Abstract
The optical properties of hybrid systems composed of silver nanoparticles (NPs) and azobenzene molecules were systematically investigated by combining the real-time time-dependent density functional theory (RT-TDDFT) approach with the classical electrodynamics finite difference time domain (FDTD) technique for the solution of Maxwell’s equations. In order to reflect the chemical interaction between azobenzene and metal more exactly, except for adsorbed molecules, a Ag cluster separated from NP was also dealt, using RT-TDDFT. We studied the different factors affecting the surface-enhanced absorption spectra. It was found that the electric field amplified by plasmon resonance of Ag NPs can have an overall enhancement to the molecular light absorption throughout the whole energy range. The resonance between the electron and the plasmon excitation results in a larger percentage of enhancement in the absorption spectrum the closer the resonance peak is. The enhancement ratio of the resonance peak is the largest. The plasmon–exciton coupling and the optical properties of different isolate isomers influence the line shape of the absorption spectra. The dipole interaction and electronic transfer between azobenzene molecules and Ag NPs also change the shape of spectroscopy from the absorption enhancement ratio and the location of the peak. Physical and chemical factors lead to photoswitching in these hybrid systems together.
1. Introduction
Azobenzene and its derivatives are one of the most frequently studied class of switchable compounds. Azobenzene can exist in two different configurations (trans and cis) in the ground state. The trans-azobenzene can be converted to cis-azobenzene upon UV light (365 nm) irradiation. The cis to trans isomerization readily occurs at room temperature and can be stimulated by visible light irradiation [1,2]. Plasmonic metal nanostructures have attracted much attention because of their broad range of applications. It is known that light can be guided and localized by metallic nanoparticles (NPs). As the light frequency is resonant with collective electron charge oscillation of NPs, the surface plasmon resonance (SPR) is formed, which provides NPs with a brilliant optical property [3,4]. When azobenzene is adsorbed on the noble metal surface, because of the electronic coupling, the photoisomerization reaction in these adlayers are often considerably affected, and photoswiching can be demonstrated in other way. Self-assembled monolayers (SAMs) of azobenzene-containing compounds on Au or Ag surfaces have been intensively studied recent years [5,6,7,8,9,10,11,12]. The photoisomerization reaction can be demonstrated in the study about the electronic coupling between the adsorbed molecules and the metal substrate. Joshi et al. described an unprecedentedly large and photoreversible localized surface plasmon resonance (LSPR) wavelength shift caused by the photoisomerization of azobenzenes attached to gold nanoprisms that act as nanoantennas in the solid state [13]. The LSPR, such as frequency, total optical cross section and line width also sensitively depend on the properties of the plasmonic nanostructures, i.e., their size, shape and material. The resonant excitation of LSPRs by an electromagnetic field results in strong light absorption and scattering an enhancement of the local electromagenetic fields [5,14].In addition to resonance and electronic coupling, charge transfer between the azobenzene molecule and metal nanoparticles can also affect the optical properties of the molecule [15,16]. When different isomers of azobenzene are adsorbed on the surface of metal nanoparticles, the photoswitching effect may also be manifested in the surface-enhanced absorption spectra. So the simulation of the optical properties of azobenzene molecule adsorbed on a noble metal theoretically and study about enhancement for different isomers is very necessary because of its different application, such as sensors, photovoltaic and photonic devices, and optical metamaterials.
The hybrid superstructures composed of noble metal NPs and the absorbed molecules represent a challenge for theoretical and computational methods because elementary excitation in molecules or semiconductors and metal NPs, an exciton and a plasmon, have very different properties. The properties of molecules or semiconductors require a quantum mechanics (QM) description. However, QM methods are unable to describe the medium- to large-size metal NPs due to the limitation of computational bottleneck. So the absorbed molecules and metal NPs cannot be treated in the same footing by the state-of-the-art methods. The plasmon interacts with the exciton to endow the hybrid superstructures a novel property, and this interaction is required to be suitably tackled. Three kinds of simplified treatments are usually adopted. The most commonly used methods are the multiscale approaches [17,18,19,20,21,22,23] wherein the plasmonic nanostructures are treated by the classical [24,25,26,27,28] or cavity quantum electromagnetic methods (EM) [20,29,30,31,32], and the molecules are described by the quantum mechanics (QM) approaches, such as the time-dependent density functional theory (TDDFT) or TD Hartree–Fock (TDHF). The second method is based on the effective model Hamiltonian [33,34,35,36,37,38,39,40,41], where the molecule or semiconductor is identified by a two-level model and the resonant plasmonic system is identified by the plasmonic resonance modes. The third one is based on a pure classical electrodynamic framework, such as the dipole–dipole coupling approximation model [42], and the coupled harmonic oscillators [43,44]. The detailed atomic distribution information of hybrid system is missed by the latter two categories of treatments.
In our previous work [45,46,47], we studied theoretically the optical properties of hybrid complexes assembled by the molecules and Ag NPs by using the hybrid quantum–classical approach, which combines the RT-TDDFT approach [48,49,50,51,52,53,54], with the numerical solution of Maxwell’s equations. The surface localized field produced by the plasmon resonance of noble metal NPs is obtained by solving classical Maxwell’s equations, and the molecular electron evolution is described by the TDDFT. The molecular dynamic responses to two driving fields, the strong surface scattered field due to LSPR and the weak incident electric field can be simultaneously well described. The main advantage of this quantum–classical treatment on the plasmon–exciton interaction in hybrid complexes is that no empirical parameters are required. In this paper, we calculate the surface-enhanced absorption spectra of different isomers of azobenzene in hybrid systems with Ag NPs. We focus on describing the coupling of the enhanced field amplified by LSPR on the different isomer’s optical signals. We aim to make a quantitative description on the changes of spectral characters arisen by the interaction between the azobenzene molecule and metal NPs. The effect of the dipole coupling and charge transfer between molecules and NPs on the absorption spectra, the formation condition of the hybrid excited state, will be demonstrated. This work is useful for understanding the nature of photoswiching in the SAMs of azobenzene-containing compounds on noble metal surfaces. It is also useful for the development of plasmonic devices, for which a key aspect is the interaction of the surface plasmon with optically active materials.
The rest of paper is arranged as follows. In Section 2, we briefly describe the hybrid scheme for molecular optical absorption spectroscopy. RT-TDDFT is coupled with the FDTD method [55] through a SRF as Chen et al. did [25]. In Section 3, the hybrid method is applied to calculate the surface-enhanced absorption of hybrid systems composed of Ag NPs and azobenzene molecules of different configurations, cis and trans, which have quite different absorption line shapes. Two computational models are used to discuss the different effects of the interaction between isomers and metal nanoparticles on the surface-enhanced spectra. Finally, a concluding remark is given in Section 4.
2. Theoretical Methods
We consider a hybrid system constructed by the NPs and azebenzene molecule. An incident field interacts with the NPs to induce the oscillating multipoles, which create an enhanced surface near field. The surface near field interacts with the molecule to induce the molecular polarization. With this treatment, the influence of the induced molecular multipoles to the NPs’ surface charges and the carrier movement between the molecule and NPs are omitted. Therefore, in this article, we calculate two computational models. In model 1, AgNP and molecule are calculated by the FDTD and TDDFT methods respectively. In model 2, part of the Ag NP is also included in the TDDFT calculation to ensure the consideration of the possible charge transfer between azobenzene and Ag NP.
It is known that the molecular absorption cross section is proportional to the polarizability as
In the linear approximation, the dynamic polarizability tensor is defined as , and denotes the Fourier transform of the induced dipole under a time-dependent external electric field. , where is the permanent dipole moment. Within the TDDFT, can be obtained from the perturbed electron density when the system is subjected to an applied field by solving the time-dependent Schrödinger equation
denotes the surface near field, which interacts with the molecule, . and denote the incident field and the scattered field produced by Ag NPs’ plasmon, respectively. The surface near field generated by NPs can be obtained by solving the Maxwell’s equations by the finite-difference time-domain (FDTD) method [55] subject to appropriate boundary conditions. By doing so, the plasmonic enhancement effect is considered on one hand, and the optical properties of the molecule close to Ag NPs is embodied on the other. Time-dependent Equation (2) can be propagated step by step in the real time and space domain by numerical integration schemes.
In order to quantitatively study the localized field near the surface of Ag NPs and connect the FDTD simulation to the TDDFT calculations to the optical properties of nearby molecules, we introduce a complex tensor SRF, , by following Chen et al. [25].
The SRF is a complex tensor and depends on both the propagation and polarization directions of the incident light. A time-shifted Gaussian wave is typically chosen as the incident field in the FDTD simulation in order to study a broad spectral range.
