Improved Solubility and Dissolution Rate of Ketoprofen by the Formation of Multicomponent Crystals with Tromethamine
Abstract
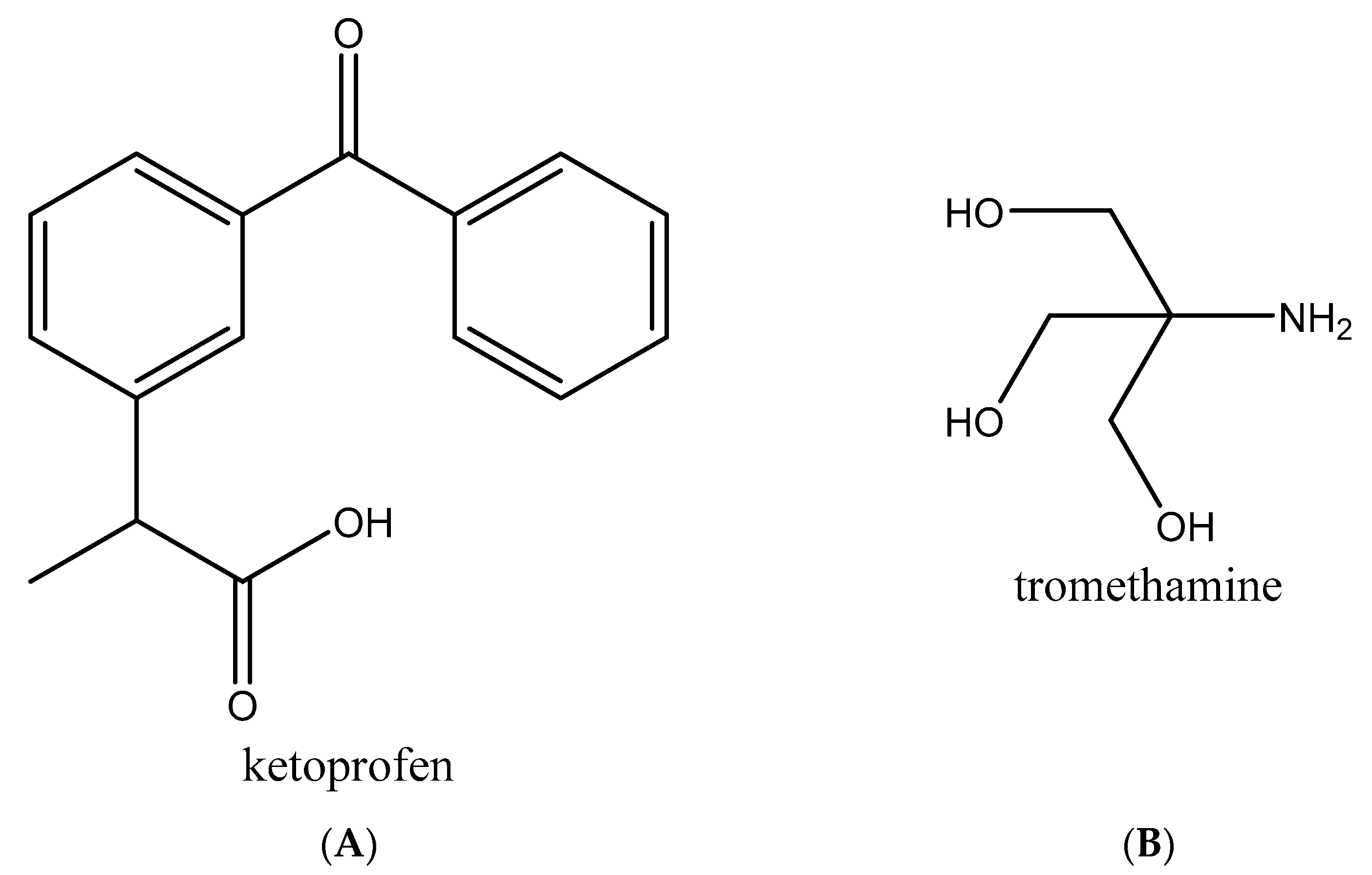
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Multicomponent Crystals of Ketoprofen–Tromethamine
2.2.2. Powder X-ray Diffraction (PXRD)
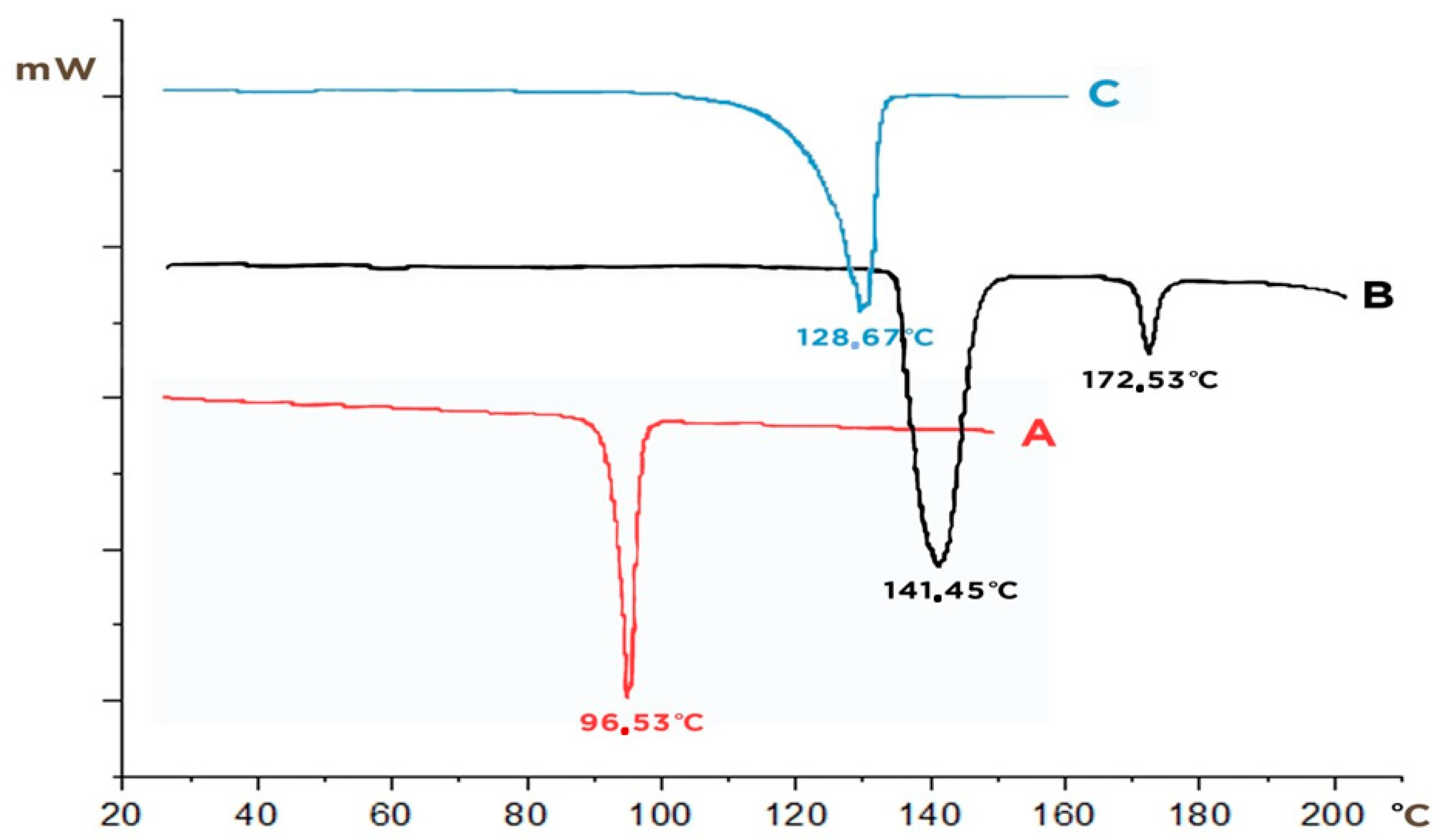
2.2.3. Differential Scanning Calorimetry (DSC)
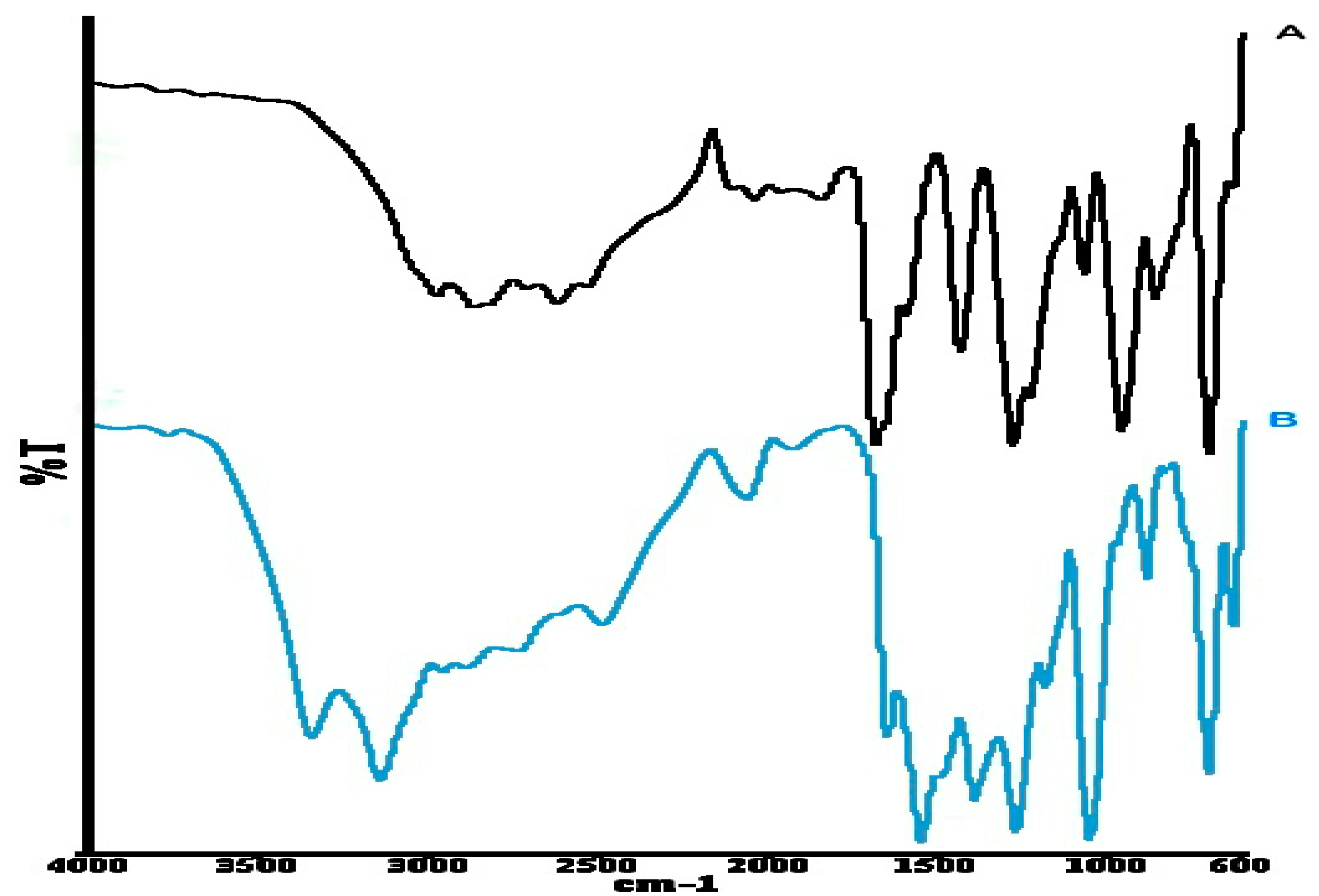
2.2.4. Fourier Transform Infrared (FT–IR) Spectroscopy
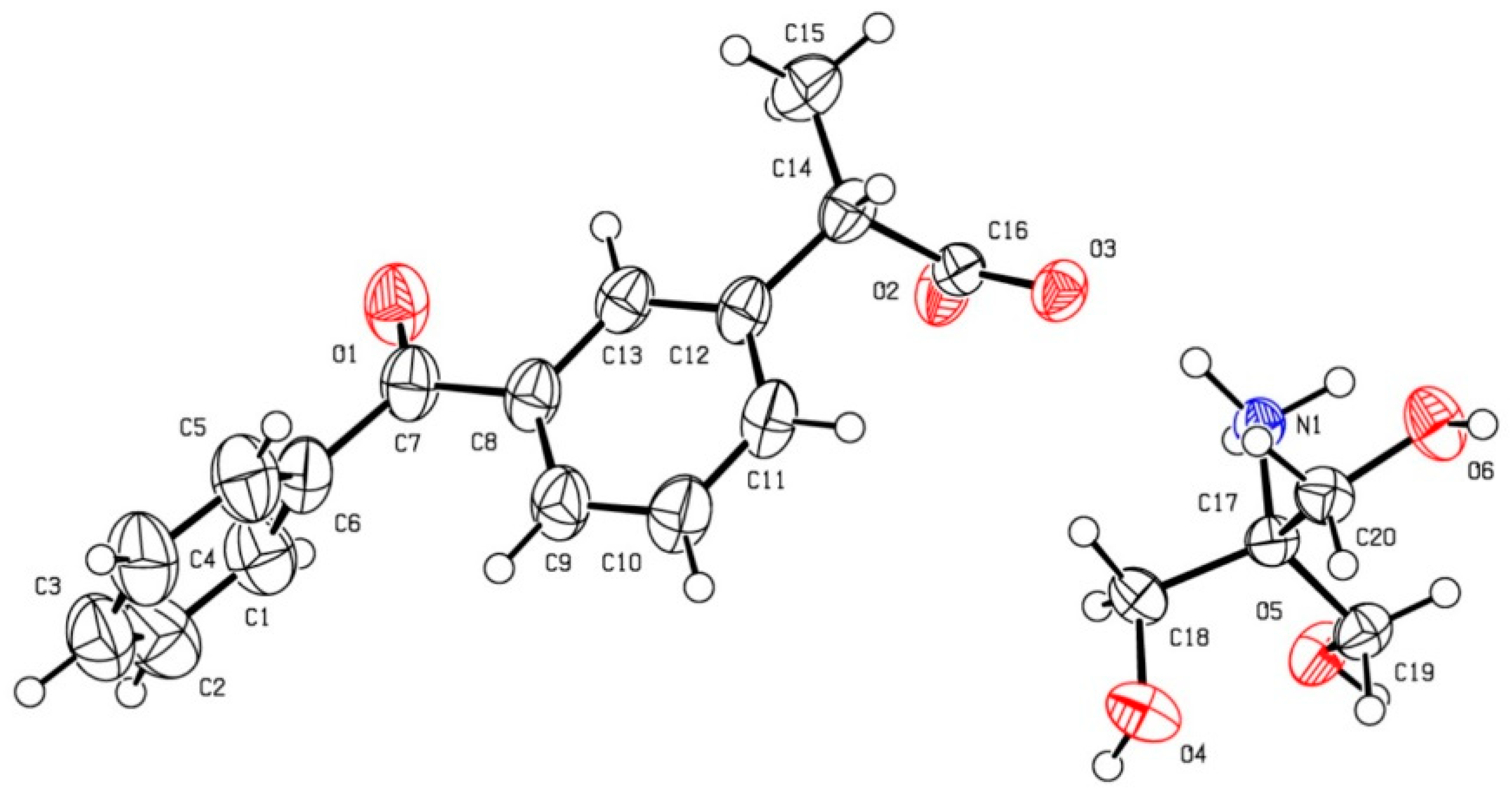
2.2.5. Single-Crystal X-ray Diffraction and Crystal Structure Refinements
2.2.6. Solubility Test
2.2.7. Dissolution Rate Study
2.2.8. Theoretical Calculation
3. Results and Discussion
3.1. Solid-State Characterization
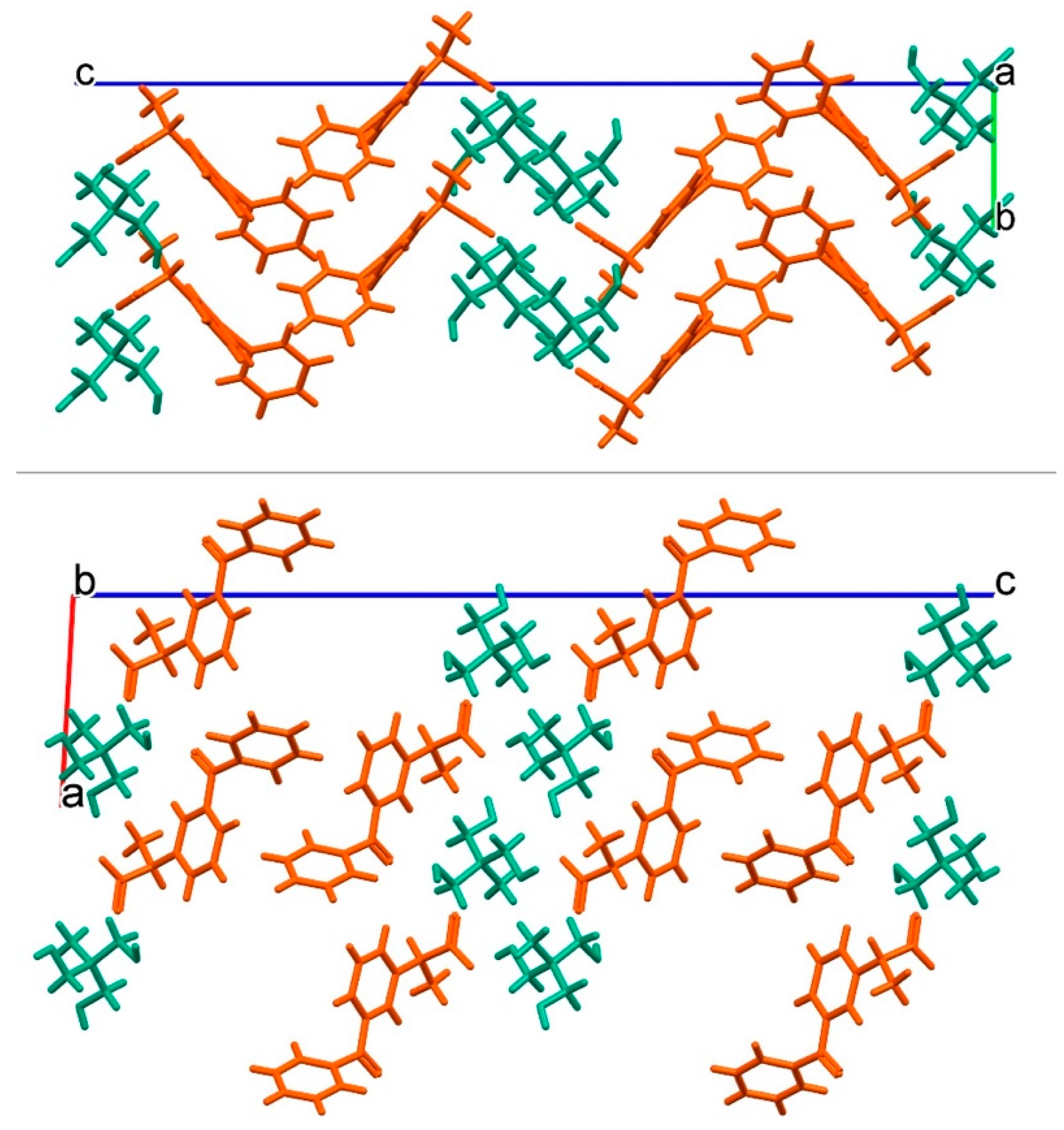
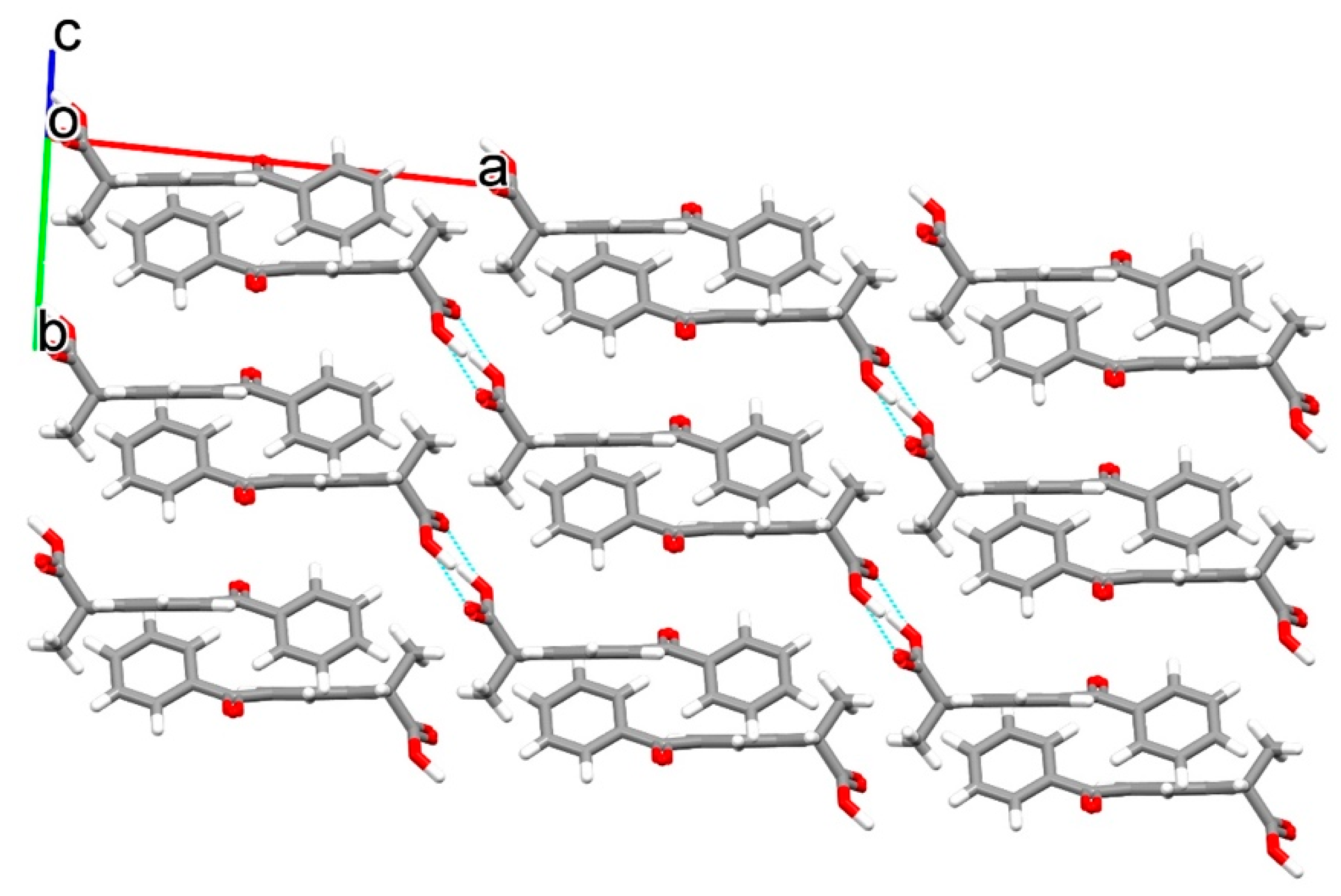
3.2. Crystal Structure of Ketoprofen–Tromethamine Multicomponent Crystal
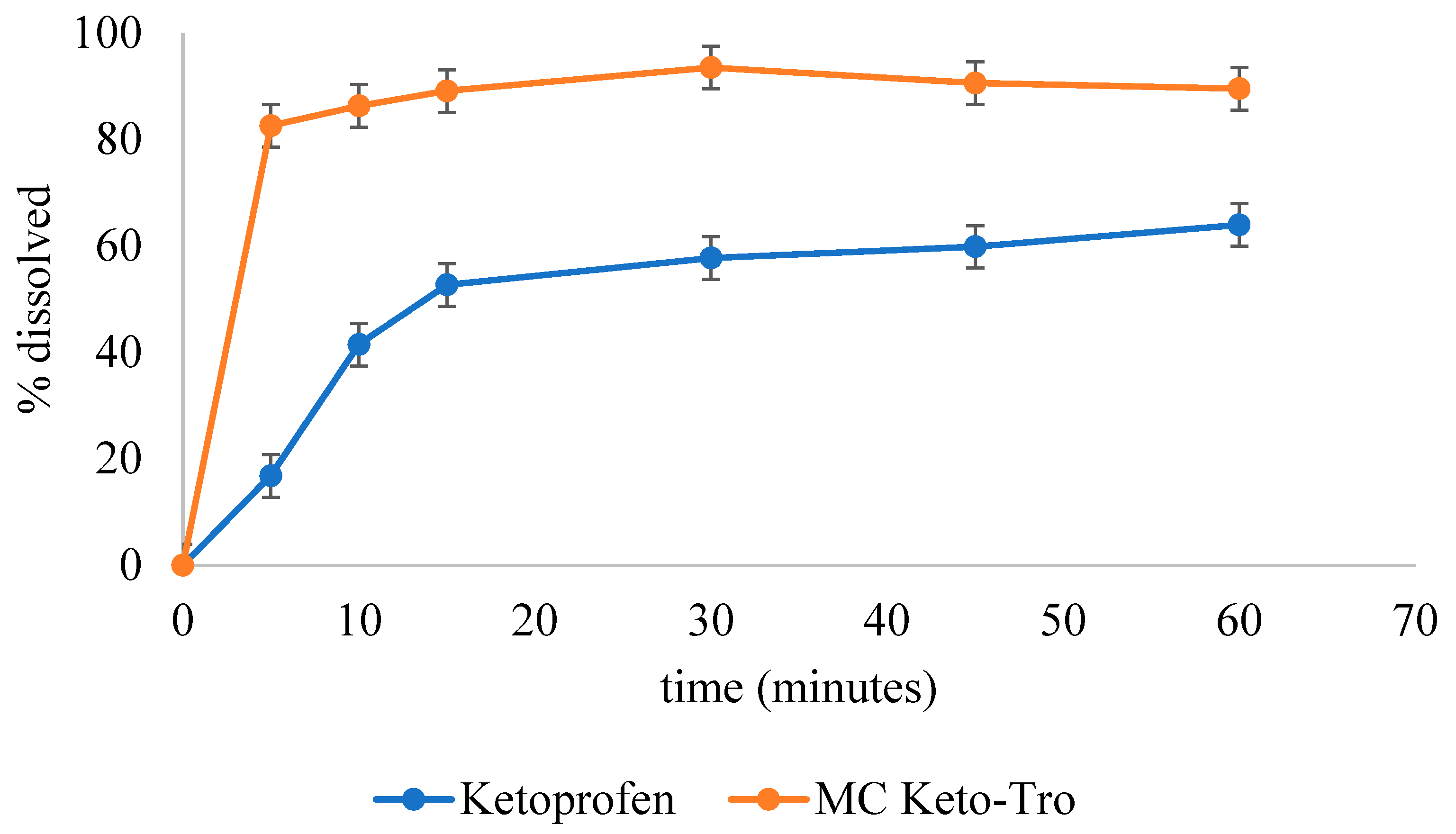
3.3. Solubility and Dissolution Rate
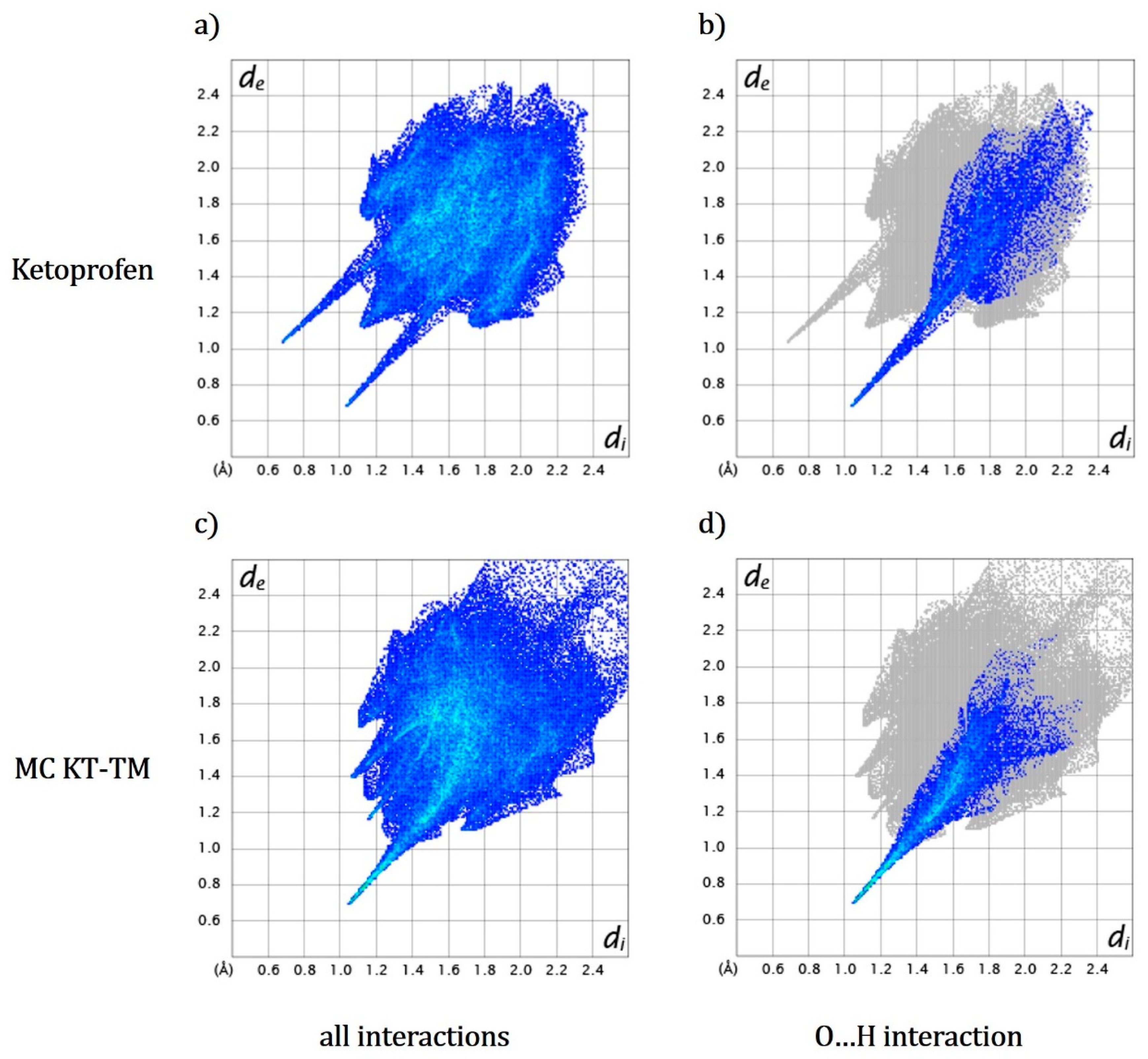
3.4. Hirshfeld Surfaces and Hydration Energy Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adeyeye, M.C.; Brittain, H.G. (Eds.) Preformulation Solid Dosage form Development; Informa Healthcare USA: New York, NY, USA, 2008. [Google Scholar]
