Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium
Abstract
:1. Introduction
2. Experimental Part
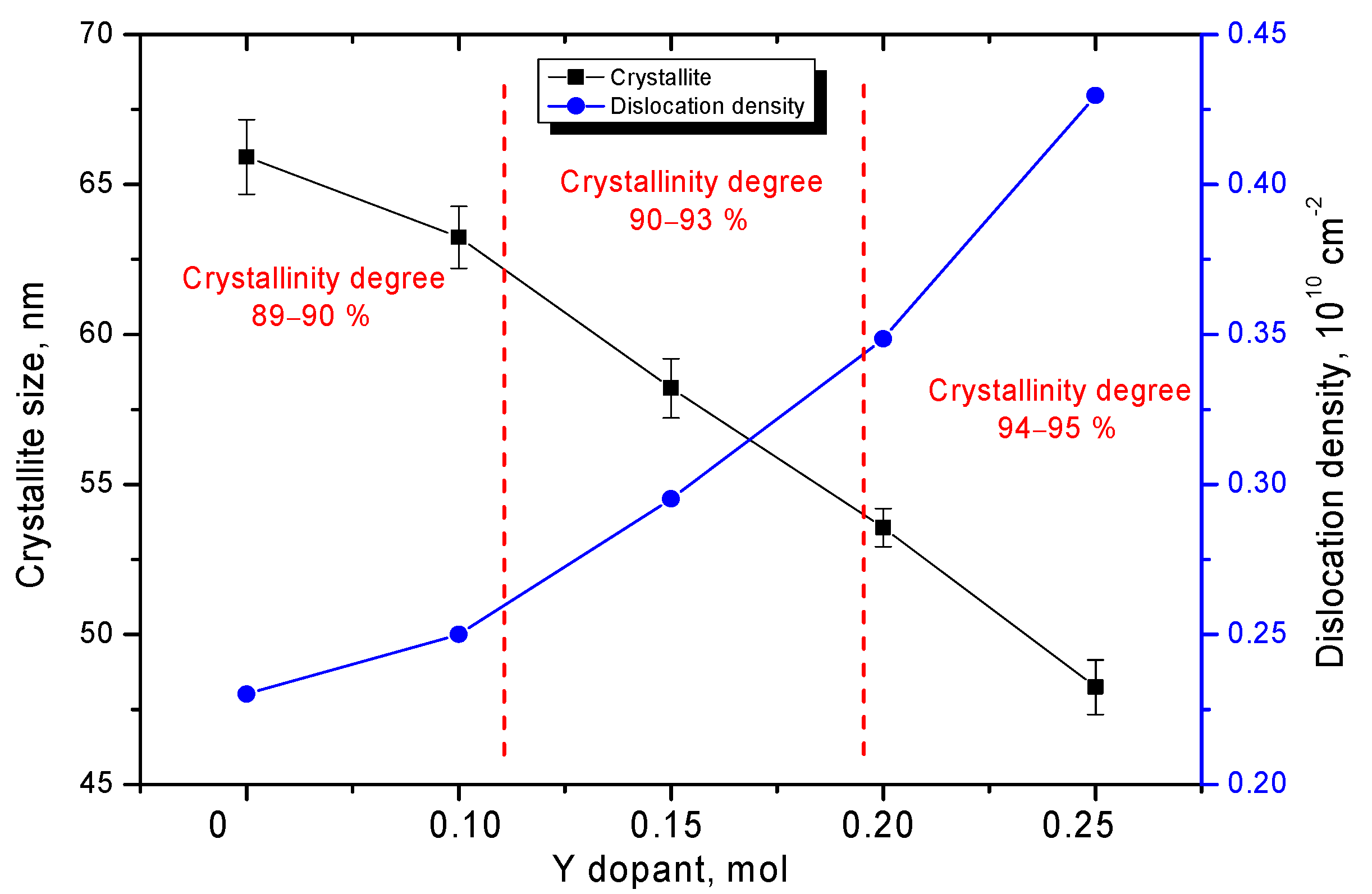
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lombardi, C.; Luzzi, L.; Padovani, E.; Vettraino, F. Thoria and inert matrix fuels for a sustainable nuclear power. Prog. Nucl. Energy 2008, 50, 944–953. [Google Scholar] [CrossRef]
- Ledergerber, G.; Degueldre, C.; Heimgartner, P.; Pouchon, M.; Kasemeyer, U. Inert matrix fuel for the utilisation of plutonium. Prog. Nucl. Energy 2001, 38, 301–308. [Google Scholar] [CrossRef]
- Nandi, C.; Grover, V.; Bhandari, K.; Bhattacharya, S.; Mishra, S.; Banerjee, J.; Prakash, A.; Tyagi, A. Exploring YSZ/ZrO2—PuO2 systems: Candidates for inert matrix fuel. J. Nucl. Mater. 2018, 508, 82–91. [Google Scholar] [CrossRef]
- Golovkina, L.S.; Orlova, A.I.; Nokhrin, A.V.; Boldin, M.S.; Lantsev, E.A.; Chuvil’deev, V.N.; Sakharov, N.V.; Shotin, S.V.; Zelenov, A.Y. Spark Plasma Sintering of fine-grained ceramic-metal composites YAG: Nd-(W, Mo) based on garnet-type oxide Y2.5Nd0.5Al5O12 for inert matrix fuel. J. Nucl. Mater. 2018, 511, 109–121. [Google Scholar] [CrossRef]
- Ang, C.; Snead, L.; Kato, Y. A logical approach for zero-rupture Fully Ceramic Microencapsulated (FCM) fuels via pressure-assisted sintering route. J. Nucl. Mater. 2020, 531, 151987. [Google Scholar] [CrossRef]