Using the definition of , can be expressed as a two-dimensional Fourier transform of and
3. Results and Discussion
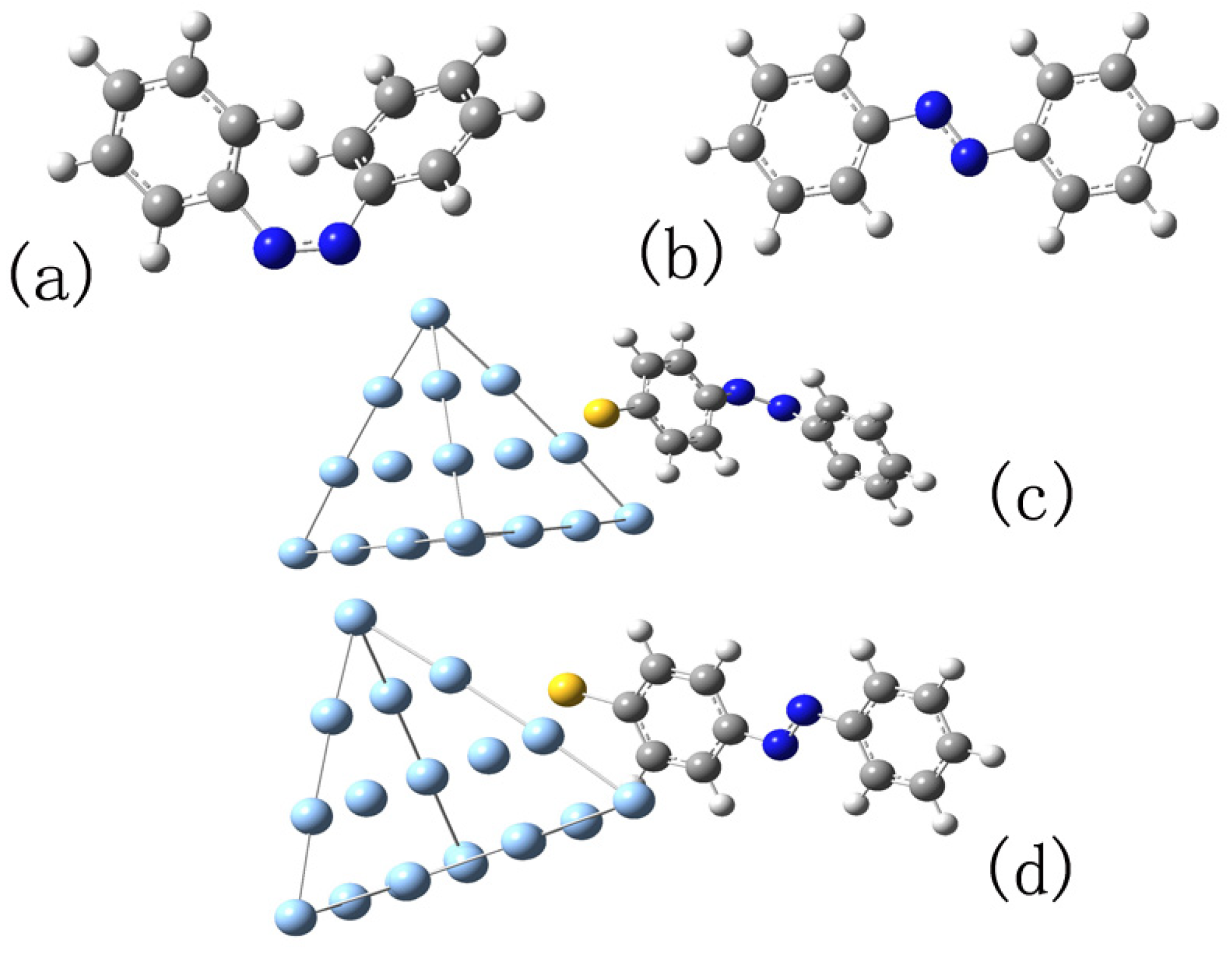
In this part, the linear optical response in the electric scattered field is calculated using the RT-TDDFT scheme in the development version of the OCTOPUS software package [56]. In order to obtain the absorption spectra, a weak Gaussian envelope external electric field, , with the amplitude a.u., fs, and fs is applied. The duration of this pulse is very small, which is like a function. For the isolated cis and trans molecules, the structures are first optimized by a DFT calculation using B3LYP/6-31G. When cis-azobenzene is converted to the trans-azobenzene, its main part rotates from a non-planar structure to one plane (Figure 1a,b).
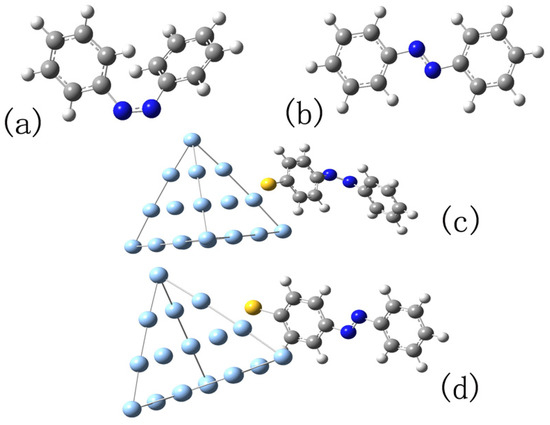
Figure 1.
(a,b) The structures of cis and trans configurations of azobenzene molecule, respectively; (c,d) the structures of the complexes in model 2 formed by cis and trans with Ag cluster, respectively. All structures are optimized at B3LYP/6-31G level.
Before adding the effect of NP, we calculate the average absorption across section of isolated azobeneze molecules using the PBE fuction when the propagation direction of incident field is fixed along the axis. As Figure 2a shows, in the range of 0–6 eV, the main excitation of the cis configuration displaces at , , , , and eV; the peak at eV has the maximum intensity. For the isolated trans azobenzene molecule, the optical absorption is stronger than the cis configuration. The first absorption peak at eV calculated theoretically is the strongest peak with a shoulder at about eV. If the damping rate decreases in the calculation, we can find that the shoulder also corresponds to an absorption band. When the azobenzene molecule converts from cis to trans, the optical properties are very different.
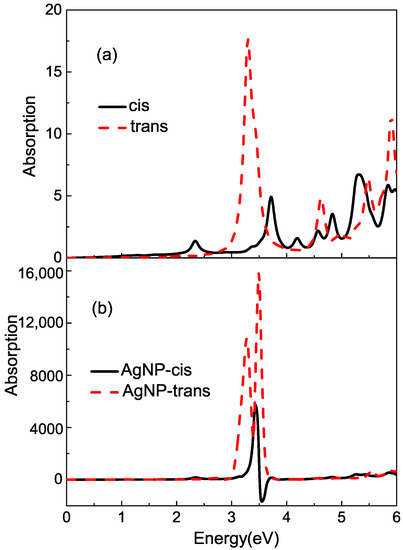
Figure 2.
(a) The average absorption cross sections of isolated cis and trans azobenzene molecules in gas phase; (b) the average absorption cross sections of bound azobenzene.
Combining with SRFs, the electric scattered field in any case can be obtained. Then, the effect of Ag NP should be introduced. In the azobenzene-NP system, except for the coupling between the molecule and NP, the process of optical excitation is often accompanied by a charge transfer. So, there are two hybrid nanostructure models that are investigated in this paper. In model 1, the adsorbed molecule and Ag sphere are studied, detached, using the QM/EM method. We mainly hope to investigate the effect of different coupling between molecules and nanoparticles and the influence of different isolated isomeric spectral line shapes on the surface-enhanced absorption spectra of bound azobenzene in the proximity of the Ag NP. In model 2, in order to describe the possible process of charge transfer between the NP and molecule, part of Ag NP is included in the calculation using QM. Thus, the effect of charge transfer on the optical properties is reflected.
3.1. Surface-Enhanced Absorption of Model 1
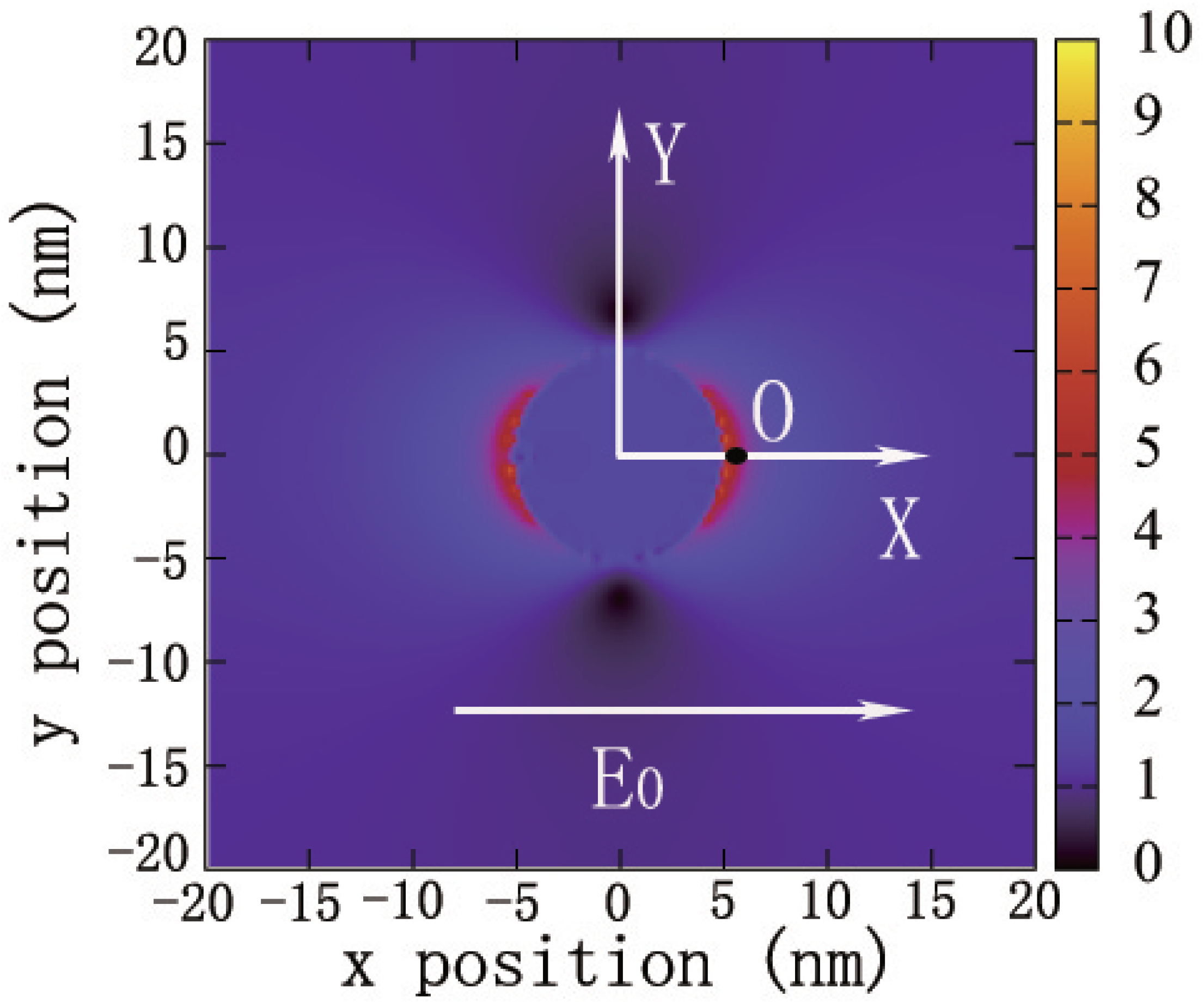
Maxwell’s equations are solved by using the FDTD method within the JFDTD3D package [57]. In our calculations, the light propagation direction is fixed along the axis in all FDTD simulations. A cubic simulation box with a side length of 40 nm and a very small grid size of nm is adopted in order to ensure the convergence of the calculation. Each grid cell is characterized by the value based on its distance to the center of the cubic box that is also the origin of the Cartesian coordination, inside the Ag NP according to the Drude–Lorentz model and in the surrounding medium. Here, is the relative permittivity, is the relative permittivity resulted from the Drude pole, is the bulk plasmon frequency, and is a width factor which includes electron–phonon and other intrinsic electron relaxation mechanisms. The last term describes the relative permittivity arising from each Lorentz oscillator pole. is the shift in relative permittivity at the electron transition at , and is the electron dephasing rate. All the parameters are determined from the experiment [58]. The incident light pulse with the functional form , is injected from the plane at , where fs, fs, and nm. These parameters of incident pulse can make the incident laser pulse cover the visible spectrum from 300 to 800 nm fully. The total simulation time is 100 fs, and the time step is fs. Figure 3 shows the contours of the surface near field of a Ag sphere with R = 5 nm observed in a plane when the incident laser pulse is assumed to have a polarization direction along +x, denoted by the arrow. The properties of SRFs are calculated at the point near the surface of Ag NP and labeled in Figure 3 as the ‘O’ point.
