Controllable Electrodeposition Adjusts the Electrochromic Properties of Co and Mo Co-Modified WO3 Films
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Preparation of Co and Mo Modified WO3 Films
2.3. Characterization
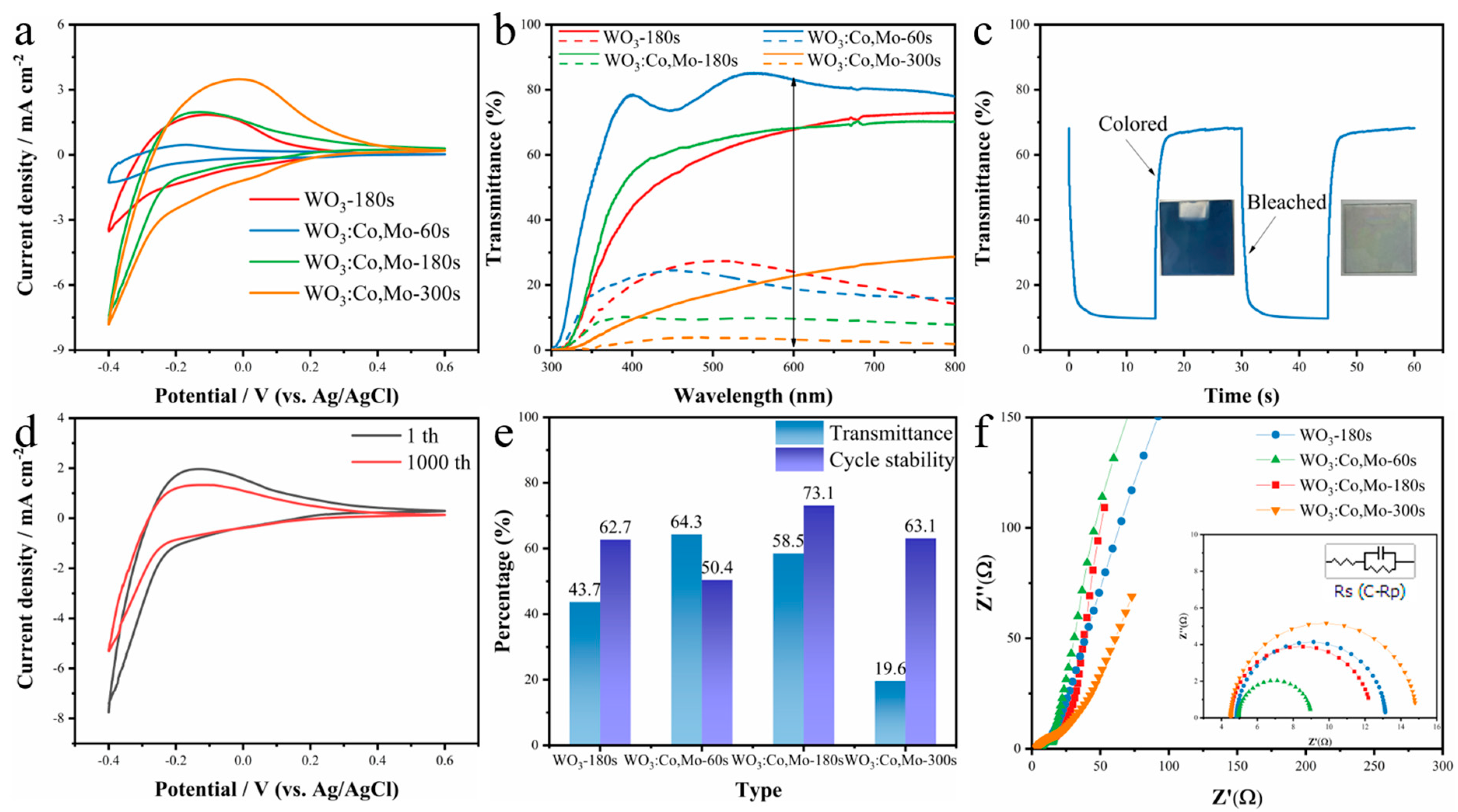
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhardt, V.; Kuhri, S.; Fleischhauer, J.; García-Iglesias, M.; González-Rodríguez, D.; Bottari, G.; Torres, T.; Guldi, D.M.; Faust, R. Light-harvesting with panchromatically absorbing BODIPY–porphyrazine conjugates to power electron transfer in supramolecular donor–acceptor ensembles. Chem. Sci. 2013, 4, 3888–3893. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, W.; Dang, J.; Wang, M.; Jian, K.; Liu, D.; Huang, D. Induction of Co2P Growth on a MXene (Ti3C2Tx)-Modified Self-Supporting Electrode for Efficient Overall Water Splitting. J. Phys. Chem. Lett. 2021, 12, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Ma, W.; Wang, M.; Dang, J.; Jian, K.; Liu, D.; Huang, D. Co-Constructing Interfaces of Multiheterostructure on MXene (Ti3C2Tx)-Modified 3D Self-Supporting Electrode for Ultraefficient Electrocatalytic HER in Alkaline Media. Adv. Funct. Mat. 2021, 31, 2102576. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, M.; Liu, D.; Jian, K.; Zhang, R.; Dang, J. Synergetic Effect of Ni2P and MXene Enhances Catalytic Activity in the Hydrogen Evolution Reaction. Inorg. Chem. 2021, 60, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, W.; Lv, Z.; Liu, D.; Jian, K.; Dang, J. Co-Doped Ni3N Nanosheets with Electron Redistribution as Bifunctional Electrocatalysts for Efficient Water Splitting. J. Phys. Chem. Lett. 2021, 12, 1581–1587. [Google Scholar] [CrossRef]
- Jian, K.; Ma, W.; Lv, Z.; Wang, M.; Lv, X.; Li, Q.; Dang, J. Tuning the Electronic Structure of the CoP/Ni2P Nanostructure by Nitrogen Doping for an Efficient Hydrogen Evolution Reaction in Alkaline Media. Inorg. Chem. 2021, 60, 18544–18552. [Google Scholar] [CrossRef]
- Granqvist, C.G. Oxide electrochromics: An introduction to devices and materials. Sol. Energy Mater. Sol. Cells 2012, 99, 1–13. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Sorar, I.; Welearegay, T.G.; Primetzhofer, D.; Österlund, L.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in Ni Oxide Thin Films Made by Advanced Gas Deposition and Sputtering: A Comparative Study Demonstrating the Significance of Surface Effects. J. Electrochem. Soc. 2020, 167, 116519. [Google Scholar] [CrossRef]
- Zhang, L.; Man, Y.; Zhu, Y. Effects of Mo Replacement on the Structure and Visible-Light-Induced Photocatalytic Performances of Bi2WO6 Photocatalyst. ACS Catal. 2011, 1, 841–848. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Bi, Q.; Zhang, X.; Guan, W.; Gao, Y. Revealing the modification mechanism of La-doped Ti/SnO2 electrodes related to the microelectronic structure by first-principles calculations. J. Alloys Compd. 2018, 747, 423–430. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; He, L.; Dong, Y.; Xu, X.; Liu, D.; Yan, H.; Yu, Q.; Huang, C.; Mai, L. In situ nitrogen-doped mesoporous carbon nanofibers as flexible freestanding electrodes for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 23620–23627. [Google Scholar] [CrossRef]
