Life from a Snowflake: Diversity and Adaptation of Cold-Loving Bacteria among Ice Crystals
Abstract
:1. The Cryosphere
2. Convergent and Divergent Aspects of Frozen Habitats at the Poles
2.1. Snow
2.2. Glaciers, Icebergs, and Ice Platforms
2.3. Sea Ice
2.4. Brines
2.5. Permafrost
3. Microbial Life at Subzero Temperatures
3.1. Cryoinjury on Bacterial Life
3.2. Cold-Adapted Bacteria: Dormant or Trapped?
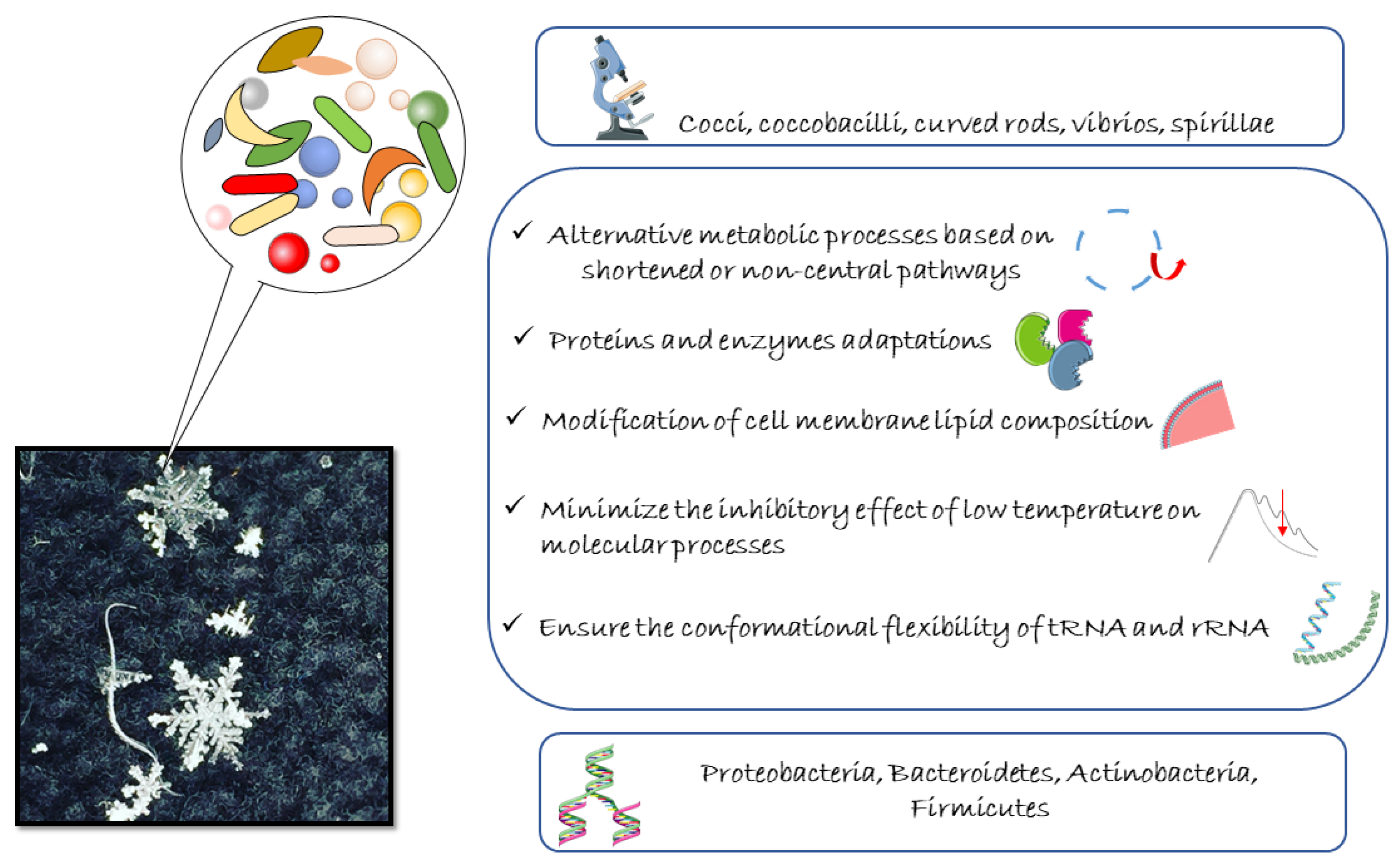
3.3. Molecular and Physiological Adaptations of Cold-Adapted Bacteria
4. Microbial Diversity in Cryoenvironments
4.1. Ice
4.2. Brines
4.3. Permafrost
4.4. Snow
5. Cryoprotection in an Icy World
5.1. Exopolymeric Substances (EPSs)
5.2. Ice-Binding Proteins (IBPs)
5.2.1. Anti-Freeze Proteins
5.2.2. Ice Nucleation Proteins
| Strain | Origin | Biomolecule | Function | Functioning/Production Temperature (°C) | Reference(s) |
|---|---|---|---|---|---|
| Pseudoalteromonas spp. | Antarctic | EPS | Emulsifying activity, heavy metal chelation, cryoprotection | 2–10 | [215,227] |
| Pseudoalteromonas sp. MER144 | Antarctic | EPS | Emulsifying activity, heavy metal chelation, Cryoprotection | 4–15 | [228] |
| Colwellia sp. GW185 Shewanella sp. CAL606 Winogradskyella sp. CAL384, CAL396 | Antarctic | EPS | Emulsifying activity, heavy metal chelation, Cryoprotection | 4–15 | [222] |
| Marinobacter sp. W1-16 | Antarctic | EPS | Emulsifying activity, heavy metal chelation, Cryoprotection | 4–15 | [229] |
| Colwellia psychrerythraea 34H | Antarctic | EPS | Cryoprotection | −4 to 10 | [230] |
| Pseudoalteromonas sp. ArcPo 1 | Arctic | EPS | Cryoprotection, ice nucleation | - | [245] |
| Colwellia sp. SLW05 | Antarctic | AFP | Cryoprotection | 0–3 | [245] |
| Flavobacterium frigoris PS1 | Antarctic | IBP | Cryoprotection | Not specified | [247] |
| Marinomonas primoryensis | Antarctic | IBP | Cryoprotection | 4 | [240,244,261] |
| Micrococcus cryophilus Rhodococcus erythropolis | AFP | Cryoprotection | 3 | [252] | |
| Pseudomonas putida GR12-2 | Arctic | AFP | Cryoprotection | 5 | [69] |
| Moraxella sp. | Antarctic | AFP | Cryoprotection | 5 | [251] |
| Flavobacterium xanthum | Antarctic | AFP | Cryoprotection | 4 | [248] |
| Pseudomonas fluorescens KUAF-68 and | Antarctic | INP | Cryoprotection | −10.6 to −8 | [259] |
| Flavobacterium sp. GL7, Chryseobacterium sp. GL8, Pseudomonas borealis DL7, Acinetobacter radioresistens DL5 | Temperate regions | INP | Cryoprotection | 4 | [260] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.A. Extremophiles in Antarctica: Life at low temperatures. In Adaption of Microbial Life to Environmental Extremes; Stan-Lotter, H., Fendrihan, S., Eds.; Springer: Vienna, Austria, 2012; pp. 87–218. [Google Scholar]
- Margesin, R.; Neuner, G.; Storey, K.B. Cold-loving microbes, plants, and animals—Fundamental and applied aspects. Naturwissenschaften 2007, 94, 77–99. [Google Scholar] [CrossRef]
- Rizzo, C.; Lo Giudice, A. The Variety and Inscrutability of Polar Environments as a Resource of Biotechnologically Relevant Molecules. Microorganisms 2020, 8, 1422. [Google Scholar] [CrossRef] [PubMed]
- Beniston, M.; Farinotti, D.; Stoffel, M.; Andreassen, L.M.; Coppola, E.; Eckert, N.; Fantini, A.; Giacona, F.; Hauck, C.; Huss, M.; et al. The European mountain cryosphere: A review of its current state, trends, and future challenges. Cryosphere 2018, 12, 759–794. [Google Scholar] [CrossRef] [Green Version]
- Bibi, S.; Wang, L.; Li, X.; Zhou, J.; Chen, D.; Yao, T. Climatic and associated cryospheric, biospheric, and hydrological changes on the Tibetan Plateau: A review. Int. J. Climatol. 2018, 38, e1–e17. [Google Scholar] [CrossRef] [Green Version]
- Huss, M.; Bookhagen, B.; Huggel, C.; Jacobsen, D.; Bradley, R.S.; Clague, J.J.; Vuille, M.; Buytaert, W.; Cayan, D.R.; Greenwood, G.; et al. Toward mountains without permanent snow and ice. Earths Future 2017, 5, 418–435. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Manucharova, N.A.; Gorlenko, M.V.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Bulat, S.A. 100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions. Extremophiles 2017, 21, 1057–1067. [Google Scholar] [CrossRef]
- Garcia-Lopez, E.; Cid, C. Glaciers and ice sheets as analog environments of potentially habitable icy worlds. Front. Microbiol. 2017, 8, 1407. [Google Scholar] [CrossRef]
- Jansson, J.K.; Tas, N. The microbial ecology of permafrost. Nat. Rev. Microbiol. 2014, 12, 414–425. [Google Scholar] [CrossRef]
- Margesin, R. Psychrophiles: From Biodiversity to Biotechnology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Doesken, N.J.; Judson, A. The Snow Booklet: A Guide to the Science, Climatology, and Measurement of Snow in the United States; Colorado State University: Fort Collins, CO, USA, 2000. [Google Scholar]
- Steele, P. Snow: Causes and Effects; Franklin Watts: New York, NY, USA, 1991. [Google Scholar]
- Jones, H.G. The ecology of snow-covered systems: A brief overview of nutrient cycling and life in the cold. Hydrol. Process. 1999, 13, 2135–2147. [Google Scholar] [CrossRef]
- Bragdon, B.; Breault, A.; Bush, B.; Gamble, J.; Hudak, R.; Johnston, E.; Kennedy, K.; Makucewicz, A.; Maynard, S.; Mohr, E.; et al. Life in the Cold: An Investigation of Polar Regions. Available online: https://dune.une.edu/marinesci_studproj/2/ (accessed on 18 January 2022).
- Haas, C. Dynamics versus thermodynamics. In Sea Ice—An Introduction to Its Physics, Chemistry, Biology and Geology; Thomas, D.N., Dieckmann, G.S., Eds.; Blackwell: Oxford, UK, 2003; pp. 82–111. [Google Scholar]