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Reval, M.I.; Ventura-Martínez, R.; Déciga-Campos, M.; Terrón, A.J.; Cabré, F.; López-Muñoz, F.J. Evidence for a central mechanism of action of S-(+)-ketoprofen. Eur. J. Pharmacol. 2004, 483, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus Topical NSAIDs in Rheumatic Diseases. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Tsume, Y.; Langguth, P.; Garcia-Arieta, A.; Amidon, G.L. In silico prediction of drug dissolution and absorption with variation in intestinal pH for BCS class II weak acid drugs: Ibuprofen and ketoprofen. Biopharm. Drug Dispos. 2012, 33, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical Properties of Poly-Vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhou, F.; Williams, G.R. Developing and scaling up fast-dissolving electrospun formulations based on poly(vinylpyrrolidone) and ketoprofen. J. Drug Deliv. Sci. Technol. 2021, 61, 102138. [Google Scholar] [CrossRef]
- Bhatia, M.; Devi, S. Development, Characterisation and Evaluation of PVP K-30/PEG Solid Dispersion Containing Ketoprofen. ACTA Pharm. Sci. 2020, 58, 1. [Google Scholar] [CrossRef]
- Trisanti, P.N.; Sumarno. The effect of water addition in inclusion formation of ketoprofen/β-cyclodextrin using supercritical CO2. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2085, p. 20052. [Google Scholar]
- Zaini, E.; Wahyuni, Y.S.; Halim, A.; Yuliandra, Y. Preparation of eutectic mixture of ketoprofen and nicotinamide for enhanced dissolution rate. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 161–164. [Google Scholar]
- Khan, J.; Bashir, S.; Khan, M.A.; Ghaffar, R.; Naz, A.; Khan, W.; Ahmad, S.; Ullah, A.; Ali, F.L.; Isreb, M. Enhanced dissolution rate of Ketoprofen by fabricating into smart nanocrystals. Pak. J. Pharm. Sci. 2019, 32, 2899–2904. [Google Scholar]
- Ramos, P.; Pedra, N.; Soares, M.; Da Silveira, E.; Oliveira, P.; Grecco, F.; Da Silva, L.; Ferreira, L.M.; Ribas, D.; Gehrcke, M.; et al. Ketoprofen-loaded rose hip oil nanocapsules attenuate chronic inflammatory response in a pre-clinical trial in mice. Mater. Sci. Eng. C 2019, 103, 109742. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999, 88, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.P.; Date, A.A.; Patravale, V.B. Overcoming poor oral bioavailability using nanoparticle formulations–opportunities and limitations. Drug Discov. Today Technol. 