- Wu, W.; Fang, H.; Ma, H.; Wu, L.; Zhang, W.; Wang, H. Boosting Transport Kinetics of Ions and Electrons Simultaneously by Ti3C2Tx (MXene) Addition for Enhanced Electrochromic Performance. Nano-Micro Lett. 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Zheng, Y.; Cui, Y. High performance electrochromic energy storage devices based on Mo-doped crystalline/amorphous WO3 core-shell structures. Sol. Energy Mater. Sol. Cells 2022, 235, 111488. [Google Scholar] [CrossRef]
- Green, S.V.; Pehlivan, E.; Granqvist, C.G.; Niklasson, G.A. Electrochromism in sputter deposited nickel-containing tungsten oxide films. Sol. Energy Mater. Sol. Cells 2012, 99, 339–344. [Google Scholar] [CrossRef]
- Green, S.V.; Kuzmin, A.; Purans, J.; Granqvist, C.G.; Niklasson, G.A. Structure and composition of sputter-deposited nickel-tungsten oxide films. Thin Solid Film. 2011, 519, 2062–2066. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Shi, F.; Xie, D.; Wang, D.H.; Xia, X.H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Bi-functional Mo-doped WO3 nanowire array electrochromism-plus electrochemical energy storage. J. Colloid Interf. Sci. 2016, 465, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Sheng, K.; Wang, Z.; Zheng, J.; Xu, C. Cobalt ions doped tungsten oxide nanowires achieved vertically aligned nanostructure with enhanced electrochromic properties. Appl. Surf. Sci. 2020, 501, 144003. [Google Scholar] [CrossRef]
- Xie, S.; Bi, Z.; Chen, Y.; He, X.; Guo, X.; Gao, X.; Li, X. Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl. Surf. Sci. 2018, 459, 774–781. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.F.; Zhou, D.; Xiong, Q.Q.; Zhang, J.H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Efficient electrochromic materials based on TiO2@WO3 core/shell nanorod arrays. Sol. Energy Mater. Sol. Cells 2013, 117, 231–238. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.P.; Cai, G.F.; Du, G.H.; Wang, X.L.; Liu, P.C. Enhanced electrochromic performance of highly ordered, macroporous WO3 arrays electrodeposited using polystyrene colloidal crystals as template. Electrochim. Acta 2013, 99, 1–8. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; Chen, Y.; He, X.; Xu, X.; Gao, X. Large-Scale Multifunctional Electrochromic-Energy Storage Device Based on Tungsten Trioxide Monohydrate Nanosheets and Prussian White. ACS Appl. Mater. Inter. 2017, 9, 29872–29880. [Google Scholar] [CrossRef]
