A Simple Population Balance Model for Crystallization of L-Lactide in a Mixture of n-Hexane and Tetrahydrofuran
Abstract
:1. Introduction
2. Modeling
3. Materials and Methods
3.1. Materials and Equipments
3.2. Solubility Measurement
3.3. Preparation of Seed Crystals
3.4. Crystallization Experimental Setup
3.5. Microscopic Analysis
4. Results and Discussion
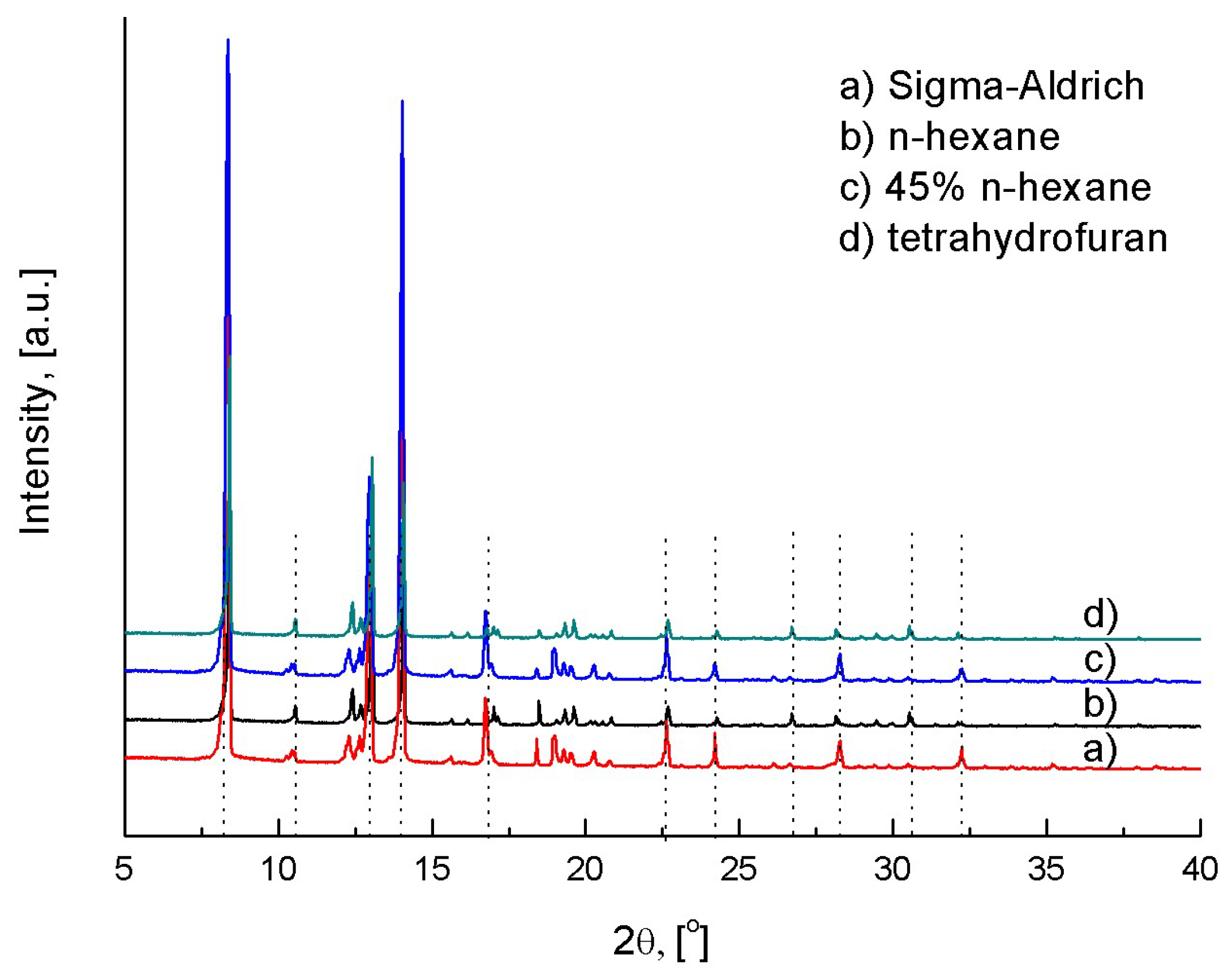
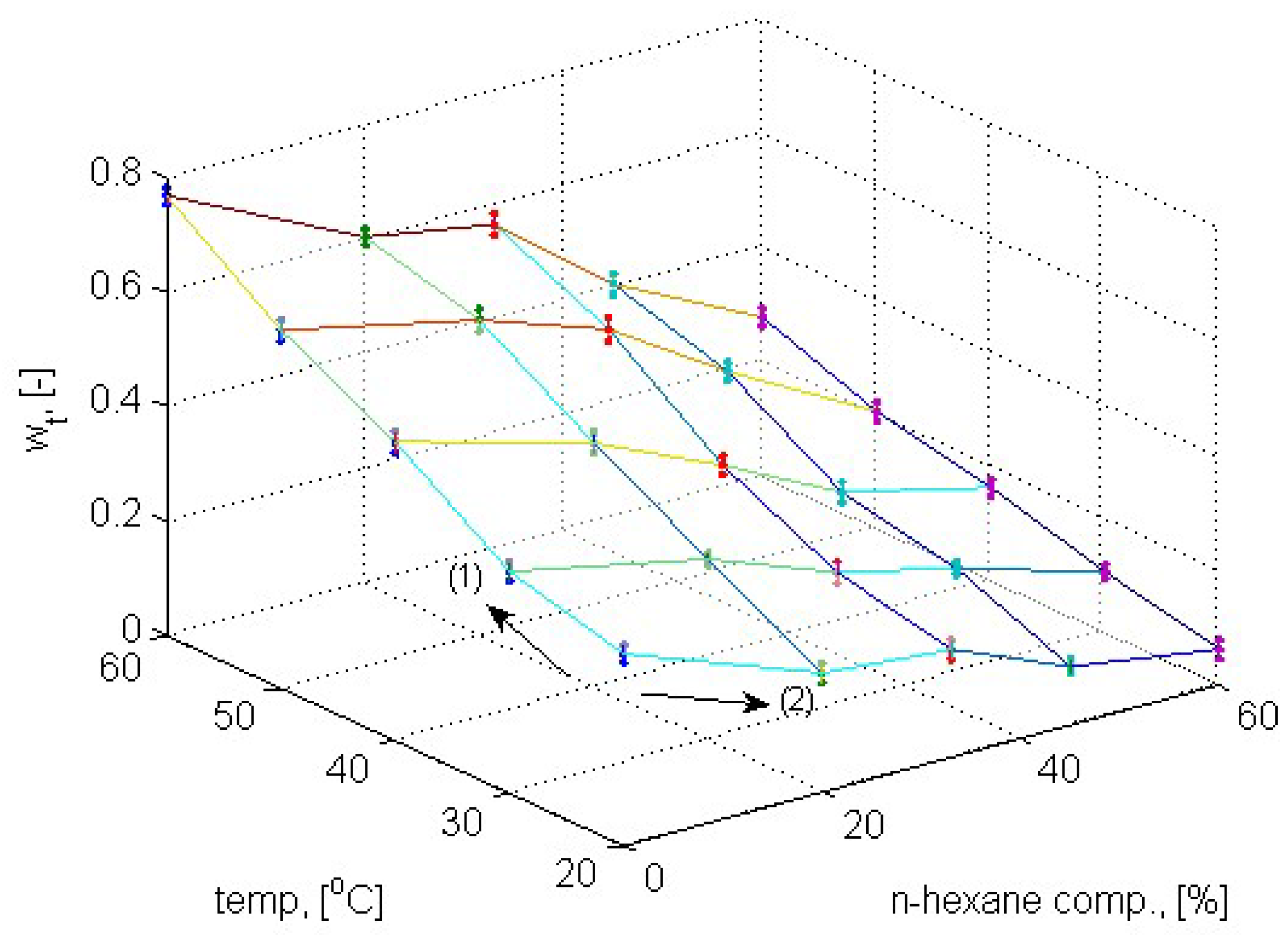
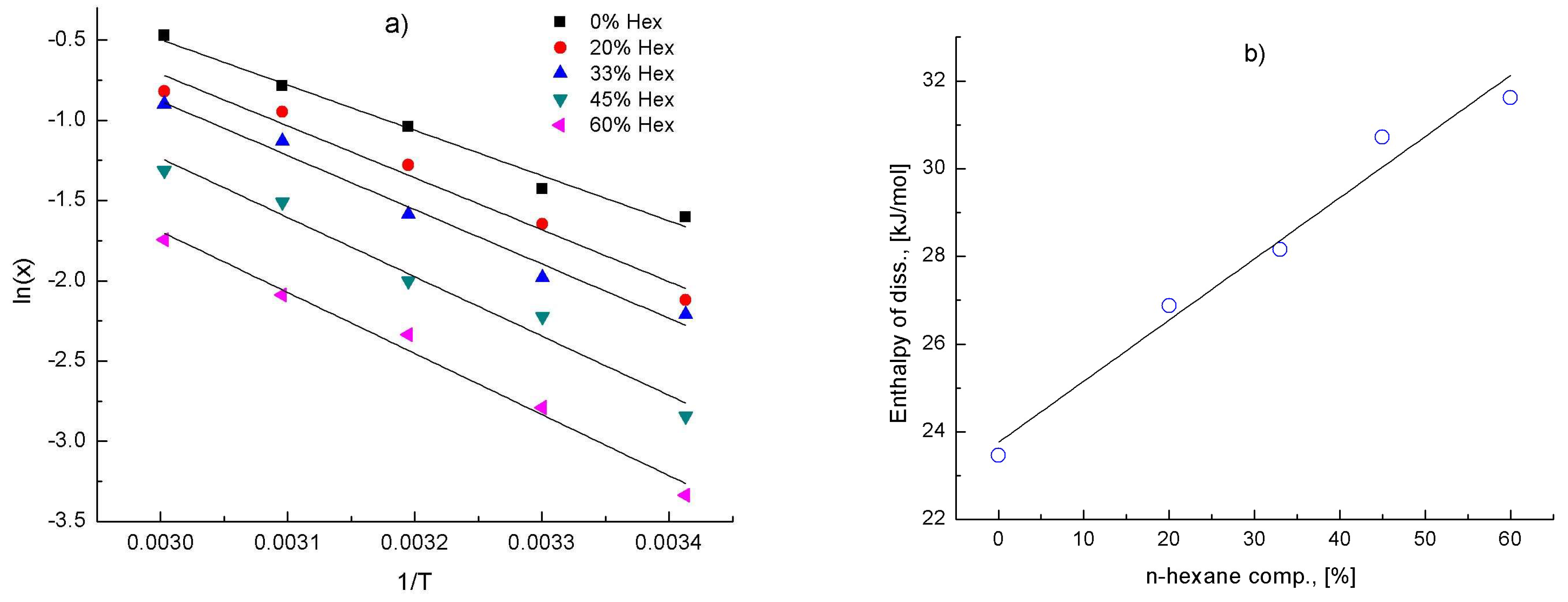
4.1. Anti-Solvent Effects of n-Hexane on L-Lactide/THF Solutions
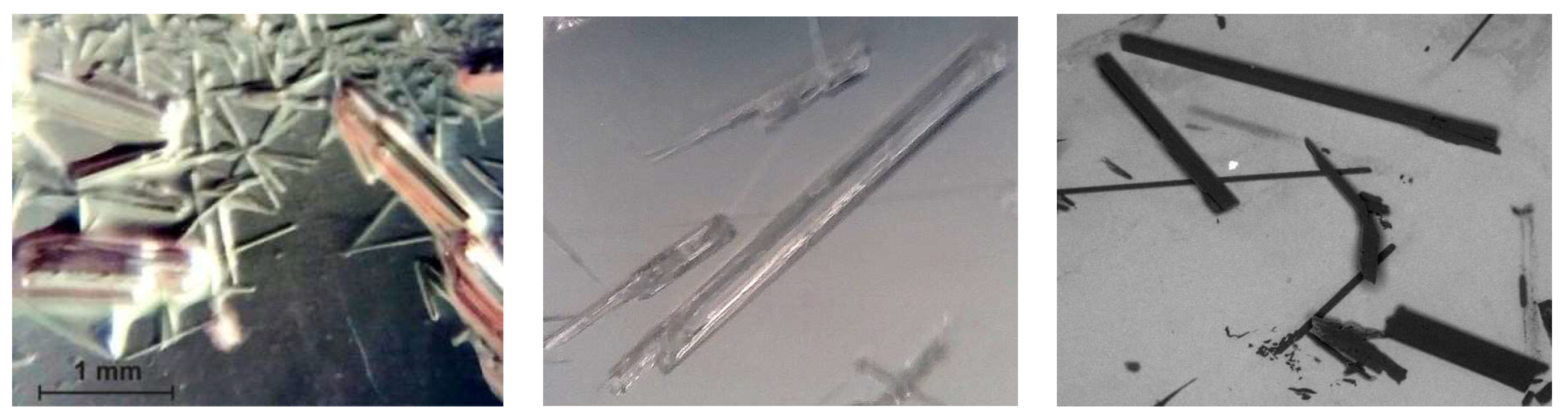
4.2. Crystal Shape of L-Lactide and the Shape Factor kv
4.3. Solving the PBE for L-Lactide System
4.4. Variation of Solute Concentration during Crystallization and Model Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Latin | ||
| B | birth rate | [#·s−1] |
| b | the exponent in birth term | [-] |
| G | growth rate | [m·s−1] |
| g | the exponent in growth term | [-] |
| i | index | [-] |
| kg | crystal growth constant | [m·s−1] |
| kb | secondary nucleation constant | [#·m−3·s−1] |
| kv | shape factor | [-] |
| L | characteristic dimension | [m] |
| m0 | mass of empty vessel | [g] |
| m1 | mass of saturated solution and empty vessel | [g] |
| m2 | mass of solute and empty vessel | [g] |