- Dieckmann, G.S.; Hellmer, H.H. The importance of sea ice: An overview. In Sea Ice—An Introduction to Its Physics, Chemistry, Biology and Geology; Thomas, D.N., Dieckmann, G.S., Eds.; Blackwell: Oxford, UK, 2003; pp. 1–21. [Google Scholar]
- Eicken, H. From the microscopic, to the macroscopic, to the regional scale: Growth, microstructure, and properties of sea ice. In Sea Ice—An Introduction to Its Physics, Chemistry, Biology and Geology; Thomas, D.N., Dieckmann, G.S., Eds.; Blackwell: Oxford, UK, 2003; pp. 22–81. [Google Scholar]
- Kirst, G.O.; Wiencke, C. Ecophysiology of polar algae. J. Phycol. 1995, 31, 181–199. [Google Scholar] [CrossRef]
- Junge, K.; Eicken, H.; Deming, J.W. Bacterial activity at −2 to −20 °C in Arctic wintertime sea ice. Appl. Environ. Microbiol. 2004, 70, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junge, K.; Eicken, H.; Swanson, B.D.; Deming, J.W. Bacterial incorporation of leucine into protein down to −20 °C with evidence for potential activity in sub-eutectic saline ice formations. Cryobiology 2006, 52, 417–429. [Google Scholar] [CrossRef]
- Mock, T.; Thomas, D.N. Sea ice—Recent advances in microbial studies. Environ. Microbiol. 2005, 7, 605–619. [Google Scholar] [CrossRef]
- Deming, J.W. Life in ice formations at very cold temperatures. In Physiology and Biochemistry of Extremophiles; Gerday, C., Glansdorff, N., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 133–145. [Google Scholar]
- Poltermann, M. Arctic sea ice as feeding ground for amphipods—Food sources and strategies. Polar Biol. 2001, 24, 89–96. [Google Scholar] [CrossRef]
- Wing, S.; McLeod, R.; Leichter, J.; Frew, R.; Lamare, M. Sea ice microbial production supports Ross Sea benthic communities: Influence of a small but stable subsidy. Ecology 2012, 93, 314–323. [Google Scholar] [CrossRef]
- Kuosa, H.; Kaartokallio, H. Experimental evidence on nutrient and substrate limitation of Baltic Sea sea-ice algae and bacteria. Hydrobiologia 2006, 554, 1–10. [Google Scholar] [CrossRef]
- Riedel, A.; Michel, C.; Gosselin, M. Grazing of large-sized bacteria by sea-ice heterotrophic protists on the Mackenzie shelf during the winter-spring transition. Aquat. Microb. Ecol. 2007, 74, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Deming, J.W. Sea ice bacteria and viruses. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2010; pp. 247–282. [Google Scholar]
- Eronen-Rasimus, E.; Lyra, C.; Rintala, J.-M.; Jürgens, K.; Ikonen, V.; Kaartokallio, H. Ice formation and growth shape bacterial community structure in Baltic Sea drift ice. FEMS Microbiol. Ecol. 2015, 91, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, M.D.; France, J.L.; Fisher, F.N.; Beine, H.J. Measurement and modelling of UV radiation penetration and photolysis rates of nitrate and hydrogen peroxide in Antarctic sea ice: An estimate of the production rate of hydroxyl radicals in first-year sea ice. J. Photochem. Photobiol. A 2005, 176, 39–49. [Google Scholar] [CrossRef]
- Junge, K.; Krembs, C.; Deming, J.; Stierler, A.; Eicken, H. A microscopic approach to investigate bacteria under in situ conditions in sea-ice samples. Ann. Glaciol. 2001, 33, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Wadhams, P. Ice in the Ocean; Gordon and Breach Science Publishers: London, UK, 2000. [Google Scholar]
- Dickson, J.; Head, J.; Levy, J.S.; Marchant, D.R. Don Juan Pond, Antarctica: Near-surface CaCl2-brine feeding Earth’s most saline lake and implications for Mars. Sci. Rep. 2013, 3, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toner, J.D.; Sletten, R.S. The formation of Ca-Cl-rich groundwaters in the Dry Valleys of Antarctica: Field measurements and modelling of reactive transport. Geochim. Cosmochim. Acta 2013, 110, 84–105. [Google Scholar] [CrossRef]
- Mikucki, J.; Auken, E.; Tulaczyk, S.; Virginia, R.A.; Schamper, C.; Sørensen, K.I.; Doran, P.T.; Dugan, H.; Foley, N. Deep groundwater and potential subsurface habitats beneath an Antarctic dry valley. Nat. Commun. 2015, 6, 6831. [Google Scholar] [CrossRef] [Green Version]
- Badgeley, J.A.; Pettit, E.C.; Carr, C.G.; Tulaczyk, S.; Mikucki, J.A.; Lyons, W.B.; MIDGE Science Team. An englacial hydrologic system of brine within a cold glacier: Blood Falls, McMurdo Dry Valleys, Antarctica. J. Glaciol. 2017, 63, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.E.; Kenig, F.; Fritsen, C.H.; McKay, C.P.; Cawley, K.M.; Edwards, R.; Kuhn, E.; McKnight, D.M.; Ostrom, N.E.; Peng, V.; et al. Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. Proc. Natl. Acad. Sci. USA 2012, 109, 20626–20631. [Google Scholar] [CrossRef] [Green Version]
- Dugan, H.A.; Doran, P.T.; Wagner, B.; Kenig, F.; Fritsen, C.H.; Arcone, S.A.; Kuhn, E.; Ostrom, N.E.; Warnock, J.P.; Murray, A.E. Stratigraphy of Lake Vida, Antarctica: Hydrologic implications of 27m of ice. Cryosphere 2015, 9, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Grima, C.; Blankenship, D.; Young, D.A.; Soderlund, K.M. Mobilization of Near-Surface Brine on Europa. In Proceedings of the Conference Held, Europa Deep Dive 1: Ice-Shell Exchange Processes, Houston, TX, USA, 1–2 November 2017. [Google Scholar]
- Mikucki, J.A.; Priscu, J.C. Bacterial diversity associated with blood falls, a subglacial outflow from the Taylor Glacier, Antarctica. Appl. Environ. Microbiol. 2007, 73, 4029–4039. [Google Scholar] [CrossRef] [Green Version]
- Siegert, M.J.; Ross, N.; Le Brocq, A.M. Recent advances in understanding Antarctic subglacial lakes and hydrology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20140306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, E.; Dalle Fratte, M.; Azzaro, M.; Guglielmin, M. Pressurized brines in continental Antarctica as a possible analogue of Mars. Sci. Rep. 2016, 6, 33158. [Google Scholar] [CrossRef] [Green Version]