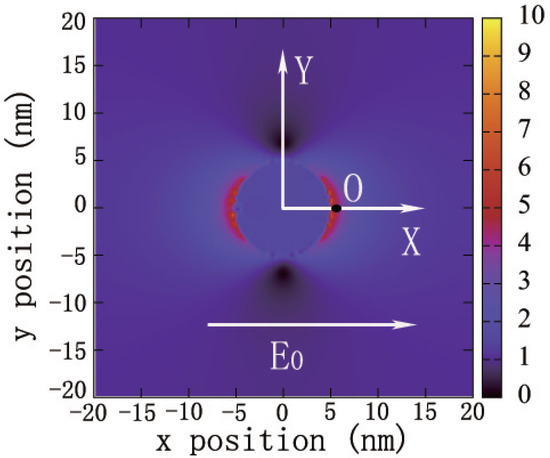
Figure 3.
The contour of a surface localized field generated by an Ag NP sphere with R = 5 nm. The arrow denotes the field polarization direction. The magnitude of the field intensity E is indicated by the color scale. The observation point ‘O’ is away from the NP surface, at a distance of L. The electric field is normalized to the incident field.
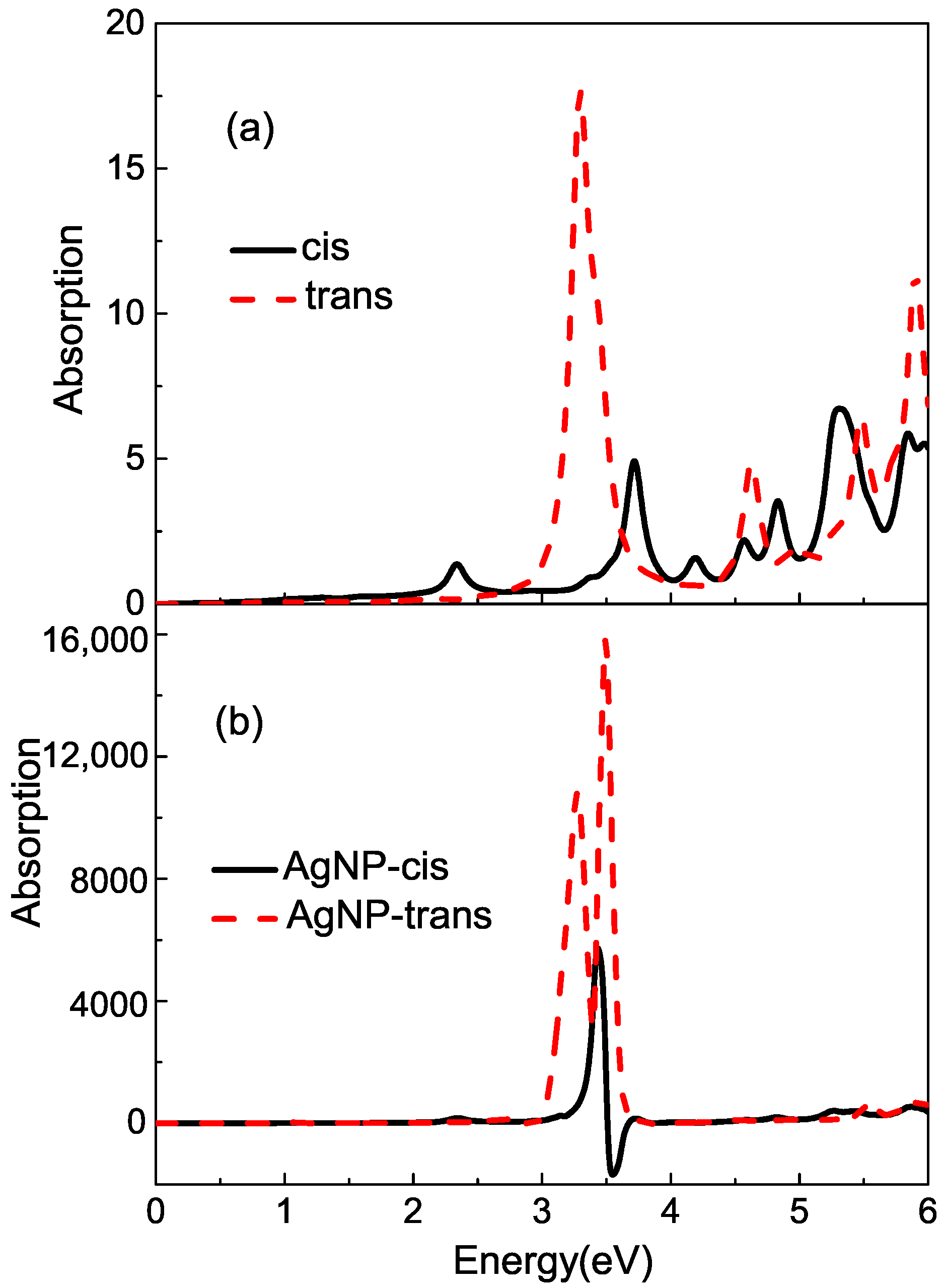
For model 1, the azobenzene molecule is set near the surface of the Ag sphere with nm. Generally, there is a layer of silver oxidation wrapped around the outside of the Ag nanospheres. However, unlike the Ag core, the intermediate Ag2O layer is a semiconducting material that does not exhibit any plasmonic features. Its presence and thickness influences the magnitude of the surface electric fields, which in turn determine the enhanced ratio of spectra. In this part, we are mainly interested in the effect of the coupling between plasmon resonance and molecular excitation on the spectra. So the silver oxidation is not considered. The observed point ‘O’ is also the mass center of the azobenzene molecule, which is at the x-axis, and the distance between the point and the surface of Ag NP is about nm. As Figure 4 shows, there is a featured peak centered at about eV in the , which is ascribed to the plasmon mode.
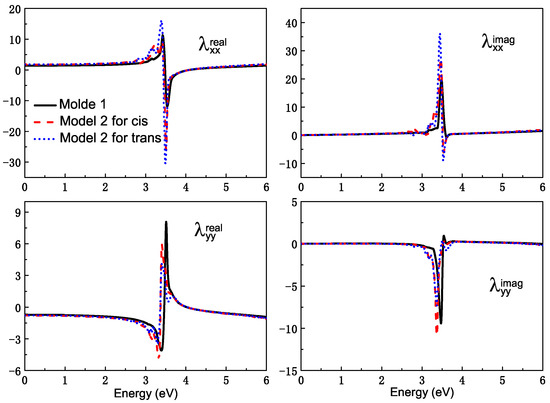
Figure 4.
The real and imaginary parts of and on the surface of Ag NP. For the solid black line, the radius of Ag sphere is 5 nm, and the observed point is set at the center of mass of the azobenzene molecule. The dash red line and dot blue line show the SPR in model 2. The observed point is set at the center of mass of Ag20-cis azobenzene and Ag20-trans azobenzene complexes, respectively. AgNP is the remaining part of a 5 nm radius nanosphere that is gouged out of a tetrahedron in model 2.
Within the RT-TDDFT theory, the absorption spectra of bound molecule in the proximity of the AgNP can be studied. FDTD simulation about SRFs reflects plasmon enhancement, which is a physical factor for surface-enhanced spectroscopies; the calculation process of RT-TDDFT ensures the consideration of some chemical factor. With an external laser pulse incident, whose propagation direction is fixed along the axis, the field will be strengthened, and the molecule’s light absorption will be changed and improved through plasmonic enhancement. In order to make our theory investigation more common, for a silver-bound cis or trans azobenzene system, two separate RT-TDDFT simulations are carried out with an electric field pulse applied along the x and y axes, respectively, and the average absorption spectra are obtained eventually. By using the SRFs tensor, the with other incident laser pulse is calculated by using Equation (4). For the off-diagonal terms in , nearly negligible compared to the diagonal terms, only and are considered in Equation (4).