| m | mass of solute in mother liquid | [g] |
| mobtained | obtained solid after crystallization | [g] |
| minitial | total solute in initial solution | [g] |
| n | number of particles/elements | [#] |
| R | the universal gas constant | [J·mol−1·K−1] |
| Pr | process productivity | [] |
| t | time variable | [s] |
| T | absolute temperature | [K] |
| w, wsat | solution and saturated concentrations | [] |
| Y | process yield | [%] |
| enthalpy of dissolution | [kJ·mol−1] | |
| Greek | ||
| µi | the moment ith | [-] |
| solid density | [g·cm−3] | |
| Ψ | number density function | [#·m−1] |
References
- El-Zhry El-Yafi, A.K.; El-Zein, H. Technical crystallization for application in pharmaceutical material engineering: Review article. Asian J. Pharm. Sci. 2015, 10, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Sato, K. Crystallization of Lipids Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.E.; Seckler, M.; Kramer, H.; van Rosmalen, G. Industrial Crystallization: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Leubner, I.H. Precision Crystallization: Theory and Practice of Controlling Crystal Size, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Woburn, MA, USA, 2001. [Google Scholar]
- Myerson, A.S.; Erdemir, D.; Lee, A.Y. Handbook of Industrial Crystallization, 3rd ed.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Randolph, A.D. Theory of Particulate Processes, 2nd ed.; Academic Press: San Diego, CA, USA, 1988. [Google Scholar]
- Hu, Q.; Rohani, S.; Wang, D.X.; Jutan, A. Nonlinear Kinetic Parameter Estimation for Batch Cooling Seeded Crystallization. AIChE J. 2004, 50, 1786–1794. [Google Scholar] [CrossRef]
- Czapla, F.; Haida, H.; Elsner, M.P.; Lorenz, H.; Seidel-Morgenstern, A. Parameterization of population balance models for polythermal auto seeded preferential crystallization of enantiomers. Chem. Eng. Sci. 2009, 64, 753–763. [Google Scholar] [CrossRef]
- Rigopoulos, S.; Jones, A.G. Finite-Element Scheme for Solution of the Dynamic Population Balance Equation. AIChE J. 2003, 49, 1127–1139. [Google Scholar] [CrossRef]
- Costa, L.I.; Storti, G.; Lazzari, S. Solution of population balance equations by logarithmic shape preserving interpolation on finite elements. Comput. Chem. Eng. 2018, 119, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Banerrjee, S.; Sengupt, B. A Stratified Monte Carlo Simulation of CSD in a Continuous Crystallizer for Size-dependent Growth. Cryst. Res. Technol. 1994, 29, 505–508. [Google Scholar] [CrossRef]