- Savchyn, V.P.; Popov, A.I.; Aksimentyeva, O.I.; Klym, H.; Horbenko, Y.Y.; Serga, V.; Moskina, A.; Karbovnyk, I. Cathodoluminescence characterization of polystyrene-BaZrO3 hybrid composites. Low Temp. Phys. 2016, 42, 597–600. [Google Scholar] [CrossRef]
- Van Vuuren, A.J.; Skuratov, V.; Ibrayeva, A.; Zdorovets, M. Microstructural Effects of Al Doping on Si3N4 Irradiated with Swift Heavy Ions. Acta Phys. Pol. A 2019, 136, 241–244. [Google Scholar] [CrossRef]
- Settar, A.; Abboudi, S.; Lebaal, N. Effect of inert metal foam matrices on hydrogen production intensification of methane steam reforming process in wall-coated reformer. Int. J. Hydrogen Energy 2018, 43, 12386–12397. [Google Scholar] [CrossRef]
- Manara, D.; Naji, M.; Mastromarino, S.; Elorrieta, J.; Magnani, N.; Martel, L.; Colle, J.-Y. The Raman fingerprint of plutonium dioxide: Some example applications for the detection of PuO2 in host matrices. J. Nucl. Mater. 2018, 499, 268–271. [Google Scholar] [CrossRef]
- Mikhailov, D.A.; Orlova, A.I.; Malanina, N.V.; Nokhrin, A.V.; Potanina, E.A.; Chuvil’deev, V.N.; Boldin, M.S.; Sakharov, N.V.; Belkin, O.A.; Kalenova, M.Y.; et al. A study of fine-grained ceramics based on complex oxides ZrO2-Ln2O3 (Ln = Sm, Yb) obtained by Spark Plasma Sintering for inert matrix fuel. Ceram. Int. 2018, 44, 18595–18608. [Google Scholar] [CrossRef]