2012, 9, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Cerreia Vioglio, P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical aspects of salt and cocrystal forms of APIs and characterization challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.D.; Uekusa, H. Pharmaceutical Multicomponent Crystals: Structure, Design, and Properties. In Advances in Organic Crystal Chemistry; Springer: Berlin, Germany, 2020; pp. 153–184. [Google Scholar]
- Zaini, E.; Afriyani, A.; Fitriani, L.; Ismed, F.; Horikawa, A.; Uekusa, H. Improved Solubility and Dissolution Rates in Novel Multicomponent Crystals of Piperine with Succinic Acid. Sci. Pharm. 2020, 88, 21. [Google Scholar] [CrossRef] [Green Version]
- Nugrahani, I.; Komara, S.W.; Horikawa, A.; Uekusa, H. Composing Novel Diclofenac Potassium and l-Proline Salt Cocrystal as a Strategy to Increase Solubility and Dissolution. J. Pharm. Sci. 2020, 109, 3423–3438. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.; Bahl, D.; Morris, K.; Stevens, L.L.; Haware, R. Structure-mechanics and improved tableting performance of the drug-drug cocrystal metformin: Salicylic acid. Eur. J. Pharm. Biopharm. 2020, 153, 23–35. [Google Scholar] [CrossRef]
- Lu, Q.; Dun, J.; Chen, J.-M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [CrossRef]