- Lina, Y.; Leeb, K.; Matsoukasa, T. Solution of the population balance equation using constant-number Monte Carlo. Chem. Eng. Sci. 2002, 57, 2241–2252. [Google Scholar] [CrossRef]
- Barrett, C.; Jheeta, J.S. Improving the accuracy of the moments method for solving the aerosol general dynamic equation. J. Aerosol Sci. 1996, 27, 1135–1142. [Google Scholar] [CrossRef]
- Madras, G.; McCoy, B.J. Reversible crystal growth–dissolution and aggregation–breakage: Numerical and moment solutions for population balance equations. Powder Technol. 2004, 143–144, 297–307. [Google Scholar] [CrossRef]
- Marchisio, D.L.; Fox, R.O. Solution of population balance equations using the direct quadrature method of moments. J. Aerosol Sci. 2005, 36, 43–73. [Google Scholar] [CrossRef]
- Févotte, F.; Févotte, G. A method of characteristics for solving Population Balance Equations (PBE) describing the adsorption of impurities during crystallization processes. Chem. Eng. Sci. 2010, 65, 3191–3198. [Google Scholar] [CrossRef] [Green Version]
- Pilon, L.; Viskanta, R. Modified method of characteristics for solving population balance equations. Int. J. Numer. Meth. Fluids 2003, 42, 1211–1236. [Google Scholar] [CrossRef]