The average absorption across of the bound cis or trans azobenzene molecule near Ag NP is calculated and displayed in Figure 2b. Obviously, the enhanced surface electric field amplifies the molecular absorption in the whole energy range of 0–6 eV and strongly alters the absorption profiles of NP-azobenzene systems, in contrast to the isolated molecules. However, the enhancement for spectra is not uniform. The smaller the energy difference of , the larger the absorption enhancement ratio becomes. As a result, the absorption peaks near the plasmon excitation at eV become more significant. In addition, the different optical properties of the isolated isomers lead to different line shapes near the resonance peaks in the absorption spectra of the bound azobenzene molecule. Due to the complex interplay between the metal NP’s LSPR and the molecule’s excitation (exciton), a set of particular characteristics in the absorption spectra, such as excitonic splitting, asymmetric line shapes, plasmon-induced absorption enhancement and transparencies, are observed in those nanosystems. In the strong plasmon–exciton coupling regime, where the plasmon–exciton coupling is much larger than the damping rates of plasmon resonance and excitons [59], two coupled oscillators are split when the bare oscillator frequencies are tuned to each other, and the interaction of plasmons and excitons results in two new mixed states of light and matter separated energetically by Rabi splitting [60,61,62]. For the isolate trans-azobenzene molecule, the narrow absorption band at eV is coupled with plasmon excitation strongly. So, there are separate absorption bands at and eV, respectively, for surface-enhanced spectra. In the weak coupling regime, where the plasmon–exciton coupling strength is much less than one of the dampings [59], the absorption spectra characterize an asymmetric Fano line shape [63,64,65,66,67,68]. The case of the cis isomer satisfies this condition. Near eV, there is only a weak excitation at eV for the isolated cis azobenzene molecule. Large energy difference and weak absorption intensity make the coupling between the plasmon and electron’s excitation not very strong. The Fano resonance occurs when a discrete quantum state interferes with a continuum band of state in this case. As a result, the Fano asymmetric line shape appears in the absorption band at eV. Hybrid systems composed by two isomers exhibit completely different surface-enhanced absorption spectra because of different optical properties of molecule and plasmon–exciton coupling.
3.2. Surface Enhanced Absorption of Model 2
In fact, for hybrid systems, chemical enhancement effects include much more than the resonance of excitation, such as charge transfer between adsorbed molecules and metals. In order to investigate the actual interaction between azobenzene and NP, the model of calculation is improved in this part. Before introducing the effect of NP, a cluster including 20 Ag atoms is cut apart from Ag NP to form a complex with the cis or trans azobenzene molecule. At first, the optical properties of the complexes are investigated by TDDFT. Then, the effect of the rest of Ag NP is considered by adding an enhanced scattering field as we do in the previous section. We expect that our investigation combines the chemical influence, including both the coupling and the charge transfer between the metal and azobenzene molecule. The cluster, including 20 Ag atoms, forms a regular tetrahedron, and the azobenzene molecule is placed near an Ag atom, which is the center of one face of the centrum. In order to ensure that there is a chemical effect between the azobenzene molecule and silver cluster, an S atom is set between the C and Ag atoms. The structure of the complexes is optimized at the B3LYP/6-31G level, and Ag atoms are dealt using the LANL2DZ pseudopotential. We call this structure model 2 as Figure 1c,d shows.
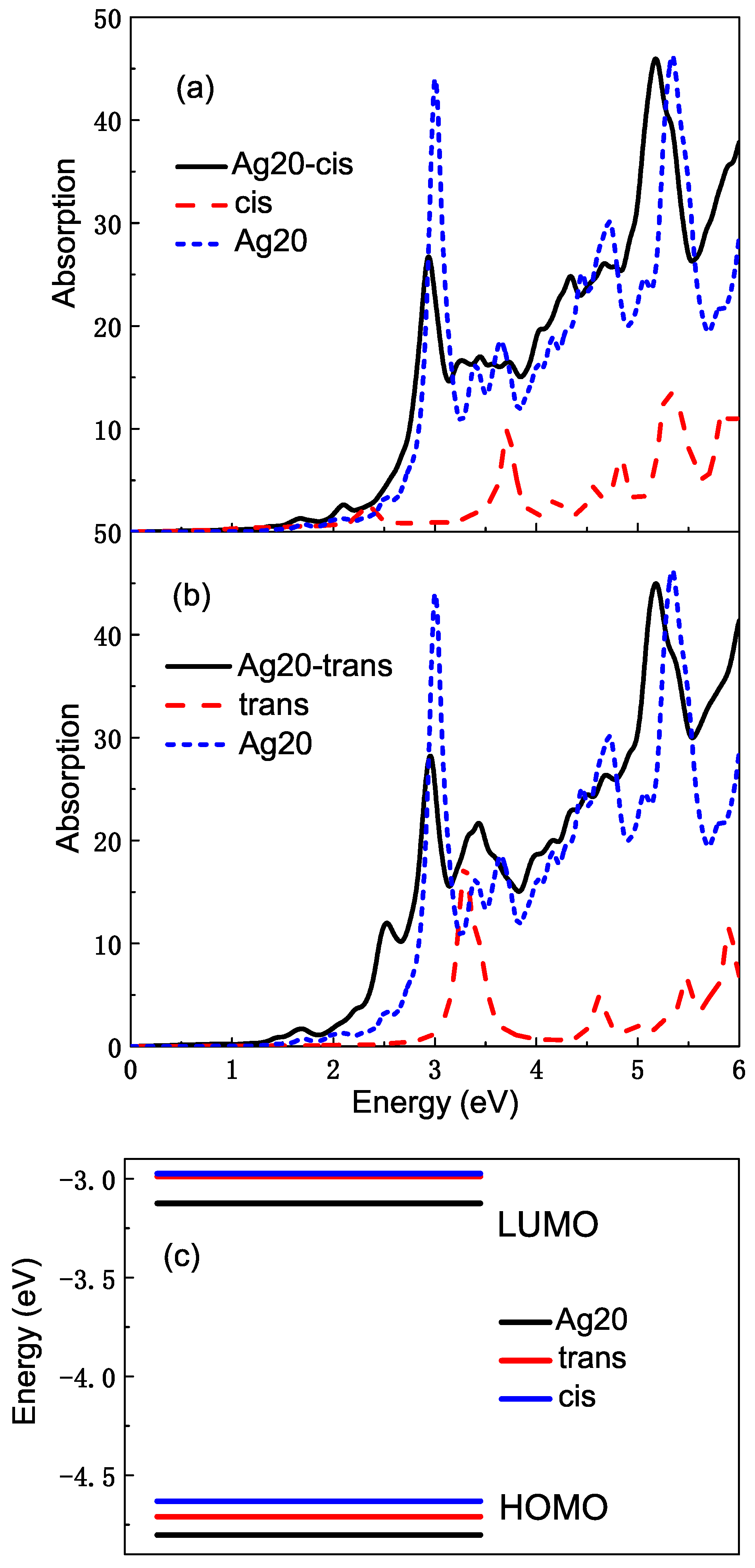
We study the optical properties of the whole complexes using TDDFT with the PBE function that can deal with the interaction between the molecule and metal more exactly. SIC correction is added in order to make the calculation more accurate. The spectra of complexes change considerably, which is not just a superposition of the absorption spectra of azobenzene molecules and Ag clusters. As Figure 5a,b shows, the participation of the Ag cluster makes the shape of the absorption spectra change very dramatically not only from the intensity, but also from the location of the absorption band. Comparing with the absorption spectra of the Ag cluster or azobenzene molecule, they are broad in general and thus represent charge transfer character. Both for Ag20-cis and Ag20-trans complexes, in the high energy region greater than eV, the excitation of azobenzene molecules and Ag clusters are mixed together to form a wide absorption band. In the range of – eV, the shift of absorption band can also be seen very clearly. For example, for the Ag20-cis complex, the absorption peak of the molecule at eV is incorporated with that of the Ag cluster, forming a broad absorption band, which also contains a number of other new excitations for the complex. The peak at eV for the cis molecule red shifts to eV. In addition, some small absorption peaks appear in the low-energy range, and there is a new high absorption peak at eV, which belongs to the excitation of the Ag cluster. The spectral intensity of the complex is much stronger than that of the isolated cis isomer, and the chemical enhancement of metal can be embodied from here. In Figure 5b, the shift and mixture for spectra of the Ag20-trans complex is also obvious. There is an absorption peak belonging to the Ag cluster at eV. The peaks at , and eV are close to each other and form a broad absorption band. There are also some differences compared to the Ag20-cis complex. A new absorption peak appears at eV, which is not found in the spectra of the Ag20-cis complex. The absorption peak at eV for isolated trans azobenzene is replaced by a stronger absorption peak at eV. Figure 5c shows the energy levels of HOMO and LUMO of the Ag20 cluster and azobenzene molecules. We find that for two isomers of azobenzene, their HOMO lie between the HOMO and LUMO of the Ag20 cluster, while the energy of LUMO is higher than that of Ag20, which provides the conditions for the charge transfer during the electron transition.
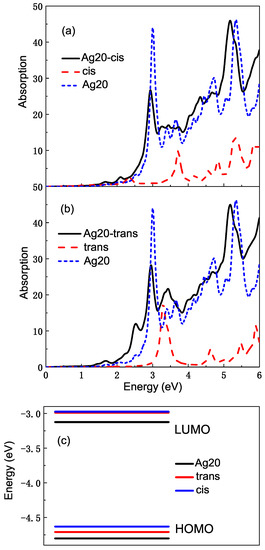
Figure 5.
