Two Polynuclear Manganese(II) Complexes Based on Multidentate N-Heterocyclic Aromatic Ligand and V-Shaped Polycarboxylate Ligand: Synthesis, Crystal Structure Analysis and Magnetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Single-Crystal X-Ray Diffraction
2.3. Preparation of Compounds 1–2
2.3.1. Synthesis of {[Mn4(ptptp)2(sdba)2(H2O)2]·2H2O}n (1)
2.3.2. Synthesis of {[Mn3(ptptp)(cpop)Br(H2O)2]·2H2O}n (2)
3. Results and Discussion
3.1. Structural Description
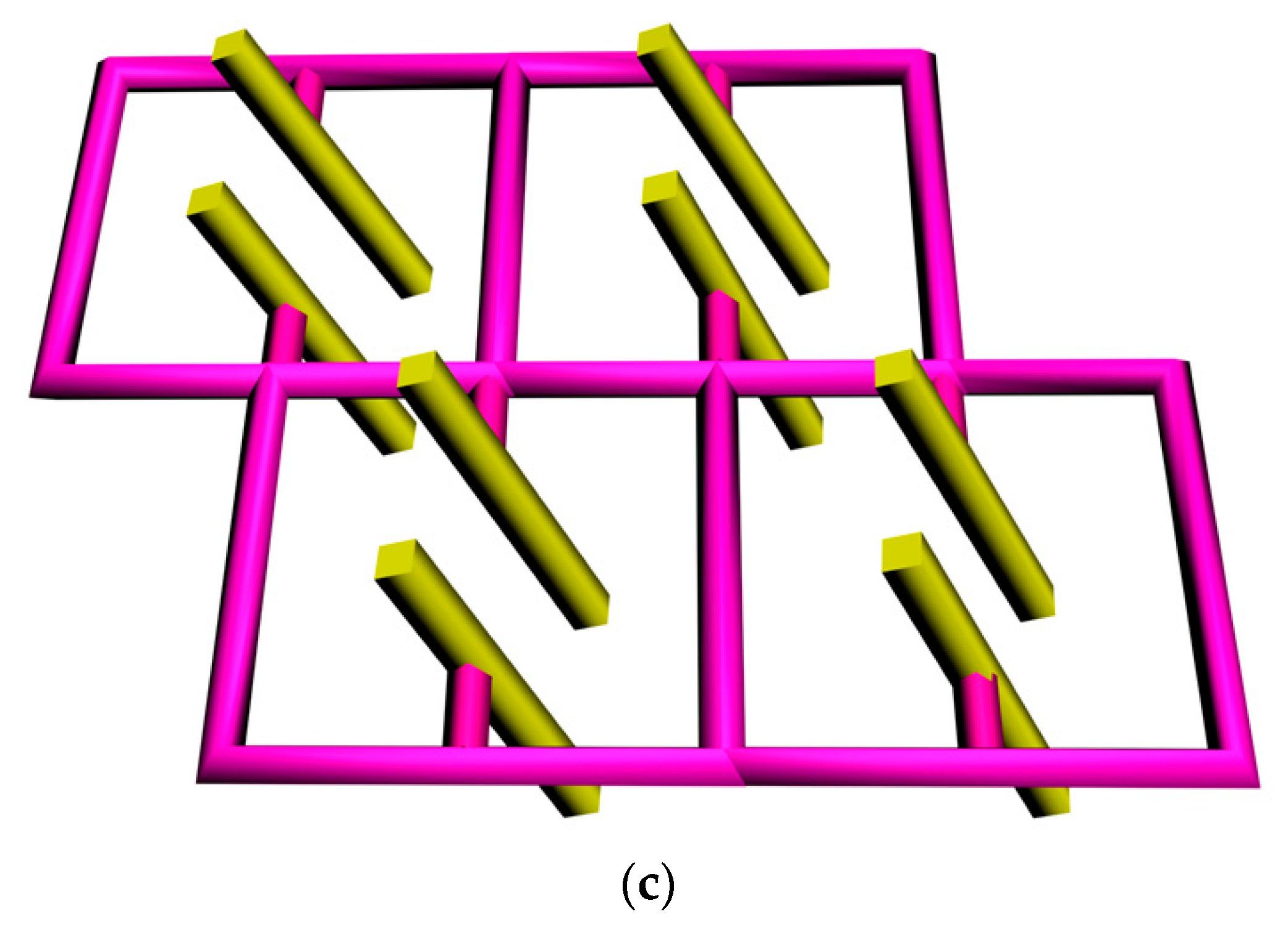
3.1.1. Crystal Structure of 1
3.1.2. Crystal Structure of 2
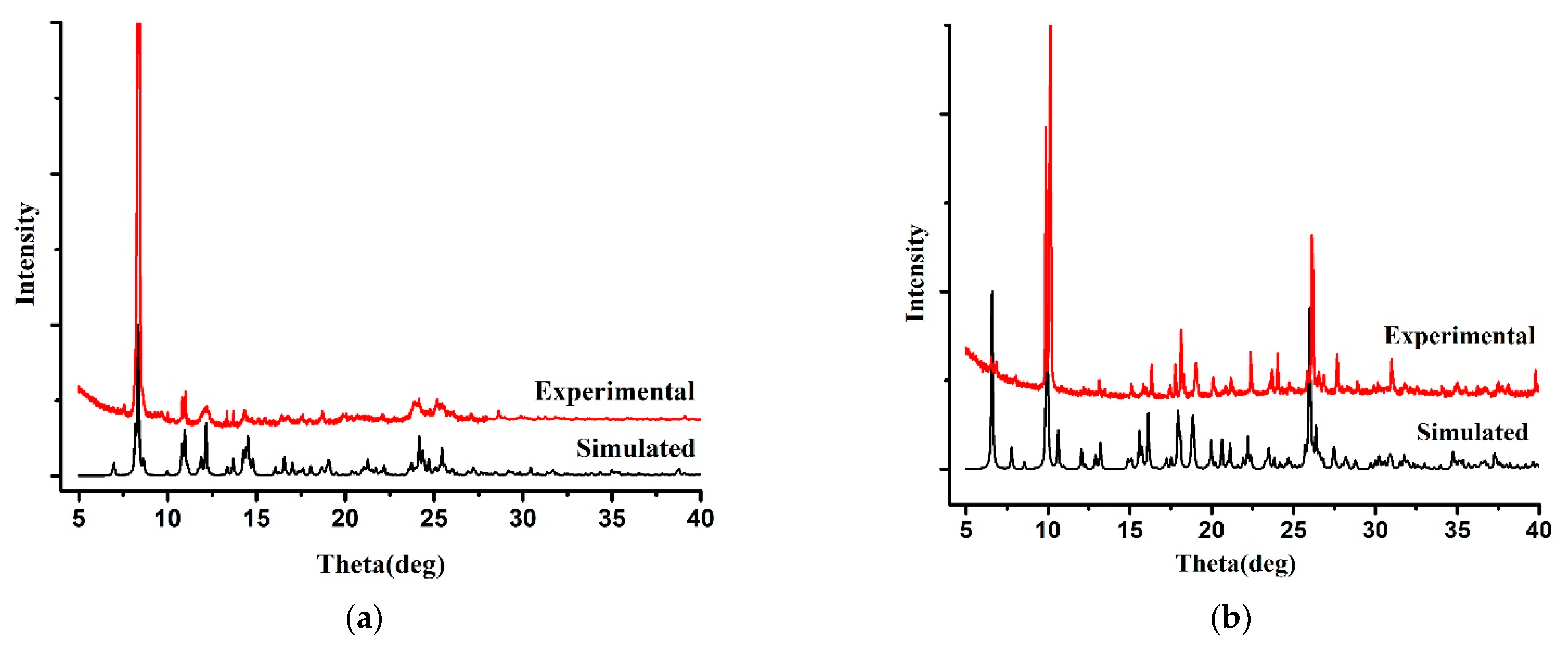
3.2. Powder X-Ray Diffraction (PXRD) and Thermal Analyses
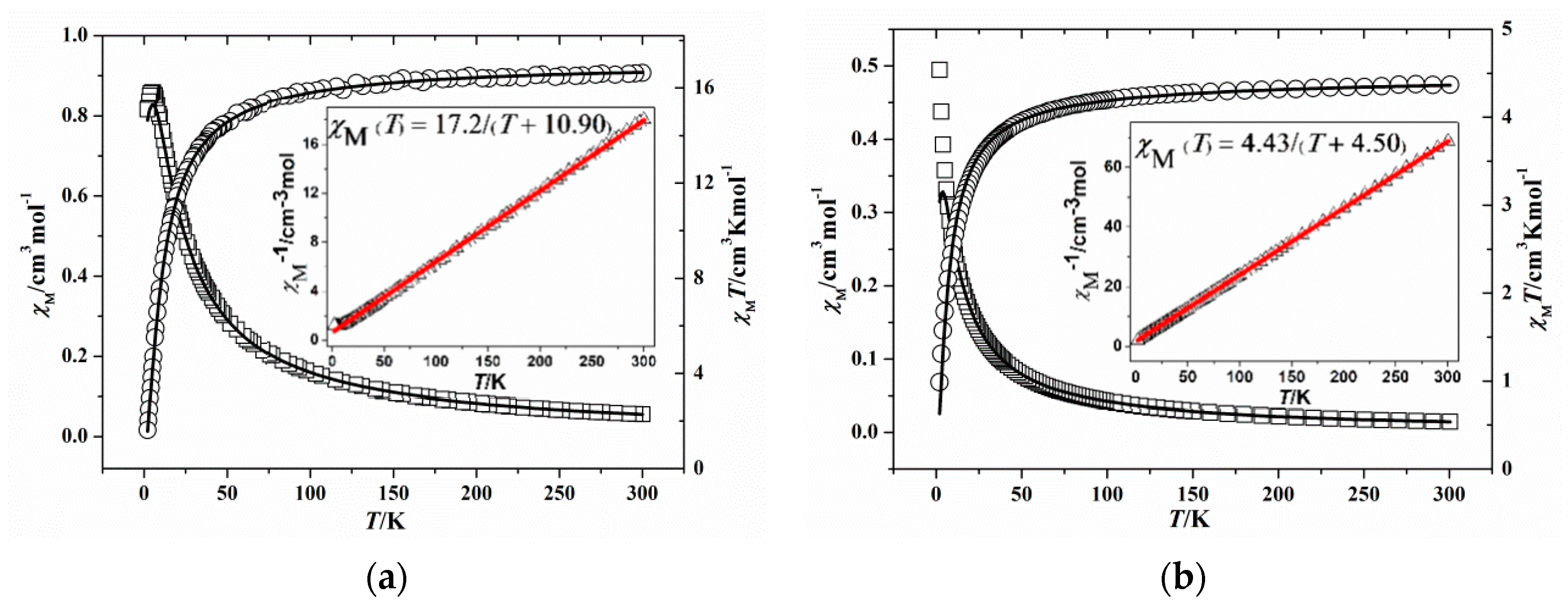
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishihara, H. Coordination programming: A new concept for the creation of multifunctional molecular systems. Chem. Lett. 2014, 43, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-D.; Liu, A.-J.; Wei, Q.; Hu, J.-X.; Pan, J.; Wang, G.-M. Quadruple photoresponsive functionality in a crystalline hybrid material: Photochromism, photomodulated fluorescence, magnetism and nonlinear optical properties. Chem. Eur. J. 2021, 27, 7842–7846. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Wang, Y.-T.; Ni, Q.-L.; Zhang, Z.-H.; Wang, X.-J.; Liang, G.-M.; Gui, L.-C. Aggregation of the metallocycles {Cu8} and {Cu20} using [Cu(bp)] units (H2bp = bis(2-hydroxybenzyl)amine): Structures and magnetic properties. Dalton Trans. 2016, 45, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ghosh, T.; Mahapatra, P.; Ghosh, A. Joining of trinuclear heterometallic CuII2–MII (M = Mn, Cd) nodes by nicotinate to form 1D chains: Magnetic properties and catalytic activities. Inorg. Chem. 2020, 59, 14989–15003. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Ma, L.-F.; Yan, D.-P. Facile synthesis of 1D organic—Inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadaoui, I.; Krichen, F.; Salah, B.; Mansour, R.; Miled, N.; Bougatef, A.; Kossentini, M. Design, synthesis and biological evaluation of Schiff bases of 4-amino-1,2,4-triazole derivatives as potent angiotensin converting enzyme inhibitors and antioxidant activities. J. Mol. Struct. 2019, 1180, 344–354. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A review: Pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Zafar, W.; Javed, H.; Zafar, M.; Hussain, M.Z.; Imran, M.; Nadeem, M.A. Facile synthesis, spectroscopic evaluation and antimicrobial screening of metal endowed triazole compounds. Biometals 2021, 34, 1329–1351. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev. 2000, 200, 131–185. [Google Scholar] [CrossRef]
- Naik, A.D.; Dirtu, M.M.; Railliet, A.P.; Marchand-Brynaert, J.; Garcia, Y. Coordination polymers and metal organic frameworks derived from 1,2,4-triazole amino acid linkers. Polymers 2011, 3, 1750–1775. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Gong, C.-H.; Zhang, J.-W.; Liu, G.-C.; Kan, X.-M.; Xu, N. Polyoxometalate-directed assembly of various multinuclear metal–organic complexes with 4-amino-1,2,4-triazole and selective photocatalysis for organic dye degradation. CrystEngComm 2015, 17, 4179–4189. [Google Scholar] [CrossRef]