- Guglielmin, M.; Biasini, A.; Smiraglia, C. The contribution of geoelectrical investigations in the analysis of periglacialand glacial landforms in ice free areas of the Northern Foothills (Northern Victoria Land, Antarctica). Geogr. Ann. Ser. A Phys. Geogr. 1997, 79, 17–24. [Google Scholar] [CrossRef]
- French, H.M.; Guglielmin, M. Observations on the ice marginal periglacial geomorphology of Terra Nova Bay, Northern Victoria Land, Antarctica. Permafr. Periglac. Process. 1999, 10, 331–348. [Google Scholar] [CrossRef]
- French, H.M.; Guglielmin, M. Frozen ground phenomena in the vicinity of Terra Nova Bay, Northern Victoria Land, Antarctica: A preliminary report. Geogr. Ann. Ser. A Phys. Geogr. 2000, 82, 513–526. [Google Scholar] [CrossRef]
- Guglielmin, M.; Lewkowicz, A.; French, H.M.; Strini, A. Lakeice blisters, Terra Nova Bay area, Northern Victoria Land, Antarctica. Geogr. Ann. Ser. A Phys. Geogr. 2009, 91, 99–111. [Google Scholar] [CrossRef]
- Doran, P.T.; Fritsen, C.H.; McKay, C.P.; Priscu, J.C.; Adams, E.E. Formation and character of an ancient 19-m ice cover and underlying trapped brine in an “ice-sealed” east Antarctic lake. Proc. Natl. Acad. Sci. USA 2003, 100, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Margesin, R. Permafrost Soils; Springer: Berlin/Heidelberg, Germany, 2009; p. 16. [Google Scholar]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhang, Q.; Tian, T.; Cheng, G.; An, L.; Feng, H. The microbial diversity, distribution, and ecology of permafrost in China: A review. Extremophiles 2015, 19, 693–705. [Google Scholar] [CrossRef]
- Jansson, J.K.; Baker, E.S. Multi-omic future for microbiome studies. Nat. Microbiol. 2016, 1, 16049. [Google Scholar] [CrossRef]
- Mitzscherling, J.; Winkel, M.; Winterfeld, M.; Horn, F.; Yang, S.Z.; Grigoriev, M.N.; Wagner, D.; Overduin, P.P.; Liebner, S. The development of permafrost bacterial communities under submarine conditions. J. Geophys. Res. Biogeosci. 2017, 122, 1689–1704. [Google Scholar] [CrossRef]
- Nikrad, M.P.; Kerkhof, L.J.; Haggblom, M.M. The subzero microbiome: Microbial activity in frozen and thawing soils. FEMS Microbiol. Ecol. 2016, 92, fiw081. [Google Scholar] [CrossRef] [PubMed]
- Vishnivetskaya, T.A.; Buongiorno, J.; Bird, J.; Krivushin, K.; Spirina, E.V.; Oshurkova, V.; Shcherbakova, V.A.; Wilson, G.; Lloyd, K.G.; Rivkina, E.M. Methanogens in the Antarctic Dry Valley permafrost. FEMS Microbiol. Ecol. 2018, 94, fiy109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goordial, J.; Davila, A.; Lacelle, D.; Pollard, W.; Marinova, M.M.; Greer, C.W.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Nearing the cold-arid limits of microbial life in permafrost of an upper dry valley, Antarctica. ISME J. 2016, 10, 1613–1624. [Google Scholar] [CrossRef]
- Goordial, J.; Raymond-Bouchard, I.; Zolotarov, Y.; de Bethencourt, L.; Ronholm, J.; Shapiro, N.; Woyke, T.; Stromvik, M.; Greer, C.W.; Bakermans, C.; et al. Cold adaptive traits revealed by comparative genomic analysis of the eurypsychrophile Rhodococcus sp. JG3 isolated from high elevation McMurdo Dry Valley permafrost, Antarctica. FEMS Microbiol. Ecol. 2016, 92, fiv154. [Google Scholar] [CrossRef] [Green Version]
- Bock, C.; Eicken, H. A magnetic resonance study of temperature-dependent microstructural evolution and self-diffusion of water in Arctic first-year sea ice. Ann. Glaciol. 2005, 40, 179–184. [Google Scholar] [CrossRef]
- Bakermans, C. Limits for microbial life at subzero temperatures. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Clarke, A. The thermal limits to life on Earth. Int. J. Astrobiol. 2014, 13, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Bakermans, C. Determining the limits of microbial life at subzero temperatures. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–38. [Google Scholar]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [Green Version]
- Tung, H.C.; Bramall, N.E.; Price, P.B. Microbial origin of excess methane in glacial ice and implications for life on Mars. Proc. Natl. Acad. Sci. USA 2005, 102, 18292–18296. [Google Scholar] [CrossRef] [Green Version]
- Tung, H.C.; Price, P.B.; Bramall, N.E.; Vrdoljak, G. Microorganisms metabolizing on clay grains in 3-km-deep Greenland basal ice. Astrobiology 2006, 6, 69–86. [Google Scholar] [CrossRef]
- Piette, F.; Leprince, P.; Feller, G. Is there a cold shock response in the Antarctic psychrophile Pseudoalteromonas haloplanktis? Extremophiles 2012, 16, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.N.; Dieckmann, G.S. Sea Ice—An Introduction to Its Physics, Chemistry, Biology and Geology; Blackwell: Oxford, UK, 2002; pp. 112–142. [Google Scholar]
- Białkowska, A.; Majewska, E.; Olczak, A.; Twarda-Clapa, A. Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry. Biomolecules 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Griffith, M.; Patten, C.L.; Galick, B.R. Isolation and characterization of an antifreeze protein with ice nucleation activity from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 1998, 44, 64–73. [Google Scholar]
- Christner, B.C.; Mikucki, J.A.; Foreman, C.M.; Denson, J.; Priscu, J.C. Glacial ice cores: A model system for developing extraterrestrial decontamination protocols. Icarus 2005, 174, 572–584. [Google Scholar]