- Monge, M.A.; González, R.; Muñoz Santiuste, J.E.; Pareja, R.; Chen, Y.; Kotomin, E.A.; Popov, A.I. Photoconversion and dynamic hole recycling process in anion vacancies in neutron-irradiated MgO crystals. Phys. Rev. B 1999, 60, 3787–3791. [Google Scholar] [CrossRef]
- Monge, M.A.; González, R.; Muñoz-Santiuste, J.E.; Pareja, R.; Chen, Y.; Kotomin, E.; Popov, A. Photoconversion of F+ centers in neutron-irradiated MgO. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2000, 166, 220–224. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Tinishbaeva, K.; Uglov, V.V. Investigation of the effect of exposure to heavy Xe22+ ions on the mechanical properties of carbide ceramics. Eurasian Phys. Tech. J. 2021, 17, 46–53. [Google Scholar] [CrossRef]
- Popov, A.; Lushchik, A.; Shablonin, E.; Vasil’Chenko, E.; Kotomin, E.; Moskina, A.; Kuzovkov, V. Comparison of the F-type center thermal annealing in heavy-ion and neutron irradiated Al2O3 single crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 433, 93–97. [Google Scholar] [CrossRef]
- Degueldre, C.; Hellwig, C. Study of a zirconia based inert matrix fuel under irradiation. J. Nucl. Mater. 2003, 320, 96–105. [Google Scholar] [CrossRef]
- Chauvin, N.; Konings, R.; Matzke, H. Optimisation of inert matrix fuel concepts for americium transmutation. J. Nucl. Mater. 1999, 274, 105–111. [Google Scholar] [CrossRef]