(a) The average absorption cross sections of the Ag20-cis azobenzene complex, isolated cis isomer and Ag20 cluster; (b) the average absorption cross sections of the Ag20-trans azobenzene complex, isolated trans isomer and Ag20 cluster; (c) the energy levels of HOMO and LUMO of Ag20, cis and trans azobenzene.
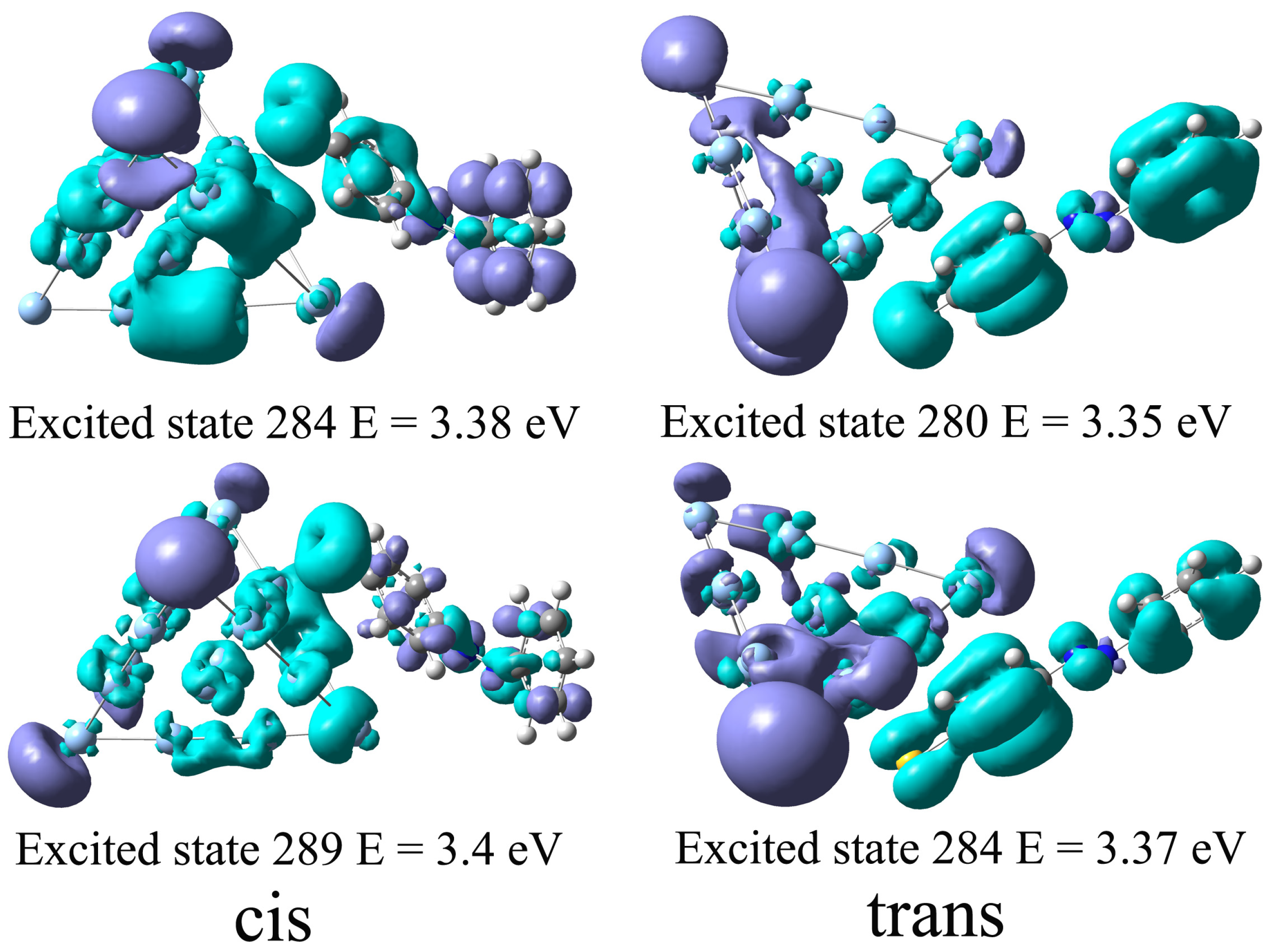
In order to demonstrate the interaction between the azobenzene molecule and the Ag cluster during the excitation, we further investigate the excited state properties of the complexes. Since the rest Ag sphere with R = 5 nm will be added to form a hybrid system, the absorption excitation of complexes near eV will resonate with the plasmon excitation near the Ag NP surface and thus be greatly enhanced. So we use the charge density difference to analyze the nature of excited states of the Ag20-azobenzene complexes near eV. Figure 6 displays the charge density difference between the ground state, and several excited states correspond to the strong excitation at and eV, and and eV for Ag20-cis azobenzene and Ag20-trans azobenzene complexes, respectively. To maintain consistency in the calculation method, the charge density difference is obtained at the PBE/6-31G level, using the Gaussian 16 package, and Ag atoms are dealt with the LANL2DZ pseudopotential. The transition of electrons for Ag20-cis azobenzene and Ag20-trans azobenzene is different. As Figure 6 shows, for the trans complex, the different distribution of positive and negative charges on the azobenzene molecule and the Ag cluster indicates that charge transfer does occur during the transition of electrons corresponding to these excited states. However, for the cis complex, the characteristics of charge transfer are not very obvious because positive and negative charges are evenly distributed on molecules and clusters. This shows that different isomers do not interact with Ag clusters in the same way. For trans configuration, the planar molecular structure is more conducive to charge transfer between it and the Ag cluster, and many excitation processes are accompanied by a significant charge transfer. Thus, the absorption spectra of the complexes formed by the two isomers are different near eV, and there is a very significant absorption peak appearing at eV for the Ag20-trans azobenzene complex.
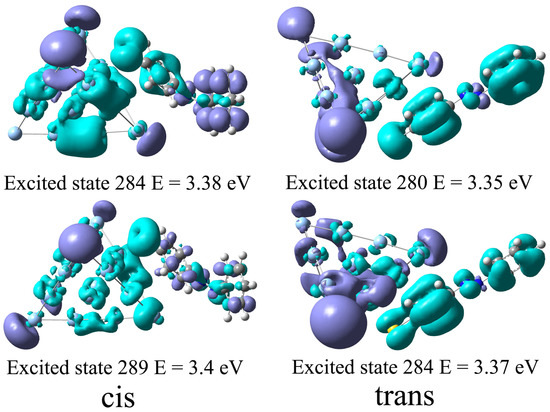
Figure 6.
The charge density difference of excited states correspond to the two strong transitions around the eV for Ag20-cis and Ag20-trans complexes, respectively.
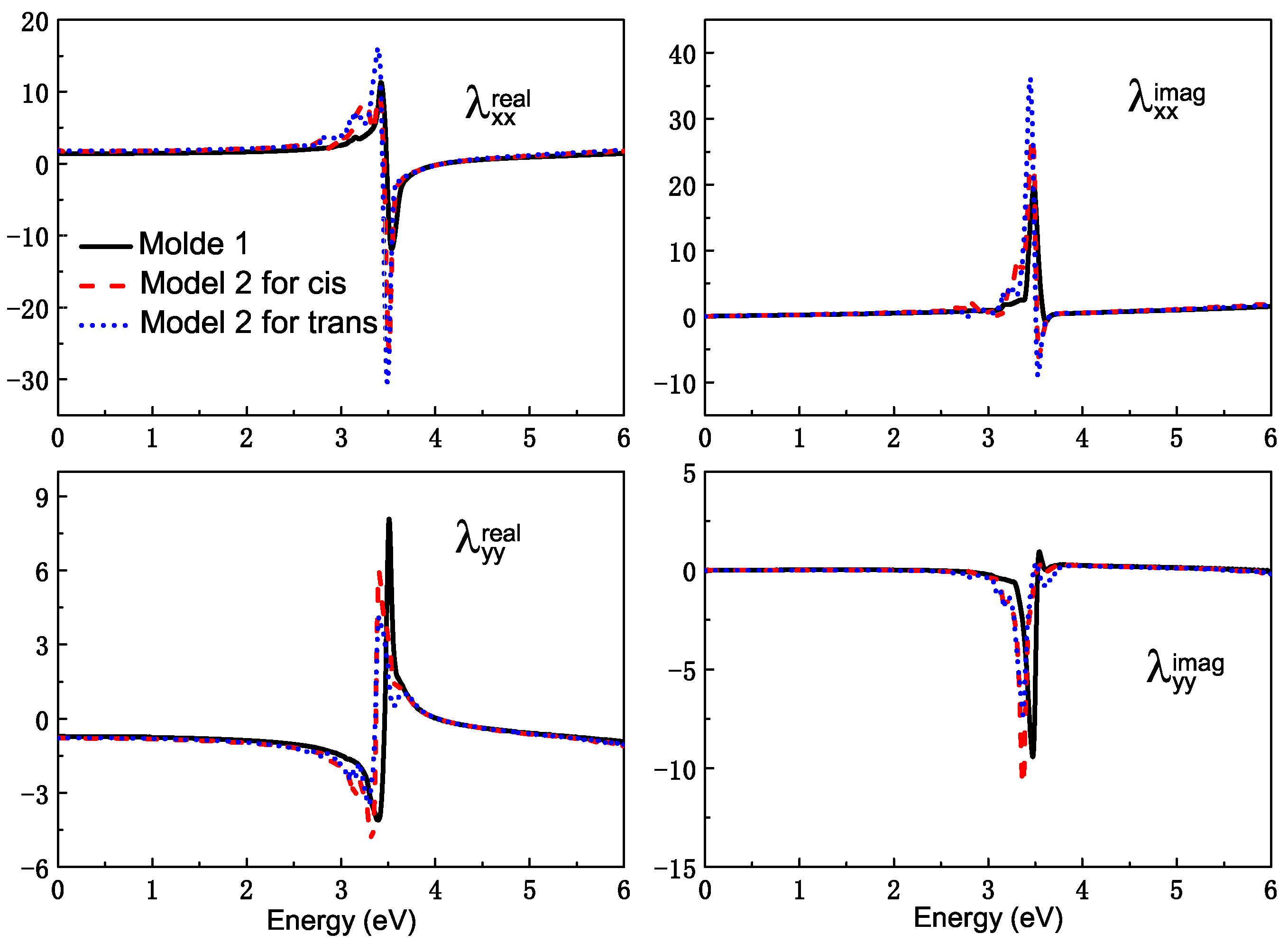
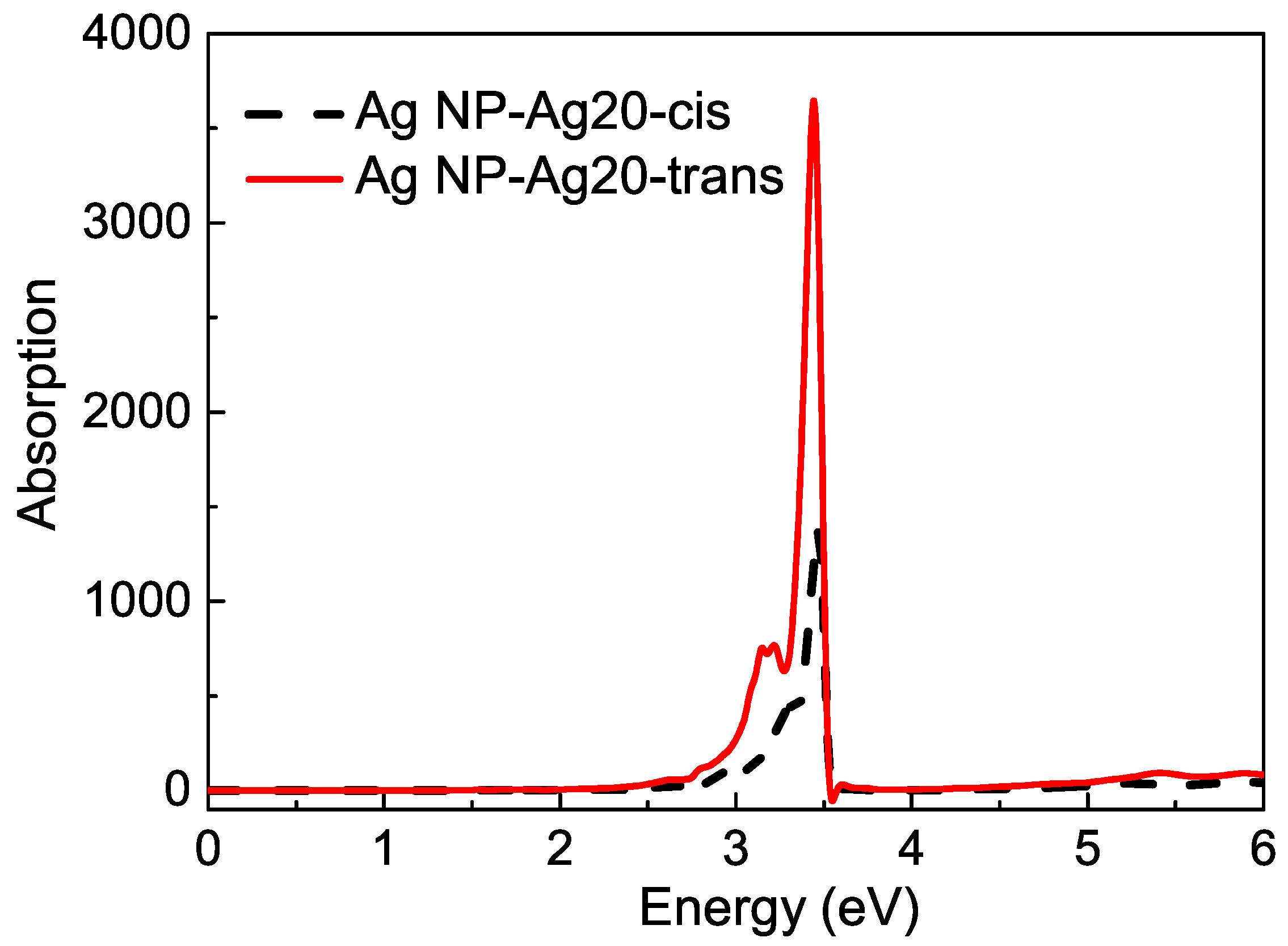
Then, the rest effect of NP is considered. The complex is also fixed on the surface of the Ag nanoparticle, and the Ag20 cluster can be seen as one part of Ag NP. The SRF of the remaining part of Ag NP, which was cut off a regular tetrahedron is also shown in Figure 4. The observation point is set at the mass center of the Ag cluster–azobenzene complex. For the remaining Ag NP, the plasmon mode red shifts a little compared with the intact Ag sphere. The effect of Ag NP is considered by adding the scattering field produced by the rest Ag NP into the TDDFT calculation. Figure 7 is the surfaced-enhanced absorption spectra in model 2. The surface enhancement effect can be found very clearly, especially for the absorption band at about the – eV energy range, which is close to the excitation of the plasmon mode at eV. The absorption enhancement ratio is also sensitive to the energy gap between the molecular excitation and plasmon modes. For the excitation, which is away from plasmon mode, the physical factor, which lies in the enhanced scattering field, is the main reason for enhancement.However, near eV, the energy gap approaches zero, and resonance play a role again. The dual physical and chemical enhancement effects cause the peaks near the plasmon mode to be enhanced much more than the other peaks. So, in Figure 7, for the AgNP-Ag20-cis azobenzene hybrid system, we observe only one strong absorption peak at eV with a shoulder at eV, and the enhancement ratio is about 80 times, compared with the Ag20-cis complex. Although the absorption spectrum of the Ag20-cis complex just has a wide absorption band near eV, the