- Rogers, S.O.; Theraisnathan, V.; Ma, L.J.; Zhao, Y.; Zhang, G.; Shin, S.G.; Castelo, J.D.; Starmer, W.T. Comparisons of protocols for decontamination of environmental ice samples for biological and molecular examinations. Appl. Environ. Microbiol. 2004, 70, 2540–2544. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.O.; Ma, L.J.; Zhao, Y.; Theraisnathan, V.; Shin, S.G.; Zhang, G.; Catranis, C.M.; Starmer, W.T.; Castelo, J.D. Recommendations for elimination of contaminants and authentication of isolates in ancient ice cores. In Life in Ancient Ice; Castello, J.D., Rogers, S.O., Eds.; Princeton University Press: Princeton, NJ, USA, 2005; pp. 5–21. [Google Scholar]
- Jepsen, S.M.; Adams, E.E.; Priscu, J.C. Fuel movement along grain boundaries in ice. Cold Reg. Sci. Technol. 2006, 45, 158–165. [Google Scholar]
- Price, P.B. A habitat for psychrophiles in deep Antarctic ice. Proc. Natl. Acad. Sci. USA 2000, 97, 1247–1251. [Google Scholar]
- Christner, B.C. Incorporation of DNA and protein precursors into macromolecules by bacteria at −15 °C. Appl. Environ. Microbiol. 2002, 68, 6435–6438. [Google Scholar]
- Bakermans, C.; Tsapin, A.I.; Souza-Egipsy, V.; Gilichinsky, D.A.; Nealson, K.H. Reproduction and metabolism at −10 °C of bacteria isolated from Siberian permafrost. Environ. Microbiol. 2003, 5, 321–326. [Google Scholar]
- Junge, K.; Eicken, H.; Deming, J.W. Motility of Colwellia psychrerythraea strain 34H at subzero temperatures. Appl. Environ. Microbiol. 2003, 69, 4282–4284. [Google Scholar] [CrossRef] [Green Version]
- Panikov, N.S.; Flanagan, P.W.; Oechel, W.C.; Mastepanov, M.A.; Christensen, T.R. Microbial activity in soils frozen to below −39 °C. Soil Biol. Biochem. 2006, 38, 785–794. [Google Scholar] [CrossRef]
- Bay, R.; Bramall, N.; Price, P.B. Search for microbes and biogenic compounds in polar ice using flourescence. In Life in Ancient Ice; Castello, J.D., Rogers, S.O., Eds.; Princeton University Press: Princeton, NJ, USA, 2005; pp. 268–276. [Google Scholar]
- Royston-Bishop, G.; Priscu, J.C.; Tranter, M.; Christner, B.C.; Siegert, M.J.; Lee, V. Incorporation of particulates into accreted ice above subglacial Vostok lake, Antarktica. Ann. Glaciol. 2005, 40, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Vorobyova, E.A.; Soina, V.S.; Manukelashvili, A.G.; Bolshakova, A.; Yaminski, I.V.; Mulyukin, A.L. Living cells in permafrost as models for astrobiology research. In Life in Ancient Ice; Castello, J.D., Rogers, S.O., Eds.; Princeton University Press: Princeton, NJ, USA; Oxford, UK, 2005; pp. 277–288. [Google Scholar]
- Abyzov, S.S.; Poglazova, M.N.; Mitskevich, I.N.; Ivanov, M.V. Common features of microorganisms in ancient layers of the Antarctic ice sheet. In Life in Ancient Ice; Castello, J.D., Rogers, S.O., Eds.; Princeton University Press: Princeton, NJ, USA, 2005; pp. 240–250. [Google Scholar]
- Karl, D.M.; Bird, D.F.; Bjõrkman, K.; Houlihan, T.; Shackelford, R.; Tupas, L. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science 1999, 286, 2144–2147. [Google Scholar] [CrossRef] [Green Version]
- Priscu, J.C.; Adams, E.E.; Lyons, W.B.; Voytek, M.A.; Mogk, D.W.; Brown, R.L.; McKay, C.P.; Takacs, C.D.; Welch, K.A.; Wolf, C.F.; et al. Geomicrobiology of subglacial ice above Lake Vostok, Antarctica. Science 1999, 286, 2141–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, P.P.; Miteva, V.I.; Brenchley, J.B. Phylogenetic analysis of anaerobic psychrophilc enrichment cultures obtained from a Greenland ice core. Appl. Environ. Microbiol. 2003, 69, 2153–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mader, H.M.; Pettitt, M.E.; Wadham, J.L.; Wolff, E.W.; Parkes, R.J. Subsurface ice as a microbial habitat. Geology 2006, 34, 169–172. [Google Scholar] [CrossRef]
- Kuhn, E.; Ichimura, A.S.; Peng, V.; Fritsen, C.H.; Trubl, G.; Doran, P.T.; Murray, A.E. Brine assemblages of ultrasmall microbial cells within the ice cover of Lake Vida, Antarctica. Appl. Environ. Microbiol. 2014, 80, 3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavicchioli, R.; Ostrowski, M. Ultramicrobacteria. In Encyclopedia of Life Sciences; Battista, J., Ed.; John Wiley & Sons: Chichester, UK, 2003; pp. 1–8. [Google Scholar]
- Ponder, M.A.; Thomashow, M.F.; Tiedje, J.M. Metabolic activity of Siberian permafrost isolates, Psychrobacter arcticus and Exiguobacterium sibiricum, at low water activities. Extremophiles 2008, 12, 481–490. [Google Scholar] [CrossRef]
- Miteva, V. Bacteria in Snow and Glacier Ice. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.-C., Gerday, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 31–50. [Google Scholar]
- Papale, M.; Conte, A.; Mikkonen, A.; Michaud, L.; La Ferla, R.; Azzaro, M.; Caruso, G.; Paranhos, R.; Anderson, S.-C.; Maimone, G.; et al. Prokaryotic assemblages within permafrost active layer at Edmonson Point (Northern Victoria Land, Antarctica). Soil Biol. Biochem. 2018, 123, 165–179. [Google Scholar] [CrossRef]
- Azzaro, M.; Maimone, G.; La Ferla, R.; Cosenza, A.; Rappazzo, A.C.; Caruso, G.; Paranhos, R.; Anderson, S.-C.; Forte, E.; Guglielmin, M. The prokaryotic community in an extreme Antarctic environment: The brines of Boulder Clay lakes (Northern Victoria Land). Hydrobiologia 2021, 848, 1837–1857. [Google Scholar] [CrossRef]