- Vu, T.M.; Hartanto, D.; Chu, T.N.; Pham, N.V.H.; Bui, T.H. Sensitivity and uncertainty analysis of neutronic and kinetic parameters for CERCER and CERMET fueled ADS using SERPENT 2 and ENDF/B-VIII.0. Ann. Nucl. Energy 2021, 168, 108912. [Google Scholar] [CrossRef]
- Costa, D.R.; Liu, H.; Lopes, D.A.; Middleburgh, S.C.; Wallenius, J.; Olsson, P. Interface interactions in UN-X-UO2 systems (X= V, Nb, Ta, Cr, Mo, W) by pressure-assisted diffusion experiments at 1773 K. J. Nucl. Mater. 2022, 561, 153554. [Google Scholar] [CrossRef]
- Alin, M.; Kozlovskiy, A.L.; Zdorovets, M.V.; Uglov, V.V. Study of the mechanisms of the t-ZrO2→ c-ZrO2 type polymorphic transformations in ceramics as a result of irradiation with heavy Xe22+ ions. Solid State Sci. 2022, 123, 106791. [Google Scholar] [CrossRef]
- Ivanov, I.A.; Alin, M.; Koloberdin, M.V.; Sapar, A.; Kurakhmedov, A.E.; Kozlovskiy, A.L.; Zdorovets, M.V.; Uglov, V.V. Effect of irradiation with heavy Xe22+ ions with energies of 165–230 MeV on change in optical characteristics of ZrO2 ceramic. Opt. Mater. 2021, 120, 111479. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.X.; Duan, L.; Fan, J.B.; Ni, L.; Ji, V. A comparison study of the structural and mechanical properties of cubic, tetragonal, monoclinic, and three orthorhombic phases of ZrO2. J. Alloys Compd. 2018, 749, 283–292. [Google Scholar] [CrossRef]