- Yuliandra, Y.; Izadihari, R.; Rosaini, H.; Zaini, E. Multicomponent crystals of mefenamic acid–tromethamine with improved dissolution rate. J. Res. Pharm. 2019, 23, 6. [Google Scholar] [CrossRef] [Green Version]
- Bruni, G.; Berbenni, V.; Maggi, L.; Mustarelli, P.; Friuli, V.; Ferrara, C.; Pardi, F.; Castagna, F.; Girella, A.; Milanese, C. Multicomponent crystals of gliclazide and tromethamine: Preparation, physico-chemical, and pharmaceutical characterization. Drug Dev. Ind. Pharm. 2018, 44, 243–250. [Google Scholar] [CrossRef]
- Bookwala, M.; Thipsay, P.; Ross, S.; Zhang, F.; Bandari, S.; Repka, M.A. Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology. Eur. J. Pharm. Biopharm. 2018, 131, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Paoli, P.; Chelazzi, L.; Milazzo, S.; Biagi, D.; Valleri, M.; Ienco, A.; Valtancoli, B.; Conti, L. Relationships between anhydrous and solvated species of dexketoprofen trometamol: A solid-state point of view. Cryst. Growth Des. 2019, 20, 226–236. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Drug solubility enhancement through the preparation of multicomponent organic materials: Eutectics of lovastatin with carboxylic acids. Pharmaceutics 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Zaini, E.; Fitriani, L.; Sari, R.Y.; Rosaini, H.; Horikawa, A.; Uekusa, H. Multicomponent