- Miteva, V.I.; Sheridan, P.P.; Brenchley, J.E. Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl. Environ. Microbiol. 2004, 70, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.; Yao, T.; An, L.; Xu, B.; Wang, J. 16S rRNA sequences and difference in bacteria isolated Muztag Ata Glacier at increasing depths. Appl. Environ. Microbiol. 2005, 71, 4619–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skidmore, M.L.; Foght, J.M.; Sharp, M.J. Microbial life beneath a high Arctic glacier. Appl. Environ. Microbiol. 2000, 66, 3214–3220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.J.; Yao, T.D.; Ma, X.J.; Wang, N.L. Microorganisms in a high altitude glacier ice in Tibet. Folia Microbiol. 2002, 47, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Papale, M.; Rizzo, C.; Caruso, G.; La Ferla, R.; Maimone, G.; Lo Giudice, A.; Azzaro, M.; Guglielmin, M. First Insights into the Microbiology of Three Antarctic Briny Systems of the Northern Victoria Land. Diversity 2021, 13, 323. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Conte, A.; Papale, M.; Rizzo, C.; Azzaro, M.; Guglielmin, M. Prokaryotic Diversity and Metabolically Active Communities in Brines from Two Perennially Ice-Covered Antarctic Lakes. Astrobiology 2021, 21, 9. [Google Scholar] [CrossRef]
- Tesán Onrubia, J.A.; Petrova, M.V.; Puigcorbe, V.; Black, E.E.; Valk, O.; Dufour, A.; Hamelin, B.; Buesseler, K.O.; Masque, P.; Le Moigne, F.A.C.; et al. Mercury Export Flux in the Arctic Ocean Estimated from 234 Th/238 U Disequilibria. ACS Earth Space Chem. 2020, 4, 795–801. [Google Scholar] [CrossRef]
- Petäjä, T.; Duplissy, E.-M.; Tabakova, K.; Schmale, J.; Altstadter, B.; Ancellet, G.; Arshinov, M.; Balin, Y.; Baltensperger, U.; Bange, J.; et al. Overview: Integrative and Comprehensive Understanding on Polar Environments (iCUPE)—Concept and initial results. Atmos. Chem. Phys. 2020, 20, 8551–8592. [Google Scholar] [CrossRef]
- Anesio, A.M.; Lutz, S.; Chrismas, N.A.M.; Benning, L.G. The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes 2017, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Perini, L.; Gostinčar, C.; Gunde-Cimerman, N. Fungal and bacterial diversity of Svalbard subglacial ice. Sci. Rep. 2019, 9, 20230. [Google Scholar] [CrossRef]
- Tribelli, P.M.; López, N.I. Reporting key features in cold-adapted bacteria. Life 2018, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Giudice, A.; Rizzo, C. Bacteria associated with marine benthic invertebrates from polar environments: Unexplored frontiers for biodiscovery? Diversity 2018, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Del-Río, H.; Chain, P.S.; Grzymski, J.J.; Ponder, M.A.; Ivanova, N.; Bergholz, P.W.; Di Bartolo, G.; Hauser, L.; Land, M.; Bakermans, C.; et al. The genome sequence of Psychrobacter arcticus 273-4, a psychroactive siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl. Environ. Microbiol. 2010, 76, 2304–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.Y.; Park, H.; Lee, J.H.; Han, S.J.; Sohn, Y.C.; Lee, S.G. Proteomic and transcriptomic investigations on cold-responsive properties of the psychrophilic Antarctic bacterium Psychrobacter sp. PAMC 21119 at subzero temperatures. Environ. Microbiol. 2017, 19, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.; De Maayer, P.; Cowan, D. The genome of the Antarctic polyextremophile Nesterenkonia sp. AN1 reveals adaptive strategies for survival under multiple stress conditions. FEMS Microbiol. Ecol. 2016, 92, fiw032. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.F.; Ivanova, N.; He, Z.; Huebner, M.; Zhou, J.; Tiedje, J.M. Architecture of thermal adaptation in an Exiguobacterium sibiricum strain isolated from 3 million year old permafrost: A genome and transcriptome approach. BMC Genom. 2008, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Mykytczuk, N.C.; Foote, S.J.; Omelon, C.R.; Southam, G.; Greer, C.W.; Whyte, L.G. Bacterial growth at −15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013, 7, 1211–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Jagannadham, M.V.; Vairamani, M.; Shivaji, S. Carotenoid pigments of an Antarctic psychrotrophic bacterium Micrococcus roseus: Temperature dependent biosynthesis, structure and interaction with synthetic membranes. Biochem. Biophys. Res. Commun. 1997, 239, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Pires, F.; Jesus, H.E.; Dos Santos, A.L.S.; Peixoto, R.; Rosado, A.S.; D’Avila-Levy, C.M.; Branquinha, M.H. Detection of proteases from Sporosarcina aquimarina and Algoriphagus antarcticus isolated from Antarctic soil. An. Acad. Bras. Ciências 2015, 87, 109–119. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Grzymski, J.J.; Carter, B.J.; DeLong, E.F.; Feldman, R.A.; Ghadiri, A.; Murray, A.E. Comparative genomics of DNA fragments from six Antarctic marine planktonic bacteria. Appl. Environ. Microbiol. 2006, 72, 1532–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangiagalli, M.; Lotti, M. Cold-active β-galactosidases: Insight into cold adaptationn mechanisms and biotechnological exploitation. Mar. Drugs 2021, 19, 43. [Google Scholar] [CrossRef]
- Chamot, D.; Owttrim, G.W. Regulation of cold shock-induced RNA helicase gene expression in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2000, 182, 1251–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadtare, S.; Inouye, M.; Severinov, K. The nucleic acid melting activity of Escherichia coli CspE is critical for transcription antitermination and cold acclimation of cells. J. Biol. Chem. 2002, 277, 7239–7245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalluge, J.J.; Hamamoto, T.; Horikoshi, K.; Morita, R.Y.; Stetter, K.O.; McCloskey, J.A. Posttransciptional modification of tRNA in psychrophilic bacteria. J. Bacteriol. 1997, 179, 1918–1923. [Google Scholar] [CrossRef] [Green Version]
- Noon, K.R.; Guymon, R.; Crain, P.F.; McCloskey, J.A.; Thomm, M.; Lim, J.; Cavicchioli, R. Influence of temperature on tRNA modification in Archaea: Methanococcoides burtonii (optimum growth temperature, 23 °C) and Stetteria hydrogenophila (Topt, 95 °C). J. Bacteriol. 2003, 185, 5483–5490. [Google Scholar] [CrossRef] [Green Version]