- Aksimentyeva, O.I.; Savchyn, V.P.; Dyakonov, V.P.; Piechota, S.; Horbenko, Y.Y.; Opainych, I.Y.; Demchenko, P.Y.; Popov, A.; Szymczak, H. Modification of Polymer-Magnetic Nanoparticles by Luminescent and Conducting Substances. Mol. Cryst. Liq. Cryst. 2014, 590, 35–42. [Google Scholar] [CrossRef]
- Kim, S.-E.; Yoon, J.-K.; Shon, I.-J. Mechanochemical Synthesis and Rapid Consolidation of Nanostructured Nb-ZrO2 Composite. J. Nanosci. Nanotechnol. 2020, 20, 4253–4256. [Google Scholar] [CrossRef] [PubMed]
- Shelyug, A.; Navrotsky, A. Thermodynamic stability of the fluorite phase in the CeO2− CaO− ZrO2 system. J. Nucl. Mater. 2019, 517, 80–85. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, V.; Gupta, D.; Upadhyay, S.; Ram, J.; Kumar, S. Advancement of High–k ZrO2 for Potential Applications: A Review. Indian J. Pure Appl. Phys. (IJPAP) 2021, 59, 811–826. [Google Scholar]
- Alekseeva, L.S.; Nokhrin, A.V.; Boldin, M.S.; Lantsev, E.A.; Orlova, A.I.; Chuvil’Deev, V.N.; Sakharov, N.V. Preparation of Fine-Grained CeO2–SiC Ceramics for Inert Fuel Matrices by Spark Plasma Sintering. Inorg. Mater. 2020, 56, 1307–1313. [Google Scholar] [CrossRef]
- Hudák, I.; Skryja, P.; Bojanovský, J.; Jegla, Z.; Krňávek, M. The Effect of Inert Fuel Compounds on Flame Characteristics. Energies 2021, 15, 262. [Google Scholar] [CrossRef]