- Rehman, M.S.; Qamar, S. Application of the Method of Characteristics to Population Balance Models Considering Growth and Nucleation Phenomena. Appl. Math. 2014, 5, 1853–1862. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Ma, C.; Xu, P. Biotechnological routes based on lactic acid production from biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Buchholz, H.; Le Minh, T.; Lorenz, H.; Seidel-Morgenstern, A. Solubility determination and prediction of the lactide species in different solvent systems. In Proceedings of the 20th International Workshop on Industrial Crystallization, Odense, Denmark, 18–20 September 2013; pp. 319–324, ISBN 978-87-92646-73-6. [Google Scholar]
- Chen, Z.; Xie, C.; Xu, Z.; Wang, Y.; Zhao, H.; Hao, H. Determination and Correlation of Solubility Data and Dissolution Thermodynamic Data of L-Lactide in Different Pure Solvents. J. Chem. Eng. Data 2013, 58, 143–150. [Google Scholar] [CrossRef]
- Elsner, M.P.; Menéndez, D.F.; Muslera, E.A.; Seidel-Morgenstern, A. Experimental study and simplified mathematical description of preferential crystallization. Chirality 2005, 17, S183–S195. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.M. Modelling and Quality Control Strategies for Batch Cooling Crystallizers. Ph.D Thesis, The University of Texas at Austin, Austin, TX, USA, 1993. [Google Scholar]