resonance absorption peak becomes strong and sharp. There is a little difference for the trans isomer. Since the charge transfer makes the Ag20-trans complex have an obvious absorption peak at eV, the resonance enhancement is more pronounced for the AgNP-Ag20-trans azobenzene hybrid system, with a resonance absorption peak at eV, which is enhanced by a factor of 167. For the two different isomers, the enhancement for the resonance absorption peak of the trans configuration is more pronounced, and we even found a negative part in the spectra, which is the nonlinear FANO effect, due to the coupling between the molecule and plasmon. Due to the different interactions between the molecule and plasmon, when the structure of the adsorbed molecule converts from cis to trans, the resonance absorption peak also red shifts from to eV. Different chemical effects, including coupling and charge transfer between the Ag and molecule, lead to different enhancement and shifts of the absorption spectra. Photoswitching is still well represented from the surface-enhanced absorption spectra.
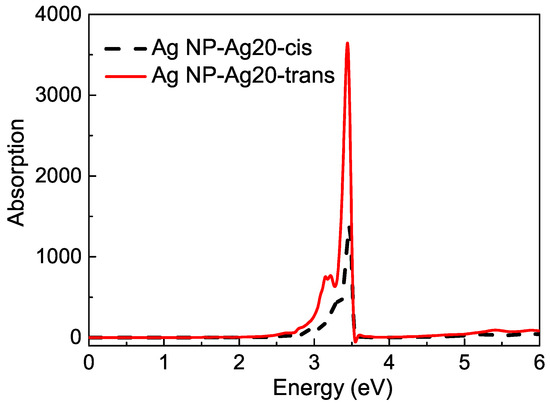
Figure 7.
The surface-enhanced absorption spectra in model 2.
4. Conclusions
In this paper, we investigate the plasmon-enhanced light absorption of the azobenzene molecule adsorbed on Ag NP, using the hybrid scheme, which combines the RT-TDDFT approach for the dynamic polarizabilities with the FDTD method for solving Maxwell’s equation. Our calculation gives consideration of the physical and chemical influence for the optical properties of the system. Two investigated models are studied in order to investigate the effect of different factors on the surface-enhanced absorption spectra. For model 1, the azobenzene molecules and AgNP are calculated using quantum chemistry and classical physics methods, respectively. The enhancement of the laser field by metal nanoparticles and the resonance of molecules with plasmon excitation make the surface-enhanced absorption spectra of two isomers different. Its absorption enhancement ratio caused by the surface localized field heavily depends on the energy gap between the molecular excitation band and the plasmon mode. For the absorption band far away from localized plasmon resonance, the coupling between the plasmon mode and the molecular transition dipole moment can be neglected, and physical enhancement, which lies in the enhanced scattering field generated by NP, is the main reason for the change of spectra. As the energy gap decreases, the chemical enhancement becomes the other main reason. The molecular excitation is strongly coupled with the plasmon excitation, which possesses a significantly amplified absorption intensity and a shifted peak position. The resonance happens when the energy gap is close to zero, and a hybrid band formed by molecule and plasmon excitation is found. In addition to the strength enhancement, the line shape of the resonance absorption peak is related to the energy gap and the peak width of the molecular and plasmon absorption spectra. We find the Fano line shape and Rabi split for the surface-enhancement absorption spectra of the cis and trans configurations, respectively.
In order to study the chemical factor adequately, the possible charge transfer between the azobenzene molecule and Ag NP is considered in our calculation of model 2. Except for the azobenzene molecule, an Ag cluster, including 20 Ag atoms, is added to the part that is dealt using RT-TDDFT. The chemical interaction between the azobenzene molecule and Ag cluster makes the absorption spectra of complexes mixed, broad and amplified. For the Ag20-trans complex, the charge transfer between the azobenzene and Ag clusters also produces a strong and broad absorption peak at eV. When this intense and broad absorption peak is coupled with plasmon excitation, the resonance absorption band is not only enhanced very dramatically, but also shifts obviously. So when the structure of the adsorbed molecule converts from cis to trans, the resonance absorption peak red shifts eV. Physical and chemical factors allow photoswiching, which could also be demonstrated in the surface-enhanced absorption spectra when the azobenzene is adsorbed on Ag nanoparticles.