- Feltham, H.L.C.; Barltrop, A.S.; Brooker, S. Spin crossover in iron(II) complexes of 3,4,5-tri-substituted-1,2,4-triazole (Rdpt), 3,5-di-substituted-1,2,4-triazolate (dpt-), and related ligands. Coord. Chem. Rev. 2017, 344, 26–53. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Zhao, B.; Guo, Q.; Hou, H.; Fan, Y. The syntheses, crystal structure, and exceptional chemical stability of three multinuclear PbII complexes. Eur. J. Inorg. Chem. 2013, 2013, 438–445. [Google Scholar] [CrossRef]
- Du, D.-Y.; Qin, J.-S.; Sun, C.-X.; Wang, X.-L.; Zhang, S.-R.; Shen, P.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Functional heterometallic coordination polymers with metalloligands as tunable luminescent crystalline materials. J. Mater. Chem. 2012, 22, 19673–19678. [Google Scholar] [CrossRef]
- Qin, J.-H.; Zhang, H.; Sun, P.; Huang, Y.-D.; Shen, Q.; Yang, X.-G.; Ma, L.-F. Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans. 2020, 49, 17772–17778. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Gonzalez, C.M.O.; Méndez, Y.P.; Lopez, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.-H.; Jia, Y.; Li, H.; Zhao, B.; Wu, D.; Zang, S.-Q.; Hou, H.; Fan, Y. Conversion from a heterochiral [2 + 2] Coaxially nested double-helical column to a cationic spiral staircase stimulated by an ionic liquid anion. Inorg. Chem. 2014, 53, 685–687. [Google Scholar] [CrossRef]
- Qin, J.-H.; Wang, H.-R.; Pan, Q.; Zang, S.-Q.; Hou, H.; Fan, Y. Influence of ionic liquids on the syntheses and structures of Mn(II) coordination polymers based on multidentate N-heterocyclic aromatic ligands and bridging carboxylate ligands. Dalton Trans. 2015, 44, 17639–17651. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 342–346. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Bu, Y.; Jiang, F.; Zhou, K.; Gai, Y.; Hong, M. From 3D interpenetrated polythreading to 3D non-interpenetrated polythreading: SBU modulation in a dual-ligand system. CrystEngComm 2014, 16, 1249–1252. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Tian, Y.; Li, N.; Liu, S.J. Ni(II)/Zn(II)-triazolate clusters based MOFs constructed from a V-shaped dicarboxylate ligand: Magnetic properties and phosphate sensing. J. Solid State Chem. 2018, 262, 100–105. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Tian, C.B.; Sun, Q.F. Coordination-assembled molecular cages with metal cluster nodes. Chem. Rec. 2021, 21, 498–522. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yamaguchi, M.; Onuki, T.; Noguchi, M.; Newton, G.N.; Shiga, T.; Oshio, H. Pentanuclear and octanuclear manganese helices. Eur. J. Inorg. Chem. 2015, 2015, 2193–2198. [Google Scholar] [CrossRef]
- Li, J.X.; Xiong, L.Y.; Fu, L.L.; Bo, W.B.; Du, Z.X.; Feng, X. Structural diversity of Mn(II) and Cu(II) complexes based on 2-carboxyphenoxyacetate linker: Syntheses, conformation comparison and magnetic properties. J. Solid State Chem. 2022, 305, 122636. [Google Scholar] [CrossRef]
- Cortés, R.; Drillon, M.; Solans, X.; Lezama, L.; Rojo, T. Alternating ferromagnetic−Antiferromagnetic interactions in a manganese(II)−Azido one-dimensional compound: [Mn(bipy)(N3)2]. Inorg. Chem. 1997, 36, 677–683. [Google Scholar] [CrossRef]




| Compound | 1 | 2 |
|---|---|---|
| Formula | C62H42Mn4N22O16S2 | C32H22BrMn3N11O10 |
| Formula weight | 1635.06 | 965.34 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P2(1)/c | P-1 |
| a (Å) | 20.435(4) | 11.454(2) |
| b (Å) | 16.136(3) | 12.093(2) |
| c (Å) | 21.182(4) | 14.054(3) |
| α (°) | 90 | 106.93(3) |