- Heryanto, R.; Hasan, M.; Abdullah, E.C.; Kumoro, A.C. Solubility of Stearic Acid in Various Organic Solvents and Its Prediction using Non-ideal Solution Models. Sci. Asia 2007, 33, 469–472. [Google Scholar] [CrossRef]
- Le Minh, T.; von Langermann, J.; Lorenz, H.; Seidel-Morgenstern, A. Enantiomeric 3-chloromandelic acid system: Binary melting point phase diagram, ternary solubility phase diagrams and polymorphism. J. Pharm. Sci. 2010, 9, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Z.; Jiang, X.; Rohani, S. Solubility of L-phenylalanine in aqueous solutions. Chem. Eng. 2021, 43, 810–813. [Google Scholar] [CrossRef]
- Nowee, S.M.; Abbas, A.; Romagnoli, J.A. Antisolvent crystallization: Model identification, experimental validation and dynamic simulation. Chem. Eng. Sci. 2008, 63, 5457–5467. [Google Scholar] [CrossRef]
- Schall, J.M.; Capellades, G.; Myerson, A.S. Methods for Estimating Supersaturation in Antisolvent Crystallization Systems. CrystEngComm 2019, 21, 5811–5817. [Google Scholar] [CrossRef] [Green Version]
- Kurup, M.; Raj, R.A. Antisolvent crystallization: A novel approach to bioavailability enhancement. Eur. J. Biomed. Pharm. Sci. 2016, 3, 230–234. [Google Scholar]
- Saleeby, E.G.; Lee, H.W. On the solution of the PBE with agglomeration and random growth rate dispersion. Chem. Eng. Sci. 1995, 50, 1971–1981. [Google Scholar] [CrossRef]