Crystal of Mefenamic Acid and N-Methyl-D-Glucamine: Crystal Structures and Dissolution Study. J. Pharm. Sci. 2019, 108, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, D.; Ballone, P.; Decherchi, S.; Cavalli, A. The Solubility Advantage of Amorphous Ketoprofen. Thermodynamic and Kinetic Aspects by Molecular Dynamics and Free Energy Approaches. J. Chem. Theory Comput. 2020, 16, 4126–4140. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, Y.A.; Nikonorova, A.A.; Zolotarev, A.A.; Ugolkov, V.L.; Kochina, T.A. Tris(hydroxymethyl)methyl ammonium salts of biologically active carboxylic acids. Synthesis, properties and crystal structure. J. Mol. Struct. 2020, 1207, 127813. [Google Scholar] [CrossRef]
- Ishihara, S.; Hattori, Y.; Otsuka, M.; Sasaki, T. Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis. Crystals 2020, 10, 760. [Google Scholar] [CrossRef]
- Briard, P.; Rossi, J.C. Kétoprofène. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 1036–1038. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Putra, O.D.; Umeda, D.; Fujita, E.; Haraguchi, T.; Uchida, T.; Yonemochi, E.; Uekusa, H. Solubility improvement of benexate through salt formation using artificial sweetener. Pharmaceutics 