- Mareš, K.V.; John, J. Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: Part 1—concept design and assessment. J. Radioanal. Nucl. Chem. 2019, 320, 227–233. [Google Scholar] [CrossRef]
- Baron, R.; Wejrzanowski, T.; Milewski, J.; Szablowski, L.; Szczęśniak, A.; Fung, K.-Z. Manufacturing of γ -LiAlO2 matrix for molten carbonate fuel cell by high-energy milling. Int. J. Hydrogen Energy 2018, 43, 6696–6700. [Google Scholar] [CrossRef]
- Sharma, S.K.; Grover, V.; Tyagi, A.; Avasthi, D.; Singh, U.; Kulriya, P. Probing the temperature effects in the radiation stability of Nd2Zr2O7 pyrochlore under swift ion irradiation. Materialia 2019, 6, 100317. [Google Scholar] [CrossRef]
- Mistarihi, Q.M.; Park, W.; Nam, K.; Yahya, M.-S.; Kim, Y.; Ryu, H.J. Fabrication of oxide pellets containing lumped Gd2O3 using Y2O3-stabilized ZrO2 for burnable absorber fuel applications. Int. J. Energy Res. 2018, 42, 2141–2151. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Tian, C.; Yan, Z.; Liu, Z.; Yu, J. Electrical conductivity behavior of ZrO2-MgO-Y2O3 ceramic: Effect of heat treatment temperature. J. Aust. Ceram. Soc. 2022, 1–7. [Google Scholar] [CrossRef]
- Marek, I.O.; Dudnik, O.V.; Korniy, S.A.; Red’ko, V.P.; Danilenko, M.I.; Ruban, O.K. Effect of Heat Treatment in the Temperature Range 400−1300 °C on the Properties of Nanocrystalline ZrO2− Y2O3− CeO2 Powders. Powder Metall. Met. Ceram. 2022, 60, 1–11. [Google Scholar] [CrossRef]