- Ouchiyama, N.; Rough, S.L.; Bridgwater, J. A population balance approach to describing bulk attrition. Chem. Eng. Sci. 2005, 60, 1429–1440. [Google Scholar] [CrossRef]


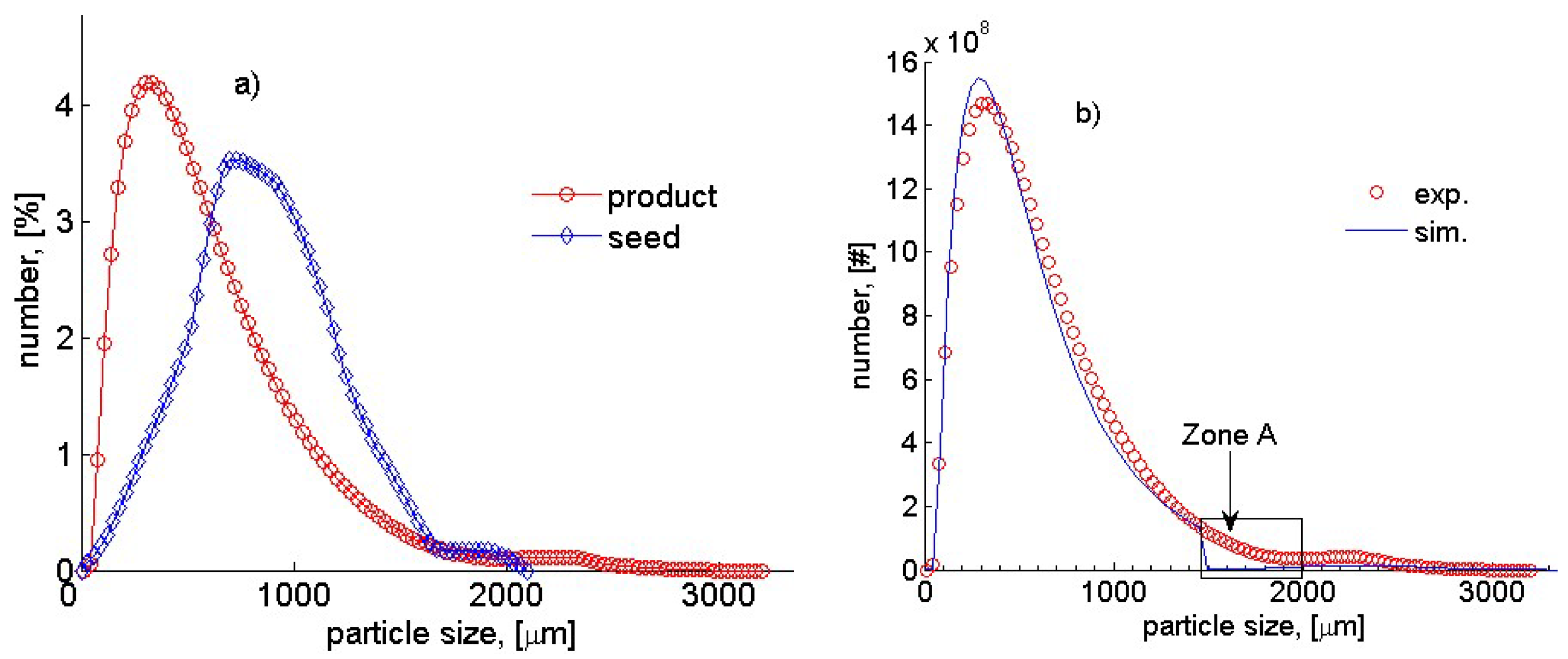
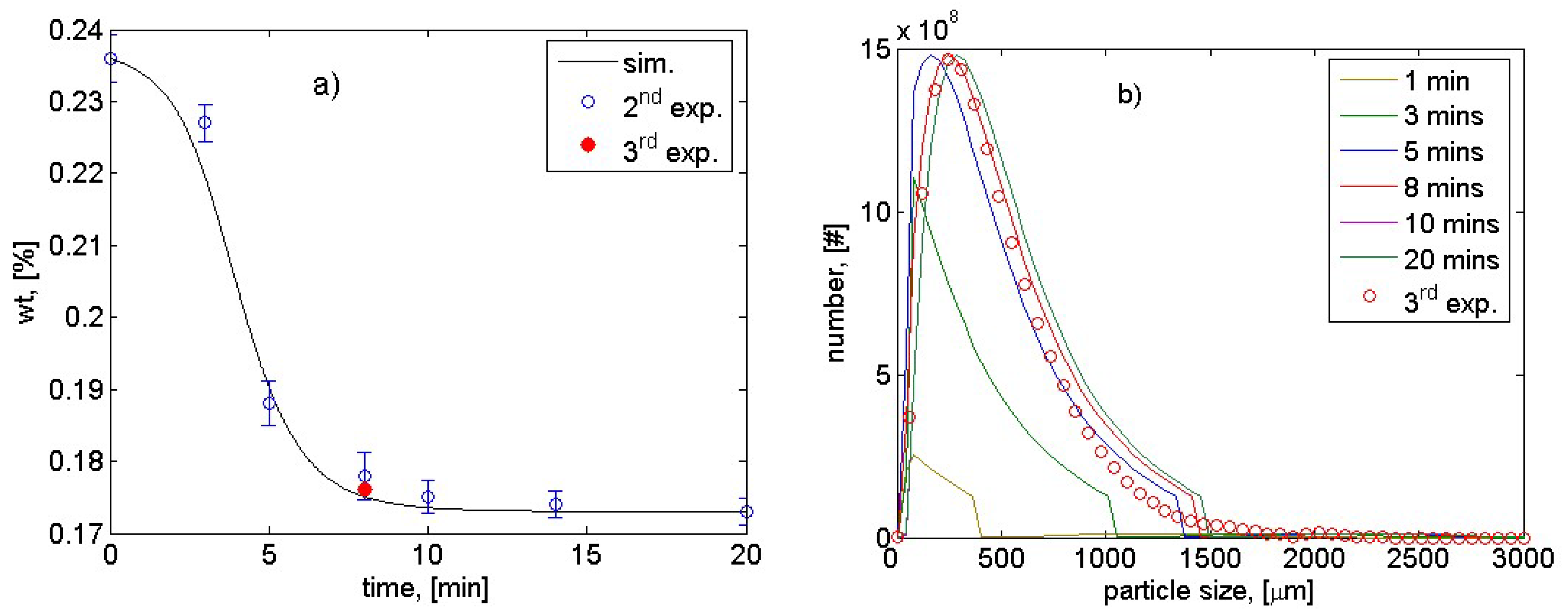
| Parameter | Estimated Value | Percentage Confidence Interval, [%] |
|---|---|---|
| kb, [#.m−3.s−1] | 2.916 × 108 | 0.3 |
| kg, [m.s−1] | 1.067 × 10−3 | 6.1 |
| b, [-] | 2.017 | 2.8 |
| g, [-] | 1.109 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Minh, T.; Phan Thanh, T.; Nguyen Thi Hong, N.; Phan Minh, V. A Simple Population Balance Model for Crystallization of L-Lactide in a Mixture of n-Hexane and Tetrahydrofuran. Crystals 2022, 12, 221. https://doi.org/10.3390/cryst12020221
Le Minh T, Phan Thanh T, Nguyen Thi Hong N, Phan Minh V. A Simple Population Balance Model for Crystallization of L-Lactide in a Mixture of n-Hexane and Tetrahydrofuran. Crystals. 2022; 12(2):221. https://doi.org/10.3390/cryst12020221
Chicago/Turabian StyleLe Minh, Tam, Thao Phan Thanh, No Nguyen Thi Hong, and Vuong Phan Minh. 2022. "A Simple Population Balance Model for Crystallization of L-Lactide in a Mixture of n-Hexane and Tetrahydrofuran" Crystals 12, no. 2: 221. https://doi.org/10.3390/cryst12020221