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Milazzo, S.; Chelazzi, L.; Giovannoni, M.P.; Guerrini, G.; Ienco, A.; Valleri, M.; Conti, L. A combined crystallographic and computational study on dexketoprofen trometamol dihydrate salt. Crystals 2020, 10, 659. [Google Scholar] [CrossRef]




| Compound | Ketoprofen–Tromethamine (KT-TM) | |
|---|---|---|
| Chemical formula | C16H13O3−, C4H12NO3+ | |
| Temperature/°C | −100 | |
| Crystal system | Monoclinic | |
| Space group | P21/c | |
| Cell parameters | a/Å | 8.6312(2) |
| b/Å | 5.9134(1) | |
| c/Å | 37.4357(7) | |
| β/° | 93.039(1) | |
| Volume/Å3 | 1908.02(7) | |
| Z, Z′ | 4, 1 | |
| Density/(g cm−3) | 1.307 | |
| R1 [I > 2σ(I)] | 0.0656 | |
| CCDC deposit number | 2130881 | |
| Compound | Solubility (mg/100 mL) | Solubility Enhancement |
|---|---|---|
| Intact ketoprofen | 22.23 ± 4.55 | - |
| Multicomponent crystal of ketoprofen–tromethamine | 65.62 ± 3.86 | 2.95 fold |
| Compound | Ehy/kJ mol−1 |
|---|---|
| Tromethamine (cation) | −266.49 |
| (S)-Ketoprofen (anion) | −268.40 |
| (R)-Ketoprofen (anion) | −271.49 |
| Ketoprofen (anion, average) | −269.95 |
| (S)-Ketoprofen (neutral) | −29.76 |
| (R)-Ketoprofen (neutral) | −33.96 |