Author Contributions
Conceptualization, J.S. and Z.D.; methodology, J.S. and Z.D.; software, J.S.; validation, J.S., Z.D. and Y.Y.; formal analysis, J.S.; investigation, J.S., Z.D., C.X.; resources, J.S.; data curation, J.S.; writing—original draft preparation, J.S.; writing—review and editing, J.S.; visualization, J.S.; supervision, J.S.; project administration, J.S.; funding acquisition, J.S. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21773001), Anhui Project (Z010118169), the Key Project of the Foundation of Anhui Education Committee, China (KJ2019A0020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
J.S. is thankful for the support in the research from the State Key Laboratory of Physical Chemistry of Solid Surfaces, Xiamen University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Wang, X. Ab initio study of photoisomerization mechanisms of push-pull p,p′-disubstituted azobenzene derivatives on S-1 excited state. J. Mol. Struct. THEOCHEM 2007, 806, 179–186. [Google Scholar] [CrossRef]
- Hamelmann, F.; Heinzmann, U.; Siemeling, U.; Bretthauer, F.; Vor der Brüggen, J. Light-Stimulated Switching of Azobenzene-Containing Self-Assembled Monolayers. Appl. Surf. Sci. 2004, 222, 1–5. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Collins, S.S.E.; Gallagher, M.J.; Bhattacharjee, U.; Zhang, R.; Chow, T.H.; Ahmadiv, A.; Ostovar, B.; Al-Zubeidi, A.; Wang, J.; et al. Single-Particle Emission Spectroscopy Resolves d-Hole Relaxation in Copper Nanocubes. ACS Energy Lett. 2019, 4, 2458–2465. [Google Scholar] [CrossRef]
- Ostovar, B.; Cai, Y.Y.; Tauzin, L.J.; Lee, S.A.; Ahmadivand, A.; Zhang, R.; Nordlander, P.; Link, S. Increased Intraband Transition in Smaller Gold Nanorods Enhance Light Emission. ACS Nano 2020, 14, 15757–15765. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Jung, U.; Gusak, V.; Ulrich, S.; Holz, M.; Herges, R.; Langhammer, C.; Magnussen, O. Localized Surface Plasmon Resonance Investigations of Photoswitching in Azobenzene-Functionalized Self-Assembled Monolayers On Au. Langmuir 2013, 29, 10693–10699. [Google Scholar] [CrossRef]
- Jung, U.; Filinova, O.; Kuhn, S.; Zargarani, D.; Bornholdt, C.; Herges, R.; Mangussen, O. Photoswitching Behavior of Azobenzene-Containing Alkanethiol Self-Assembled Monalayers on Au Surfaces. Langmuir 2010, 26, 13913–13923. [Google Scholar] [CrossRef]
- Song, H.; Jing, C.; Ma, W.; Xie, T.; Long, Y.T. Reversible Photoisomerization of Azobenzene Molecules on a Single Gold Nanoparticle Surface. Chem. Commun. 2016, 52, 2984–2987. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Wang, X.; Shao, F.; Hegner, M.; Zenobi, R. Nanoscale Chemical Imaging of Reversible Photoisomerization of an Azobenzene-Thiol Self-Assembled Monolayer by Tip-Enhanced Raman Spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 1025–1029. [Google Scholar] [CrossRef]
- Kunfi, A.; Vlocsko, R.B.; Keresztes, Z.; Mohai, M.; Bertoti, I.; Abraham, A.; Kiss, E.; London, G. Photoswitchable Macroscopic Solid Surfaces Based On Azobenzene-Functionalized Polydopamine/Gold Nanoparticle Composite Materials: Formation, Isomerization and Ligand Exchange. ChemPlusChem 2020, 85, 797–805. [Google Scholar] [CrossRef]
- Fast, E.; Schlimm, A.; Lautenschlager, I.; Clausen, K.U.; Strunskus, T.; Spormann, C.; Lindhorst, T.K.; Tuczek, F. Improving the Switching Capacity of Glyco-Self-Assembled Monolayers on Au(111). Chem. Eur. J. 2019, 26, 485–501. [Google Scholar] [CrossRef]
- Liu, Q.W.; Zhang, J.W.; Xing, F.S.; Cheng, C.C.; Wu, Y.W.; Huang, C.J. Plasmon-enhanced and controllable synthesis of azobenzene and hydrazobenzene using Au/TiO2 composite. Appl. Surf. Sci. 2020, 500, 144214. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, S.; Wang, C.K.; Luo, Y. Finding the true pathway for reversible isomerization of a single azobenzene molecule tumbling on Au(111) surface. Nanoscale 2020, 12, 10474–10479. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.K.; Blodgett, K.N.; Muhoberac, B.B.; Johnson, M.A.; Smith, K.A.; Saradar, R. Ultrasensitive Photoreversible Molecular Sensors of Azobenzene-Functionalized Plasmonic Nanoantennas. Nano Lett. 2014, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Link, S.; Halas, N.J. Nano-Optics from Sensing to Waveguiding. Nat. Photonics 2007, 1, 641–648. [Google Scholar] [CrossRef]
- Gahl, C.; Schmidt, R.; Brete, D.; Paarmann, S.; Weinelt, M. Charge-transfer dynamics in azobenzene alkanethoiolate self-assembled monolayers on gold. Surf. Sci. 2015, 643, 183–189. [Google Scholar] [CrossRef][Green Version]
- Lim, C.K.; Li, X.; Li, Y.; Drew, K.L.M.; Palafox-Hernandez, J.P.; Tang, Z.; Baev, A.; Kuzmin, A.N.; Knecht, M.R.; Walsh, T.R.; et al. Plasmon-enhanced two-photon-induced isomerization for highly-localized light-based actuation of inorganic/organic interfaces. Nanoscale 2016, 8, 4194–4202. [Google Scholar] [CrossRef]
- Wright, L.B.; Rodger, P.M.; Corni, S.; Walsh, T.R. GolP-CHARMM: First-Principles Based Force Fields for the Interaction of Proteins with Au(111) and Au(100). J. Chem. Theory Comput. 2013, 9, 1616–1630. [Google Scholar] [CrossRef]
- Andreussi, O.; Biancardi, A.; Corni, S.; Mennucci, B. Plasmon-Controlled Light-Harvesting: Design Rules for Biohybrid Devices via Multiscale Modeling. Nano Lett. 2013, 13, 4475–4484. [Google Scholar] [CrossRef]
- Smith, H.T.; Karam, T.E.; Haber, L.H.; Lopata, K. Capturing Plasmon-Molecule Dynamics in Dye Monolayers on Metal Nanoparticles Using Classical Electrodynamics with Quantum Embedding. J. Phys. Chem. C 2017, 121, 16932–16942. [Google Scholar] [CrossRef]
- Fregoni, J.; Granucci, G.; Coccia, E.; Persico, M.; Corni, S. Manipulating Azobenzene Photoisomerization Through Strong Light-Molecule Coupling. Nat. Commun. 2018, 9, 4688. [Google Scholar] [CrossRef]
- Mennucci, B.; Corni, S. Multiscale Modelling of Photoinduced Processes in Composite Systems. Nat. Rev. Chem. 2019, 3, 315–330. [Google Scholar] [CrossRef]
- Morton, S.M.; Jensen, L. A discrete ineraction model/quantum mechanical method to describe the interaction of metal nanoparticles and molecular absorption. J. Chem. Phys. 2011, 135, 134103. [Google Scholar] [CrossRef]
- Gao, Y.; Neuhauser, D. Dynamical quantum-electrodynamics embedding: Combining timedependent density functional theory and the near-field method. J. Chem. Phys. 2012, 137, 074113. [Google Scholar] [CrossRef]
- Chen, H.N.; McMahon, J.M.; Ratner, M.A.; Schatz, G.C. Classical Electrodynamics Coupled to Quantum Mechanics for Calculation of Molecular Optical Properties: A RT-TDDFT/FDTD Approach. J. Phys. Chem. C 2010, 114, 14384–14392. [Google Scholar] [CrossRef]
- Yam, C.; Meng, L.; Chen, G.; Chen, Q.; Wong, N. Multiscale Quantum Mechanics/Electromagnetics Simulation for Electronic Devices. Phys. Chem. Chem. Phys. 2011, 13, 14365–14369. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yam, C.; Koo, S.; Chen, Q.; Wong, N.; Chen, G. Dynamic Multiscale Quantum Mechanics/Electromagnetics Simulation Method. J. Chem. Theory Comput. 2012, 8, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Payton, J.L.; Morton, S.M.; Moore, J.E.; Jensen, L. A Hybrid Atomistic Electrodynamics-Quantum Mechanical Approach for Simulating Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2013, 47, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Purcell, T.A.R.; Seideman, T. Modeling the Chiral Imprinting Response of Oriented Dipole Moments on Metal Nanostructures. ACS Photonics 2018, 5, 4801–4809. [Google Scholar] [CrossRef]
- Nascimento, D.R.; Prince, A.E.D. Modeling Molecule-Plasmon Interactions Using Quantized Radiation Fields within Time-Dependent Electronic Structure Theory. J. Chem. Phys. 2015, 143, 214104. [Google Scholar] [CrossRef]
- Luk, H.L.; Feist, J.; Toppari, J.J.; Groenhof, G. Multiscale Molecular Dynamics Simulations of Polaritonic Chemistry. J. Chem. Theory Comput. 2017, 13, 4324–4335. [Google Scholar] [CrossRef]
- Vendrell, O. Coherent Dynamics in Cavity Femtochemistry: Application of the Multi-Configuration Time-Dependent Hartree Method. Chem. Phys. 2018, 509, 55–65. [Google Scholar] [CrossRef]