- Cai, G.F.; Gu, C.D.; Zhang, J.; Liu, P.C.; Wang, X.L.; You, Y.H.; Tu, J.P. Ultra fast electrochromic switching of nanostructured NiO films electrodeposited from choline chloride-based ionic liquid. Electrochim. Acta 2013, 87, 341–347. [Google Scholar] [CrossRef]
- Au, B.W.C.; Tamang, A.; Knipp, D.; Chan, K.Y. Post-annealing effect on the electrochromic properties of WO3 films. Opt. Mater. 2020, 108, 110426. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Zhou, D.; Zhang, J.; Xiong, Q.; Gu, C.; Tu, J. Hierarchical structure Ti-doped WO3 film with improved electrochromism in visible-infrared region. RSC Adv. 2013, 3, 6896–6905. [Google Scholar] [CrossRef]
- Llordés, A.; Garcia, G.; Gazquez, J.; Milliron, D.J. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites. Nature 2013, 500, 323–326. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; To, B.; Mahan, A.H.; Dillon, A.C. Crystalline WO3 Nanoparticles for Highly Improved Electrochromic Applications. Adv. Mater. 2006, 18, 763–766. [Google Scholar] [CrossRef]
- Cai, G.; Cui, M.; Kumar, V.; Darmawan, P.; Wang, J.; Wang, X.; Lee-Sie Eh, A.; Qian, K.; Lee, P.S. Ultra-large optical modulation of electrochromic porous WO3 film and the local monitoring of redox activity. Chem. Sci. 2016, 7, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Sun, P.; Chai, Z.; Huang, L.; Cai, X.; Tan, S.; Song, J.; Mai, W. Large-Scale Fabrication of Pseudocapacitive Glass Windows that Combine Electrochromism and Energy Storage. Angew. Chem. Int. Edit. 2014, 53, 11935–11939. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.R.; Kim, K.H.; Ahn, H.J. Switching electrochromic performance improvement enabled by highly developed mesopores and oxygen vacancy defects of Fe-doped WO3 films. Appl. Surf. Sci. 2018, 453, 238–244. [Google Scholar] [CrossRef]
- Bon-Ryul, K.; Kim, K.H.; Ahn, H.J. Novel tunneled phosphorus-doped WO3 films achieved using ignited red phosphorus for stable and fast switching electrochromic performances. Nanoscale 2019, 11, 3318–3325. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Ma, W.; Zhuang, Q.; Zhang, Y.; Dang, J. Controllable Electrodeposition Adjusts the Electrochromic Properties of Co and Mo Co-Modified WO3 Films. Crystals 2022, 12, 190. https://doi.org/10.3390/cryst12020190
Jia L, Ma W, Zhuang Q, Zhang Y, Dang J. Controllable Electrodeposition Adjusts the Electrochromic Properties of Co and Mo Co-Modified WO3 Films. Crystals. 2022; 12(2):190. https://doi.org/10.3390/cryst12020190
Chicago/Turabian StyleJia, Lihong, Wansen Ma, Qianyu Zhuang, Yani Zhang, and Jie Dang. 2022. "Controllable Electrodeposition Adjusts the Electrochromic Properties of Co and Mo Co-Modified WO3 Films" Crystals 12, no. 2: 190. https://doi.org/10.3390/cryst12020190
APA StyleJia, L., Ma, W., Zhuang, Q., Zhang, Y., & Dang, J. (2022). Controllable Electrodeposition Adjusts the Electrochromic Properties of Co and Mo Co-Modified WO3 Films. Crystals, 12(2), 190. https://doi.org/10.3390/cryst12020190






