, A.; Nafisi, A.; Evans, T.; Montoya, B. The effect of grain size and shape on mechanical behavior of MICP treated sand ii: Numerical study. In Bio-Mediated and Bio-Inspired Geotechnics; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cunningham, A.; Gerlach, R.; Spangler, L.; Mitchell, A.; Parks, S.; Phillips, A. Reducing the risk of wellbore leakage of CO2 using engineered biomineralization barriers. Energy Procedia 2011, 4, 5178–5185. [Google Scholar] [CrossRef]
- Mitchell, A.; Phillips, A.; Schultz, L.; Parks, S.; Spangler, L.; Cunningham, A.; Microbial, R. Microbial CaCO3 mineral formation and stability in an experimentally simulated high pressure saline aquifer with supercritical CO2. Int. J. Greenh. Gas Control. 2013, 15, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Afrapoli, M.S.; Alipour, S.; Torsaeter, O. Fundamental study of pore scale mechanisms in microbial improved oil recovery processes. Transp. Porous Media 2011, 90, 949–964. [Google Scholar] [CrossRef]
- Li, M.; Guo, H.; Cheng, X. Application of response surface methodology for carbonate precipitation production induced by a mutant strain of Sporosarcina pasteurii. GeoFrontiers Adv. Geotech. Eng. 2011, 4079–4088. [Google Scholar]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. ECOL ENG 2010, 36, 99–111. [Google Scholar] [CrossRef]
- Fredrickson, J.; Fletcher, M.; Frederickson, J.; Fletcher, M. Subsurface Microbiology and Biogeochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- ASTM. Standard practice for classification of soils for engineering purposes (Unified Soil Classification System). In ASTM Standard D2487; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Iqbal, D.; Wong, L.; Kong, S. Bio-cementation in construction materials: A review. Materals 2021, 14, 2175. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Capping Cylindrical Concrete Specimens. In ASTM Standard C617; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Vandevivere, P.; Baveye, P.; Sanchez de Lozada, D.; DeLeo, P. Microbial clogging of saturated soils and aquifer materials: Evaluation of mathematical models. Water Resour. Res. 1995, 31, 2173–2180. [Google Scholar] [CrossRef]
- Ebigbo, A.; Phillips, R.; Gerlach, R.; Helmig, A.; Cunningham, H.; Class, L. Darcy-scale modeling of microbiallyinduced carbonate mineral precipitation in sand columns. Water Resour. Res. 2012, 48, W07519. [Google Scholar] [CrossRef]
- Qin, C.; Hassanizadeh, S. Pore-network modeling of solute transport and biofilm growth in porous media. Transp. Porous Media 2015, 110, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Hassanizadeh, S.; Ebigbo, A. Pore-scale network modeling of microbially induced calcium carbonate precipitation: Insight into scale dependence of biogeochemical reaction rates. Water Resour. Res. 2016, 52, 8794–8810. [Google Scholar] [CrossRef]
- Fan, S. Preliminary Research on a Novel Soil Improvement Technique by Microbial Denitrification; Huazhong University of Science and Technology: Wuhan, China, 2012. (In Chinese) [Google Scholar]
- Lioliou, M.; Paraskeva, C.; Koutsoukos, P.; Payatakes, A. Heterogeneous nucleation and growth of calcium carbonate on calcite and quartz. J. Colloid Interface Sci. 2007, 308, 421–428. [Google Scholar] [CrossRef]
- Noiriel, C.; Steefel, L.; Yang, J.; Ajo-Franklin, J. Upscaling calcium carbonate precipitation rates from pore to continuum scale. Chem. Geol. 2012, 318–319, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Mahabadi, N.; Jang, J.; Paassen, L.A. Assessing the Kinetics and Pore Scale Characteristics of Biological Calcium Carbonate Precipitation in Porous Media using a Microfluidic Chip Experiment. Water Resour. Res. 2020, 56, e2019WR025420. [Google Scholar] [CrossRef]
- Mitchell, A.; Grant Ferris, F. The influence of bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
- Chou, L.; Garrels, R.M.; Wollast, R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 1989, 78, 269–282. [Google Scholar] [CrossRef]
- Castanier, S.; Metayer-Levrel, G.; Perthuisot, J. Bacterial roles in the precipitation of carbonate minerals. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. [Google Scholar]
- Van Paassen, L. Biogrout Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Zavarzin, G. Microbial geochemical calcium cycle. Microbiology 2002, 71, 1–17. [Google Scholar] [CrossRef]
- Rebata-landa, V.; Santamarina, J. Mechanical effects of biogenic nitrogen gas bubbles in soils. J. Geotech. Geoenviron. Eng. 2012, 138, 128–137. [Google Scholar] [CrossRef]