- Khachane, A.N.; Timmis, K.N.; dos Santos, V.A.P.M. Uracil content of 16S rRNA of thermophilic and psychrophilic prokaryotes correlates inversely with their optimal growth temperatures. Nucleic Acids Res. 2005, 33, 4016–4022. [Google Scholar] [CrossRef] [Green Version]
- Mangiagalli, M.; Lapi, M.; Maione, S.; Orlando, M.; Brocca, S.; Pesce, A.; Barbiroli, A.; Camilloni, C.; Pucciarelli, S.; Lotti, M. The co-existence of cold activity and thermal stability in an Antarctic GH42 β-galactosidase relies on its hexameric quaternary arrangement. FEBS J. 2020, 288, 546–565. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Xing, M. A New β-Galactosidase from the Antarctic Bacterium Alteromonas sp. ANT48 and Its Potential in Formation of Prebiotic Galacto-Oligosaccharides. Mar. Drugs 2019, 17, 599. [Google Scholar] [CrossRef] [Green Version]
- Hoyoux, A.; Jennes, I.; Dubois, P.; Genicot, S.; Dubail, F.; François, J.-M.; Baise, E.; Feller, G.; Gerday, C. Cold-adapted βgalactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl. Environ. Microbiol. 2001, 67, 1529–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkiewicz, M.; Kur, J.; Białkowska, A.; Cieśliński, H.; Kalinowska, H.; Bielecki, S. Antarctic marine bacterium Pseudoalteromonas sp. 22b as a source of cold-adapted β-galactosidase. Biomol. Eng. 2003, 20, 317–324. [Google Scholar] [CrossRef]
- Ding, H.; Zeng, Q.; Zhou, L.; Yu, Y.; Chen, B. Biochemical and structural insights into a novel thermostable β-1, 3-galactosidase from Marinomonas sp. BSi20414. Mar. Drugs 2017, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Stougaard, P. Identification, cloning and expression of a cold-active β-galactosidase from a novel Arctic bacterium, Alkalilactibacillus ikkense. Environ. Technol. 2010, 31, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Woś, A.; Bartasun, P.; Cieśliński, H.; Kur, J. Cloning and characterization of a novel cold-active glycoside hydrolase family 1 enzyme with β-glucosidase, β-fucosidase and β-galactosidase activities. BMC Biotechnol. 2013, 13, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Sun, J.; Wang, W.; Zhuang, Z.; Liu, J.; Hao, J. A novel cold-adapted β-galactosidase from Alteromonas sp. ML117 cleaves milk lactose effectively at low temperature. Process Biochem. 2019, 82, 94–101. [Google Scholar] [CrossRef]
- Sun, J.; Yao, C.; Wang, W.; Zhuang, Z.; Liu, J.; Dai, F.; Hao, J. Cloning, Expression and Characterization of a Novel Cold-adapted β-galactosidase from the Deep-sea Bacterium Alteromonas sp. ML52. Mar. Drugs 2018, 16, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Wang, W.; Yao, C.; Dai, F.; Zhu, X.; Liu, J.; Hao, J. Overexpression and characterization of a novel cold-adapted and salt-tolerant GH1 β-glucosidase from the marine bacterium Alteromonas sp. L82. J. Microbiol. 2018, 56, 656–664. [Google Scholar] [CrossRef]
- Russell, N.J. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 2000, 4, 83–90. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.M.; Dhakephalkar, P. Diversity, cold active enzymes and adaptation strategies of bacteria inhabiting glacier cryoconite holes of High Arctic. Extremophiles 2014, 18, 229–242. [Google Scholar] [CrossRef]
- Prasad, S.; Manasa, P.; Buddhi, S.; Tirunagari, P.; Begum, Z.; Rajan, S.; Shivaji, S. Diversity and Bioprospective Potential (Cold-Active Enzymes) of Cultivable Marine Bacteria from the Subarctic Glacial Fjord, Kongsfjorden. Curr. Microbiol. 2013, 68, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Petrovskaya, L.E.; Novototskaya-Vlasova, K.A.; Spirina, E.V.; Khokhlova, G.V.; Rivkina, E.; Gilichinsky, D.A.; Dolgikh, D.; Kirpichnikov, M.P. Lipolytic enzymes of microorganisms from permafrost cryopegs. Dokl. Biol. Sci. 2012, 445, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Conte, A.; Azzaro, M.; Papale, M.; Rappazzo, A.C.; Battistel, D.; Roman, M.; Lo Giudice, A.; Guglielmin, M. Cultivable Bacterial Communities in Brines from Perennially Ice-Covered and Pristine Antarctic Lakes: Ecological and Biotechnological Implications. Microorganisms 2020, 8, 819. [Google Scholar] [CrossRef] [PubMed]
- Boetius, A.; Anesio, A.M.; Deming, J.W.; Mikucki, J.A.; Rapp, J.Z. Microbial ecology of the cryosphere: Sea ice and glacial habitats. Nat. Rev. Microbiol. 2015, 13, 677–690. [Google Scholar] [CrossRef]
- Hotaling, S.; Finn, D.S.; Giersch, J.J.; Weisrock, D.W.; Jacobsen, D. Climate change and alpine stream biology: Progress, challenges, and opportunities for the future. Biol. Rev. 2017, 92, 2024–2045. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; McMinn, A. Sea ice, extremophiles and life on extraterrestrial ocean worlds. Int. J. Astrobiol. 2018, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Segawa, T.; Ushida, K.; Narita, H.; Kanda, H.; Kohshima, S. Bacterial communities in two Antarctic ice cores analysed by 16S rRNA gene sequencing analysis. Polar Sci. 2010, 4, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.S. The relationship between sea ice bacterial community structure and biogeochemistry: A synthesis of current knowledge and known unknowns. Elem. Sci. Anthr. 2015, 3, 000072. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.S.; Berthiaume, C.T.; Armbrust, E.V.; Deming, J.W. The genetic potential for key biogeochemical processes in Arctic frost flowers and young sea ice revealed by metagenomic analysis. FEMS Microbiol. Ecol. 2014, 89, 376–387. [Google Scholar] [CrossRef]