| β (°) | 91.315(2) | 91.07(3) |
| γ (°) | 90 | 99.90(3) |
| V (Å3) | 6983(2) | 1829.9(6) |
| Z | 4 | 2 |
| Dcalc (g·cm−3) | 1.555 | 1.752 |
| μ (mm−1) | 0.850 | 2.184 |
| F(000) | 3312 | 962 |
| θ range for data collection (°) | 2.30/25.50 | 1.97/25.50 |
| Data/restraints/parameters | 12997/6/955 | 6694/12/523 |
| R1a, wR2b (I > 2σ(I)) | R1 = 0.0620 wR2 = 0.1843 | R1 = 0.0597 wR2 = 0.1569 |
| R1, wR2 (all data) | R1 = 0.0683 wR2 = 0.1903 | R1 = 0.1184 wR2 = 0.1921 |
| GOF | 1.019 | 1.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Qin, J. Two Polynuclear Manganese(II) Complexes Based on Multidentate N-Heterocyclic Aromatic Ligand and V-Shaped Polycarboxylate Ligand: Synthesis, Crystal Structure Analysis and Magnetic Properties. Crystals 2022, 12, 278. https://doi.org/10.3390/cryst12020278
Wang H, Qin J. Two Polynuclear Manganese(II) Complexes Based on Multidentate N-Heterocyclic Aromatic Ligand and V-Shaped Polycarboxylate Ligand: Synthesis, Crystal Structure Analysis and Magnetic Properties. Crystals. 2022; 12(2):278. https://doi.org/10.3390/cryst12020278
Chicago/Turabian StyleWang, Huarui, and Jianhua Qin. 2022. "Two Polynuclear Manganese(II) Complexes Based on Multidentate N-Heterocyclic Aromatic Ligand and V-Shaped Polycarboxylate Ligand: Synthesis, Crystal Structure Analysis and Magnetic Properties" Crystals 12, no. 2: 278. https://doi.org/10.3390/cryst12020278
APA StyleWang, H., & Qin, J. (2022). Two Polynuclear Manganese(II) Complexes Based on Multidentate N-Heterocyclic Aromatic Ligand and V-Shaped Polycarboxylate Ligand: Synthesis, Crystal Structure Analysis and Magnetic Properties. Crystals, 12(2), 278. https://doi.org/10.3390/cryst12020278