| Ketoprofen (neutral, average) | −31.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriani, L.; Firdaus, W.A.; Sidadang, W.; Rosaini, H.; Putra, O.D.; Oyama, H.; Uekusa, H.; Zaini, E. Improved Solubility and Dissolution Rate of Ketoprofen by the Formation of Multicomponent Crystals with Tromethamine. Crystals 2022, 12, 275. https://doi.org/10.3390/cryst12020275
Fitriani L, Firdaus WA, Sidadang W, Rosaini H, Putra OD, Oyama H, Uekusa H, Zaini E. Improved Solubility and Dissolution Rate of Ketoprofen by the Formation of Multicomponent Crystals with Tromethamine. Crystals. 2022; 12(2):275. https://doi.org/10.3390/cryst12020275
Chicago/Turabian StyleFitriani, Lili, Wahyu Alfath Firdaus, Wahyu Sidadang, Henni Rosaini, Okky Dwichandra Putra, Hironaga Oyama, Hidehiro Uekusa, and Erizal Zaini. 2022. "Improved Solubility and Dissolution Rate of Ketoprofen by the Formation of Multicomponent Crystals with Tromethamine" Crystals 12, no. 2: 275. https://doi.org/10.3390/cryst12020275
APA StyleFitriani, L., Firdaus, W. A., Sidadang, W., Rosaini, H., Putra, O. D., Oyama, H., Uekusa, H., & Zaini, E. (2022). Improved Solubility and Dissolution Rate of Ketoprofen by the Formation of Multicomponent Crystals with Tromethamine. Crystals, 12(2), 275. https://doi.org/10.3390/cryst12020275