- Koh, E.Y.; Atamna-Ismaeel, N.; Martin, A.; Cowie, R.O.M.; Beja, O.; Davy, S.K.; Maas, E.W.; Ryan, K.G. Proteorhodopsin-bearing bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 2010, 76, 5918–5925. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, H.; Abbas, B.; Witte, H.; Muyzer, G. Genetic diversity of “satellite” bacteria present in cultures of marine diatoms. FEMS Microbiol. Ecol. 2002, 42, 25–35. [Google Scholar] [CrossRef]
- Jasti, S.; Sieracki, M.E.; Poulton, N.J.; Giewat, W.; Rooney-Varga, J.N. Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton. Appl. Environ. Microbiol. 2002, 71, 3483–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.V.; Bowman, J.P. A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol. Ecol. 2001, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.E.; Rocap, G.; Deming, J.W. Persistence of bacterial and archaeal communities in sea ice through an Arctic winter. Environ. Microbiol. 2010, 12, 1828–1841. [Google Scholar] [CrossRef] [Green Version]
- Cowie, R.; Maas, E.; Ryan, K. Archaeal diversity revealed in Antarctic sea ice. Antarct. Sci. 2011, 23, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Cowie, R.; Williams, G.; Maas, E.; Voyles, K.; Ryan, K. Antarctic sea-ice microbial communities show distinct patterns of zonation in response to algal-derived substrates. Aquat. Microb. Ecol. 2014, 73, 123–134. [Google Scholar] [CrossRef]
- Baer, S.E.; Connelly, T.L.; Bronk, D.A. Nitrogen uptake dynamics in landfast sea ice of the Chukchi Sea. Polar Biol. 2015, 38, 781–797. [Google Scholar] [CrossRef]
- Christner, B.C.; Royston-Bishop, G.; Foreman, C.M.; Arnold, B.R.; Tranter, M.; Welch, K.A.; Lyons, W.B.; Tsapin, A.I.; Studinger, M.; Priscu, J.C. Limnological conditions in subglacial Lake Vostok, Antarctica. Limnol. Oceanogr. 2006, 51, 2485–2501. [Google Scholar] [CrossRef]
- Hatam, I.; Lange, B.; Beckers, J.; Haas, C.; Lanoil, B. Bacterial communities from Arctic seasonal sea ice are more compositionally variable than those from multi-year sea ice. ISME J. 2016, 10, 2543–2552. [Google Scholar] [CrossRef] [Green Version]
- Hatam, I.; Charchuk, R.; Lange, B.; Beckers, J.; Haas, C.; Lanoil, B. Distinct bacterial assemblages reside at different depths in Arctic multilayer sea ice. FEMS Microbiol. Ecol. 2014, 90, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.S.; Rasmussen, S.; Blom, N.; Deming, J.W.; Rysgaard, S.; Sicheritz-Ponten, T. Microbial community structure of Arctic multiyear sea ice and surface seawater by 454 sequencing of the 16S RNA gene. ISME J. 2012, 6, 11–20. [Google Scholar] [CrossRef]
- Petri, R.; Imhoff, J. Genetic analysis of sea-ice bacterial communities of the Western Baltic Sea using an improved double gradient method. Polar Biol. 2001, 24, 252–257. [Google Scholar] [CrossRef]
- Junge, K.; Imhoff, F.; Staley, T.; Deming, J.W. Phylogenetic diversity of numerically important Arctic sea-ice bacteria cultured at subzero temperature. Microb. Ecol. 2002, 43, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Brinkmeyer, R.; Knittel, K.; Jürgens, J.; Weyland, H.; Amann, R.; Helmke, E. Diversity and structure of bacterial communities in Arctic versus Antarctic Pack Ice. Appl. Environ. Microbiol. 2003, 69, 6610–6619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, D.G.; Ehn, J.K.; Pućko, M.; Rysgaard, S.; Deming, J.W.; Bowman, J.S.; Papakyriakou, T.; Galley, R.J.; Søgaard, D.H. Frost flowers on young Arctic sea ice: The climatic, chemical and microbial significance of an emerging ice type. J. Geophys. Res. Atmos. 2014, 119, 11593–11612. [Google Scholar] [CrossRef]
- Eronen-Rasimus, E.; Kaartokallio, H.; Lyra, C.; Autio, R.; Kuosa, H.; Dieckmann, G.S.; Thomas, D.N. Bacterial community dynamics and activity in relation to dissolved organic matter availability during sea-ice formation in a mesocosm experiment. MicrobiologyOpen 2014, 3, 139–156. [Google Scholar] [CrossRef] [Green Version]
- Petrich, C.; Eicken, H. Growth, structure and properties of sea ice. In Sea Ice, 2nd ed.; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2010; pp. 23–77. [Google Scholar]
- Paun, V.I.; Icaza, G.; Lavin, P.; Marin, C.; Tudorache, A.; Persoiu, A.; Dorador, C.; Purcarea, C. Total and Potentially Active Bacterial Communities Entrapped in a Late Glacial through Holocene Ice Core from Scarisoara Ice Cave, Romania. Front. Microbiol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Papale, M.; Lo Giudice, A.; Conte, A.; Rizzo, C.; Rappazzo, A.C.; Maimone, G.; Caruso, G.; La Ferla, R.; Azzaro, M.; Gugliandolo, C.; et al. Microbial Assemblages in Pressurized Antarctic Brine Pockets (Tarn Flat, Northern Victoria Land): A Hotspot of Biodiversity and Activity. Microorganisms 2019, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Bakermans, C.; Ayala-del-Río, H.L.; Ponder, M.A. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol. 2006, 56, 1285–1291. [Google Scholar] [CrossRef]
- Pecheritsyna, S.A.; Rivkina, E.M.; Akimov, V.N. Desulfovibrio arcticus sp. nov., a psychrotolerant sulfate-reducing bacterium from a cryopeg. Int. J. Syst. Evol. Microbiol. 2012, 62, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Vishnivetskaya, T.; Kathariou, S.; McGrath, J.; Gilichinsky, D.; Tiedje, J.M. Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 2000, 4, 165–173. [Google Scholar] [CrossRef]
- Gilichinsky, D.A.; Rivkina, E.; Shcherbakova, V.; Laurinavichuis, K.; Tiedje, J.M. Supercooled water brines within permafrost—An unknown ecological niche for microorganisms: A model for astrobiology. Astrobiology 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Mondino, L.J.; Asao, M.; Madigan, M.T. Cold-active halophilic bacteria from the ice-sealed Lake Vida, Antarctica. Arch. Microbiol. 2009, 191, 785–790. [Google Scholar] [CrossRef]