- Dehm, G.; Jaya, B.; Raghavan, R.; Kirchlechner, C. Overview on micro- and nanomechanical testing: New insights in interface plasticity and fracture at small length scales. Acta Mater. 2018, 142, 248–282. [Google Scholar] [CrossRef]
- Nykyforchyn, H.; Kyryliv, V.; Maksymiv, O.; Zvirko, O. Mechanical fabrication methods of nanostructured surfaces. In Handbook of Modern Coating Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–67. [Google Scholar]
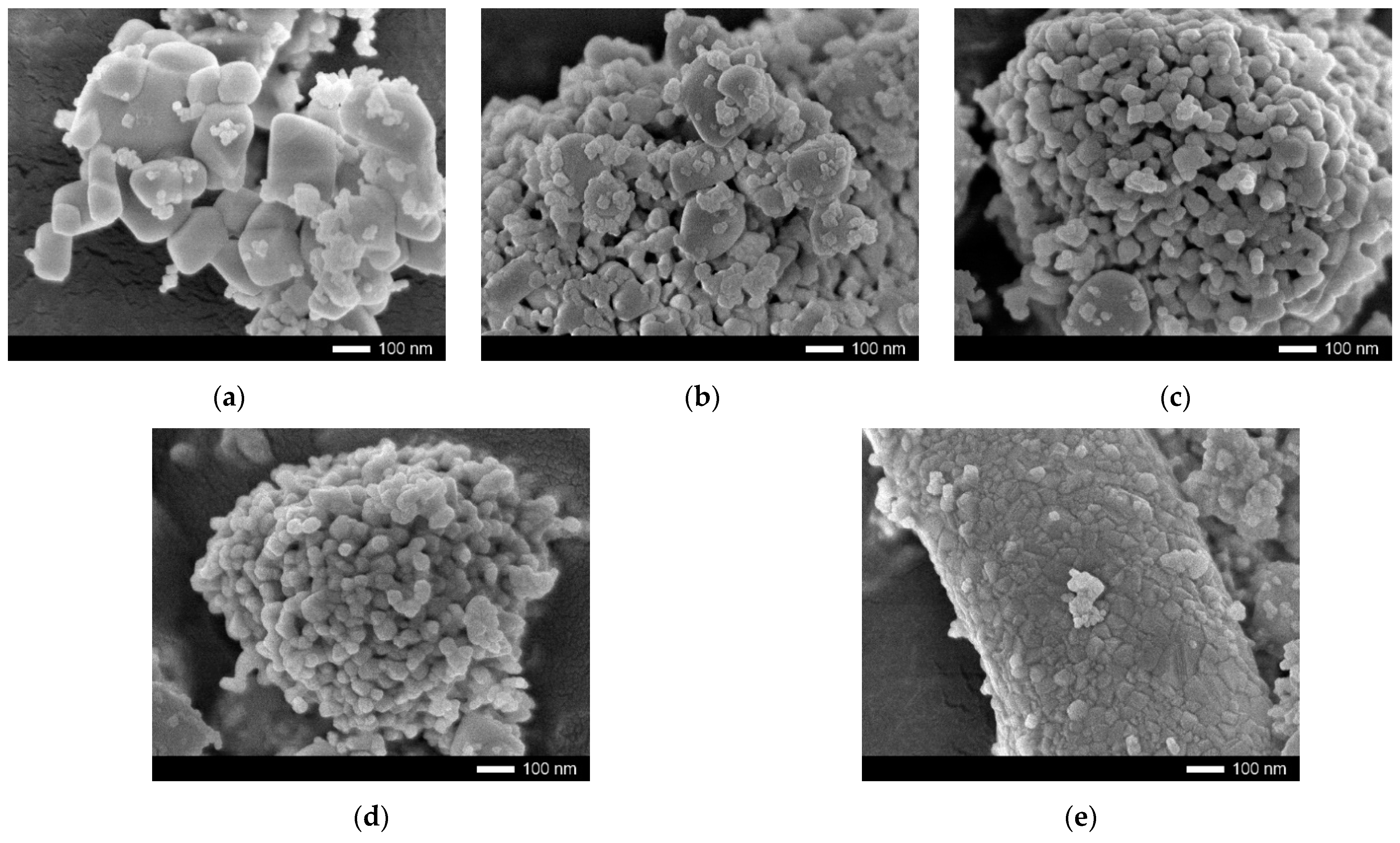
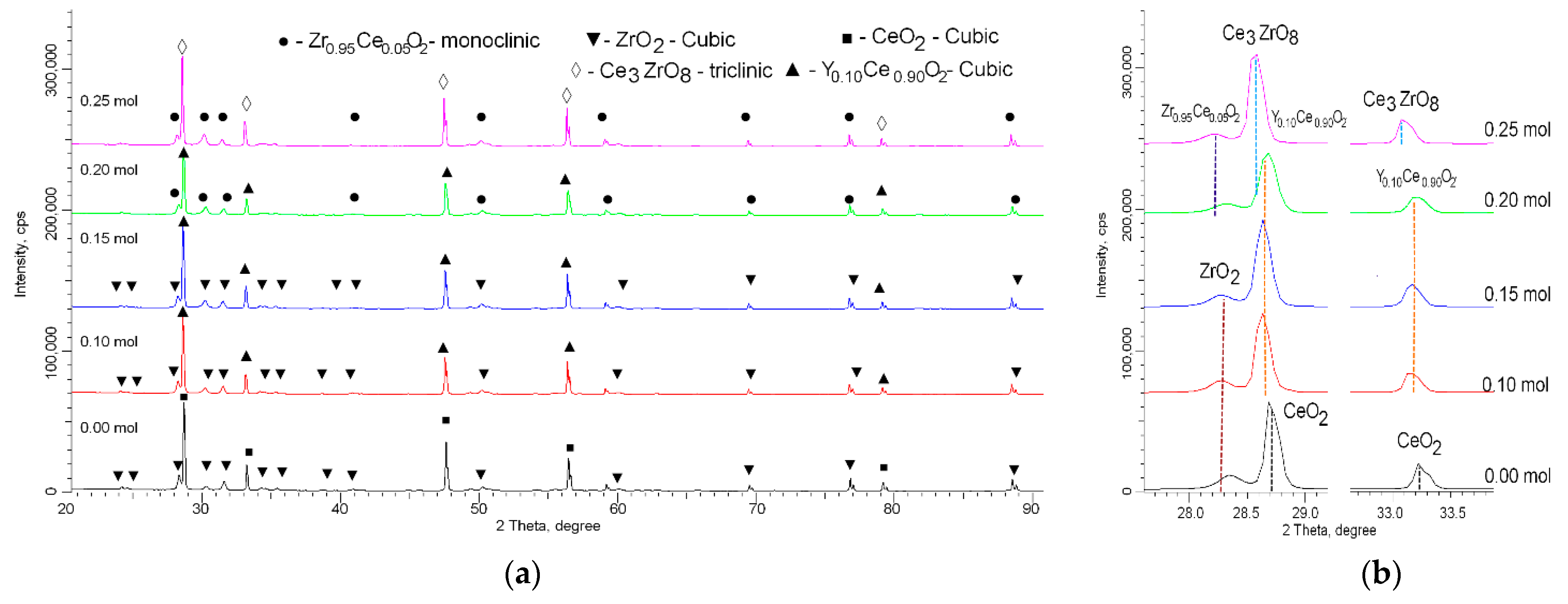
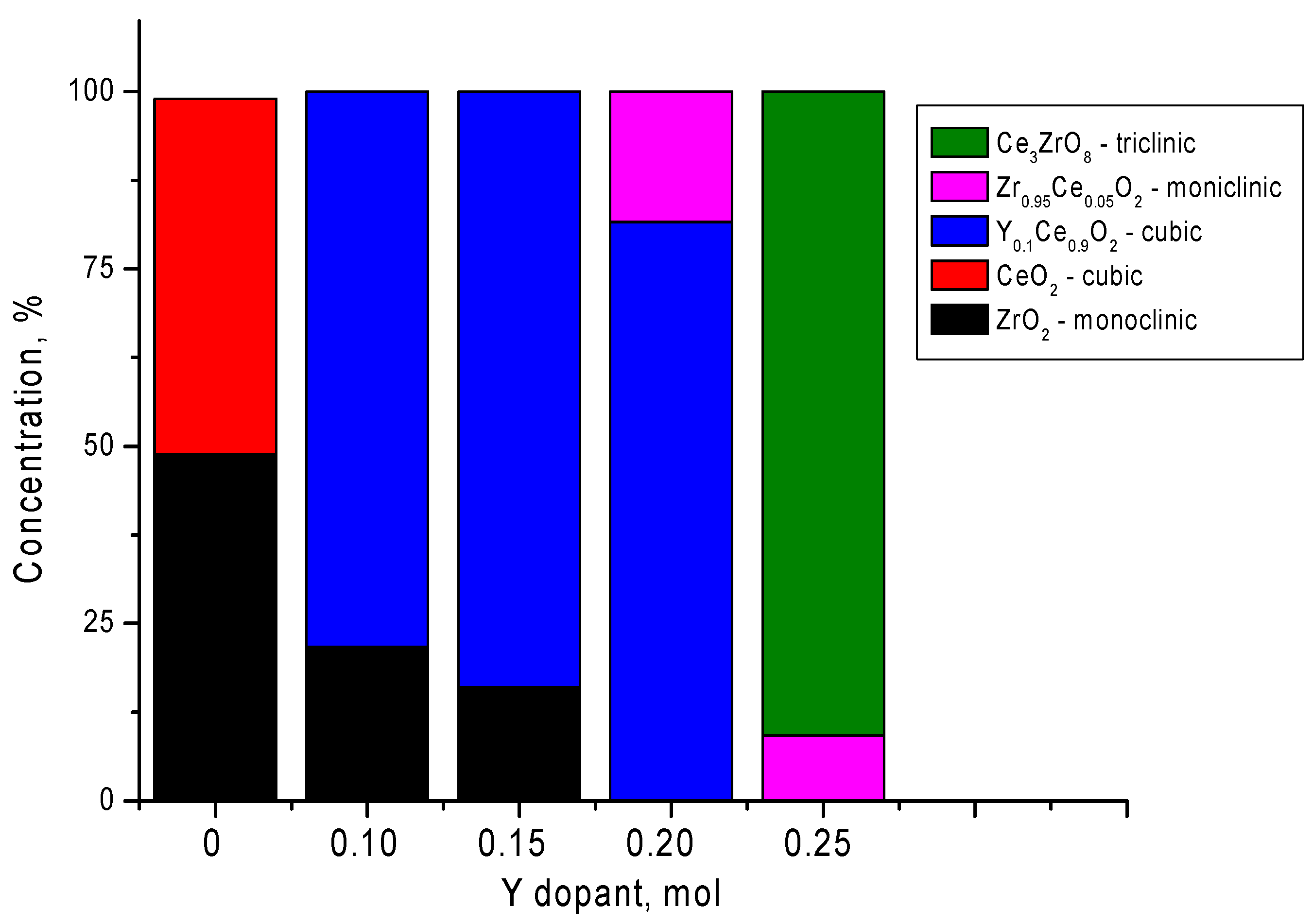
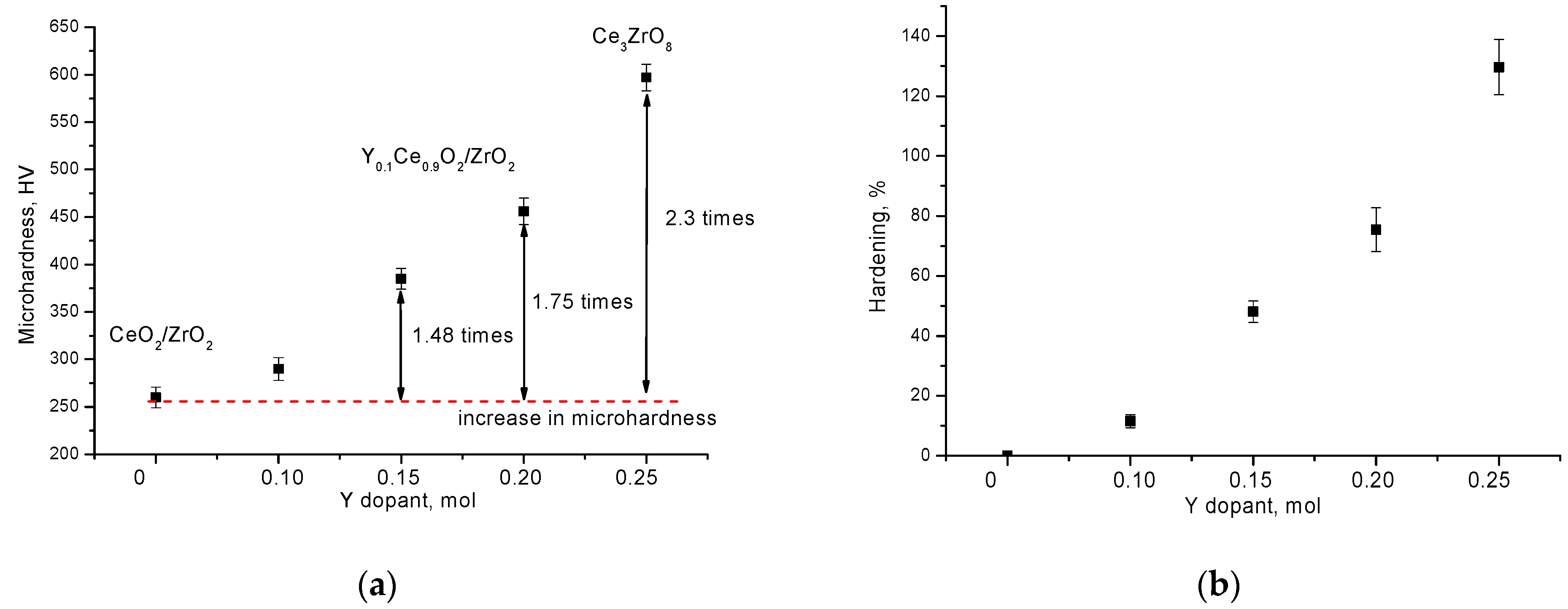

| Parameter | Y Dopant (mol) | ||||
|---|---|---|---|---|---|
| 0 | 0.10 | 0.15 | 0.20 | 0.25 | |
| ZrO2–monoclinic, P21/a 14 | a = 5.2961 ± 0.0013 Å, b = 5.1896 ± 0.0015 Å, c = 5.1238 ± 0.0012 Å, β = 99.056°, V = 139.07 Å3 | a = 5.3129 ± 0.0017 Å, b = 5.1907 ± 0.0012 Å, c = 5.1229 ± 0.0015 Å, β = 99.135°, V = 139.49 Å3 | a = 5.3172 ± 0.0015 Å, b = 5.1876 ± 0.0012 Å, c = 5.1380 ± 0.0016 Å, β = 99.131°, V = 139.93 Å3 | - | - |
| CeO2–Cubic, (225) | a = 5.3851 ± 0.0016 Å, V = 156.16 Å3 | - | - | - | - |
| Y0.1Ce0.9O2-Cubic, (225) | - | a = 5.3966 ± 0.0021 Å, V = 157.17 Å3 | a = 5.3945 ± 0.0019 Å, V = 156.98 Å3 | a = 5.3873 ± 0.0009 Å, V = 156.35 Å3 | - |