- Neuman, T.; Esteban, R.; Casanova, D.; García-Vidal, F.J.; Aizpurua, J. Coupling of Molecular Emitters and Plasmonic Cavities beyond the Point-Dipole Approximation. Nano Lett. 2018, 18, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Govorov, A.O.; Bryant, G.W. Semiconductor-Metal Nanoparticle Molecules: Hybrid Excitons and Non-linear Fano effect. Phys. Rev. Lett. 2006, 97, 146804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, W.Z. Theoretical Investigation of Resonance Raman Scattering of Dye Molecules Absorbed on Semiconductor Surfaces. J. Chem. Phys. 2011, 135, 044108. [Google Scholar] [CrossRef] [PubMed]
- Zelinskyy, Y.; Zhang, Y.; May, V. Supramolecular Complex Coupled to a Metal Nanoparticle: Computational Studies on the Optical Absorption. J. Phys. Chem. A 2012, 116, 11330–11340. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O.; Zhang, H.; Gun’ko, Y.K. Theory of Photoinjection of Hot Plasmonic Carriers from Metal Nanostructures into Semiconductors and Surface Molecules. J. Phys. Chem. C 2013, 117, 16616–16631. [Google Scholar] [CrossRef]
- Ye, C.X.; Zhao, Y.; Liang, W.Z. Resonance Raman Spectra of Organic Molecules Absorbed on Inorganic Semiconducting Surfaces: Contribution From Both Localized Intramolecular Excitation and Intermolecular Charge Transfer Excitation. J. Chem. Phys. 2015, 143, 154105. [Google Scholar] [CrossRef]
- Wang, L.X.; May, V. Control of Intermolecular Electronic Excitation Energy Transfer: Application of Metal Nanoparticle Plasmons. J. Phys. Chem. C 2017, 121, 13428–13433. [Google Scholar] [CrossRef]
- You, X.Y.; Ramakrishna, S.; Seideman, T. Plasmon-Mediated Absorption and Photocurrent Spectra in Sensitized Solar Cells. ACS Photonics 2017, 4, 1178–1187. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Liang, W.Z. Collaborative effect of plasmon-induced resonance energy and electron transfer on the interfacial electron injection dynamics of dye-sensitized solar cell. J. Chem. Phys. 2019, 151, 044702. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Liang, W.Z. Joint Effects of Exciton-Exciton and Exciton-Photon Couplings on the Singlet Fission Dynamics in Organic Aggregates. J. Phys. Chem. C 2021, 125, 1654–1664. [Google Scholar] [CrossRef]
- Faucheaux, J.A.; Fu, J.Y.; Jain, P.K. Unified Theoretical Framework for Realizing Diverse Regimes of Strong Coupling between Plasmons and Electronic Transitions. J. Phys. Chem. C 2014, 118, 2710–2717. [Google Scholar] [CrossRef]
- Wu, C.H.; Khanikaev, A.B.; Adato, R.; Arju, N.; Yanik, A.A.; Altug, H.; Shvets, G. Fano-Resonant Asymmetric Metamaterials for Ultrasensitive Spectroscopy and Identification of Molecular Monolayers. Nat. Mater. 2011, 11, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Adato, R.; Artar, A.; Erramilli, S.; Altug, H. Engineered Absorption Enhancement and Induced Transparency in Coupled Molecular and Plasmonic Resonator Systems. Nano Lett. 2013, 13, 2584–2591. [Google Scholar] [CrossRef]
- Sun, J.; Li, G.; Liang, W.Z. How does the plasmonic enhancement of molecular absorption depend on the energy gap between molecular excitation and plasmon modes: A mixed TDDFT/FDTD investigation. Phys. Chem. Chem. Phys. 2015, 17, 16835–16845. [Google Scholar] [CrossRef]
- Sun, J.; Ding, Z.L.; Yu, Y.Q.; Liang, W.Z. Plasmon-enhanced high order harmonic generation of open-ended finite-sized carbon nanotubes: The effects of incident field’s intensity and frequeny and the interference between the incident and scattered fields. J. Chem. Phys. 2020, 152, 224708. [Google Scholar] [CrossRef]
- Sun, J.; Ding, Z.L.; Yu, Y.Q.; Liang, W.Z. Nonlinear features of Fano resonance: A QM/EM study. Phys. Chem. Chem. Phys. 2021, 23, 15994. [Google Scholar] [CrossRef] [PubMed]
- Yabana, K.; Bertsch, G.F. Time-Dependent Local-Density Approximation in real time. Phys. Rev. B 1996, 54, 4484–4487. [Google Scholar] [CrossRef]
- Yam, C.Y.; Yokojima, S.; Chen, G.H. Linear-Scaling Time-Dependent Density-Functional Theory. Phys. Rev. B 2003, 68, 153105. [Google Scholar] [CrossRef]
- Yam, C.Y.; Yokojima, S.; Chen, G.H. Localized-Density-Matrix Implementation of Time-Dependent Density-Functional Theory. J. Chem. Phys. 2003, 119, 8794. [Google Scholar] [CrossRef]
- Castro, A.; Marques, M.A.L.; Rubio, A. Propagators for the Time-Dependent Kohn-Sham equations. J. Chem. Phys. 2004, 121, 3425. [Google Scholar] [CrossRef] [PubMed]
- Yabana, K.; Nakatsukasa, T.; Iwata, J.-I.; Bertsch, G.F. Real-Time, Real-Space Implementation of the Linear Response Time-Dependent Density-Functional Theory. Phys. Status Solidi B 2006, 243, 1121. [Google Scholar] [CrossRef]
- Cheng, C.L.; Evans, J.S.; Voorhis, T.V. Simulating Molecular Conductance Using Real-Time Density Functional Theory. Phys. Rev. B 2006, 74, 155112. [Google Scholar] [CrossRef]
- Pi, M.; Ancilotto, F.; Lipparini, E.; Mayol, R. Magneto-Optics of Three-Dimensional Quantum Dots: A Real Time, Time-Dependent Local Spin-Density Approach. Phys. E 2004, 24, 297–307. [Google Scholar] [CrossRef]
- Miller, E.K. Time-Domain Modeling in Electromagetics. J. Electromagn. Waves Appl. 1994, 8, 1125. [Google Scholar] [CrossRef]
- Castro, A.; Appel, H.; Oliveira, M.; Rozzi, C.A.; Andrade, X.; Lorenzen, F.; Marques, M.; Gross, E.; Rubio, A. Octopus: A tool for the application of time-dependent density functional theory. Phys. Stat. Sol. B 2006, 243, 2465–2488. [Google Scholar] [CrossRef]
- McMahon, J.M.; Wang, Y.; Sherry, L.J.; Van Duyne, R.P.; Marks, L.D.; Gray, S.K.; Schatz, G.C. Correlating the Structure, Optical Spectra, and Electrodynamics of Single Silver Nanocubes. J. Phys. Chem. C 2009, 113, 2731–2735. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Limonov, M.F.; Rybin, M.V.; Poddubny, A.N.; Kivshar, Y.S. Fano resonances in photonics. Nature 2017, 11, 543. [Google Scholar] [CrossRef]
- Fofang, N.T.; Park, T.H.; Neumann, O.; Mirin, N.A.; Nordlander, P.; Halas, N.J. Plexcitonic Nanoparticles: Plasmon-Exciton Coupling in Nanoshell-J-Aggregate Complexes. Nano Lett. 2008, 8, 3481–3487. [Google Scholar] [CrossRef]
- Fofang, N.T.; Grady, N.K.; Fan, Z.Y.; Govorov, A.O.; Halas, N.J. Plexciton Dynamics: Exciton-Plasmon Coupling in a J-Aggregate-Au Nanoshell Complex Provides a Mechanism for Nonlinearity. Nano Lett. 2011, 11, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Manjavacas, A.; Abajo, F.J.G.; Nordlander, P. Quantum Plexcitonics: Strongly Interacting Plasmons and Excitons. Nano Lett. 2011, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.W.; Wang, H.Y.; Jiang, Y.; Chen, Q.D.; Ueno, K.; Wang, W.Q.; Misawa, H.; Sun, H.B. Hybrid-State Dynamics of Gold Nanorods/Dye J-Aggregates under Strong Coupling. Angew. Chem. Int. Ed. 2011, 50, 7824. [Google Scholar] [CrossRef] [PubMed]
- Eizner, E.; Avayu, O.; Ditcovski, R.; Ellenbogen, T. Aluminum Nanoantenna Complexes for Strong Coupling between Excitons and Localized Surface Plasmons. Nano Lett. 2015, 15, 6215. [Google Scholar] [CrossRef] [PubMed]
- Neubrech, F.; Huck, C.; Weber, K.; Pucci, A.; Giessen, H. Surface-enhanced infrared spectroscopy using resonant nanoantennas. Chem. Rev. 2017, 117, 5110–5145. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.G.; Cai, W.B.; Wan, L.J.; Osawa, M. Infrared absorption enhancement for CO adsorbed on Au films in perchloric acid solutions and effects of surface structure studied by cyclic voltammetry, scanning tunneling microscopy, and surface-enhanced IR spectroscopy. J. Phys. Chem. B 1999, 103, 2460–2466. [Google Scholar] [CrossRef]
- Krauth, O.; Fahsold, G.; Magg, N.; Pucci, A. Anomalous infrared transmission of adsorbates on ultrathin metal films: Fano effect near the percolation threshold. J. Chem. Phys. 2000, 113, 6330. [Google Scholar] [CrossRef]
- Priebe, A.; Sinther, M.; Fahsold, G.; Pucci, A. The correlation between film thickness and adsorbate line shape in surface enhanced infrared absorption. J. Chem. Phys. 2003, 119, 4887. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).