- Fukue, M.; Ono, S.; Sato, Y. Cementation of sands due to microbiologically-induced carbonate precipitation. Soils Found. 2011, 51, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.; Jaffé, P. Substrate and biomass transport in a porous medium. Water Resour. Res. 1990, 26, 2181–2194. [Google Scholar] [CrossRef]
- Hommel, J.; Lauchnor, A.; Phillips, R.; Gerlach, A.; Cunningham, R.; Helmig, A.; Ebigbo, H. A revised model for microbially induced calcite precipitation: Improvements and new insights based on recent experiments. Water Resour. Res. 2015, 51, 3695–3715. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Sarmast, M.; Farpoor, M.H.; Sarcheshmehpoor, M.; Eghbal, M. Micromorphological and biocalcification effects of sporosarcina pasteurii and sporosarcina ureae in sandy soil columns. J. Agric. Sci. Technol. 2014, 16, 681–693. [Google Scholar]
- Sun, X.; Miao, L.; Chen, R. Adding Aluminum Oxide to Improve the Repairing Effect of Cracks Based on Bio-Remediation. J. Adv. Concr. Technol. 2019, 17, 177–187. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. In ASTM Standard D2166; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM. Standard Test Method for Obtaining and Testing Drilled Cores and Sawed Beams of Concrete. In ASTM Standard D2166; American Society for Testing and Materials: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Wong, L.S. Formulation of an optimal mix design of stabilized peat columns with fly ash as a pozzolan. Arab. J. Sci. Eng. 2015, 40, 1015–1025. [Google Scholar] [CrossRef]
- Uenishi, R.; Matsubara, H. Calcium carbonate growth with the ring structure of stalactite-type minerals in a tuff breccia. Crystals. 2021, 11, 1117. [Google Scholar] [CrossRef]
- Whiffin, V. Microbial CaCO3 Precipitation for the Production of Biocement; Murdoch University: Perth, Australia, 2004. [Google Scholar]
- Joana, A.; Nuno, F.; Romain, B.; Nuno, C.; Tom, C.; Ana, R.; Mickaël, D.; Giovanni, D.; Michel, H.; Zoran, J.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.; Faucher, S. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Lauchnor, E.; Topp, D.; Parker, A.; Gerlach, R. Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J. Appl. Microbiol. 2015, 118, 1321–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeJong, J.; Martinez, B.; Mortensen, B.; Nelson, D.; Waller, J.; Weil, M.; Ginn, T.; Weathers, T.; Barkouki, T.; Fujita, Y. Upscaling of bio-mediated soil improvement. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; pp. 2300–2303. [Google Scholar]
- Rodriguez-Navarro, C.; Rodriguez-Gallego, M.; Ben Chekroun, K.; Gonzalez-Munoz, M. Conservation of ornamental stone by myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microbiol. 2003, 69, 2182–2193. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Chen, W.; Yu, L.; Li, W.; Yu, H. Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Process Biochem. 2010, 45, 1017–1021. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of temperature on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl. Microbiol. Biotechnol. 2013, 97, 1335–1347. [Google Scholar] [CrossRef]

| Parameter | Value | Reference |
|---|---|---|
| 4.1667 × 10−5 l/s | [51] | |
| 2.0 × 10−5 g/L | [37] | |
| 7.99 × 10−4 g/L | [52] | |
| 3.18 × 10−7 g/L | [52] | |
| 6.15 × 10−10 | [39] | |
| 6.15 × 10−7 mm/s | [39] | |
| 1.0 | [53] | |
| 2.0 g/L | [53] | |
| 2710 g/L | [37] | |
| 0.1 | [39] | |
| 100.09 g/mol | [39] | |
| Kinetic order n | 2 | [47] |
| Name | Concentration of Yeast Extract | Concentration of Polypeptone | Concentration of Ammonium Sulfate | Optical Density (OD600) |
|---|---|---|---|---|
| Optimized medium | 20 g/L | 10 g/L | 5 g/L | 1.68 |
| A medium | 20 g/L | 10 g/L | 0 g/L | 1.72 |
| B medium | 20 g/L | 20 g/L | 5 g/L | 2.12 |
| C medium | 33.63 g/L | 33.63 g/L | 0 g/L | 2.79 |
| D medium | 5 g/L | 5 g/L | 5 g/L | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Dai, S. Influence of Culture Medium on Cementation of Coarse Grains Based on Microbially Induced Carbonate Precipitation. Crystals 2022, 12, 188. https://doi.org/10.3390/cryst12020188
Gao H, Dai S. Influence of Culture Medium on Cementation of Coarse Grains Based on Microbially Induced Carbonate Precipitation. Crystals. 2022; 12(2):188. https://doi.org/10.3390/cryst12020188
Chicago/Turabian StyleGao, Hong, and Shuhong Dai. 2022. "Influence of Culture Medium on Cementation of Coarse Grains Based on Microbially Induced Carbonate Precipitation" Crystals 12, no. 2: 188. https://doi.org/10.3390/cryst12020188
APA StyleGao, H., & Dai, S. (2022). Influence of Culture Medium on Cementation of Coarse Grains Based on Microbially Induced Carbonate Precipitation. Crystals, 12(2), 188. https://doi.org/10.3390/cryst12020188