| Zr0.95Ce0.05O2-monoclinic, P21/a 14 | - | - | - | a = 5.3136 ± 0.0011 Å, b = 5.1828 ± 0.0009 Å, c = 5.3081 ± 0.0015 Å, β = 99.158°, V = 138.88 Å3 | a = 5.3308 ± 0.0014 Å, b = 5.1886 ± 0.0016 Å, c = 5.1531 ± 0.0012 Å, β = 99.310°, V = 140.65 Å3 |
| Ce3ZrO8–triclinic, P1 (1) | - | - | - | - | a = 7.6343 ± 0.0019 Å, b = 7.6313 ± 0.0012 Å, c = 5.4209 ± 0.0014 Å, α = 89.841°, β = 89.771°, γ = 90.299°, V = 315.81 Å3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giniyatova, S.G.; Sailaukhanov, N.A.; Nesterov, E.; Zdorovets, M.V.; Kozlovskiy, A.L.; Shlimas, D.I. Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium. Crystals 2022, 12, 242. https://doi.org/10.3390/cryst12020242
Giniyatova SG, Sailaukhanov NA, Nesterov E, Zdorovets MV, Kozlovskiy AL, Shlimas DI. Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium. Crystals. 2022; 12(2):242. https://doi.org/10.3390/cryst12020242
Chicago/Turabian StyleGiniyatova, Sholpan G., Nurzhan A. Sailaukhanov, Eugeniy Nesterov, Maxim V. Zdorovets, Artem L. Kozlovskiy, and Dmitriy I. Shlimas. 2022. "Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium" Crystals 12, no. 2: 242. https://doi.org/10.3390/cryst12020242
APA StyleGiniyatova, S. G., Sailaukhanov, N. A., Nesterov, E., Zdorovets, M. V., Kozlovskiy, A. L., & Shlimas, D. I. (2022). Research of Structural, Strength and Thermal Properties of ZrO2—CeO2 Ceramics Doped with Yttrium. Crystals, 12(2), 242. https://doi.org/10.3390/cryst12020242