- Peeters, K.; Hodgson, D.A.; Convey, P.; Willems, A. Culturable diversity of heterotrophic bacteria in Forlidas Pond (Pensacola Mountains) and Lundström Lake (Shackleton Range), Antarctica. Microb. Ecol. 2011, 62, 399. [Google Scholar] [CrossRef]
- Franzmann, P.D.; Hopfl, P.; Weiss, N.; Tindall, B.J. Psychrotrophic, lactic acid producing bacteria from anoxic waters in Ace Lake, Antarctica: Carnobacterium funditum sp. nov. and Carnobacterium aherfunditum sp. nov. Arch. Microbiol. 1991, 156, 255–262. [Google Scholar] [CrossRef]
- Bratina, B.J.; Stevenson, B.S.; Green, W.J.; Schmidt, T.M. Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Appl. Environ. Microbiol. 1998, 64, 3791–3797. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.S.; Matsumoto, G.I.; Shivaji, S. Sporosarcina macmurdoensis sp. nov., from a cyanobacterial mat sample from a pond in the McMurdo Dry Valleys, Antarctica. Int. J. Syst. Evol. Microbiol. 2003, 53, 1363–1367. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Marsic, D.; Bej, A.; Tang, J.; Krader, P.; Hoover, R.B. Carnobacterium pleistocenium sp. nov., a novel psychrotolerant, facultative anaerobe isolated from permafrost of the Fox tunnel in Alaska. Int. J. Syst. Evol. Microbiol. 2005, 55, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Kobabe, S.; Liebner, S. Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta, northeastern Siberia. Can. J. Microbiol. 2009, 55, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, R.C.; Niederberger, T.D.; Greer, C.; Whyte, L.G. Microbial diversity of active layer and permafrost in an acidic wetland from the Canadian High Arctic. Can. J. Microbiol. 2011, 57, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Frank-Fahle, B.A.; Yergeau, È.; Greer, C.W.; Lantuit, H.; Wagner, D. Microbial functional potential and community composition inpermafrost-affected soils of the NW Canadian Arctic. PLoS ONE 2014, 9, e84761. [Google Scholar] [CrossRef] [Green Version]
- Ganzert, L.; Bajerski, F.; Wagner, D. Bacterial community composition and diversity of five different permafrost-affected soils of Northeast Greenland. FEMS Microbiol. Ecol. 2014, 89, 426–441. [Google Scholar] [CrossRef]
- Deng, J.; Gu, Y.; Zhang, J.; Xue, K.; Qin, Y.; Yuan, M.; Yin, H.; He, Z.; Wu, L.; Schuur, E.A.; et al. Shifts of tundra bacterial and archaeal communities along a permafrost thaw gradient in Alaska. Mol. Ecol. 2015, 24, 222–234. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B. Multi-omics of permafrost, active layer and thermokarst bogsoil microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Shivaji, S.; Reddy, G.S.; Aduri, R.P.; Kutty, R.; Ravenschlag, K. Bacterial diversity of a soil sample from Schirmacher Oasis, Antarctica. Cell. Mol. Biol. 2004, 50, 525–536. [Google Scholar]
- Aislabie, J.M.; Chhour, K.; Saul, D.J.; Miyauchi, S.; Ayton, J.; Paetzold, R.F.; Balks, M.R. Dominant bacterial groups in soils of marble point and wright valley, Victoria Land, Antarctica. Soil Biol. Biochem. 2006, 38, 3041–3056. [Google Scholar] [CrossRef]
- Hansen, A.A.; Herbert, R.A.; Mikkelsen, K.; Jensen, L.L.; Kristoffersen, T.; Tiedje, J.M.; Lomstein, B.A.; Finster, K.W. Viability, diversity and composition of the bacterial community in a high Arctic permafrost soil from Spitsbergen, Northern Norway. Environ. Microbiol. 2007, 9, 2870–2884. [Google Scholar] [CrossRef]
- Steven, B.; Briggs, G.; McKay, C.P.; Pollard, W.H.; Greer, C.W.; Whyte, L.G. Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods. FEMS Microbiol. Ecol. 2007, 59, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Martineau, C.; Whyte, L.G.; Greer, C.W. Stable isotope probing analysis of the diversity and activity of methanotrophic bacteria in soils from the Canadian High Arctic. Appl. Environ. Microbiol. 2010, 76, 5773–5784. [Google Scholar] [CrossRef] [Green Version]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.; Greer, C.W.; Aerts, R.; Kowalchuk, G.A. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 2012, 6, 692–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.S.; Hebsgaard, M.B.; Christensen, T.R.; Mastepanov, M.; Nielsen, R.; Munch, K.; Brand, T.; Gilbert, M.T.P.; Zuber, M.T.; Bunce, M.; et al. Ancient bacteria show evidence of DNA repair. Proc. Natl. Acad. Sci. USA 2007, 104, 14401–14405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Liebner, S.; Winkel, M.; Alawi, M.; Horn, F.; Dorfer, C.; Ollivier, J.; He, J.-S.; Jin, H.; Kuhn, P.; et al. In-depth analysis of core methanogenic communities from high elevation permafrost-affected wetlands. Soil Biol. Biochem. 2017, 111, 66–77. [Google Scholar] [CrossRef]
- Harding, T.; Jungblut, A.D.; Lovejoy, C.; Vincent, W.F. Microbes in high arctic snow and implications for the cold biosphere. Appl. Environ. Microbiol. 2011, 77, 3234–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuvochina, M.S.; Alekhina, I.A.; Normand, P.; Petit, J.-R.; Bulat, S.A. Three events of Saharan dust deposition on the Mont Blanc glacier associated with different snow-colonizing bacterial phylotypes. Microbiology 2011, 80, 125–131. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Lin, S.; Capone, D.G. Bacterial activity in South Pole snow. Appl. Environ. Microbiol. 2000, 66, 4514–4517. [Google Scholar] [CrossRef] [Green Version]
- Larose, C.; Berger, S.; Ferrari, C.; Navarro, E.; Dommergue, A.; Schneider, D.; Vogel, T.M. Microbial sequences retrieved from environmental samples from seasonal arctic snow and meltwater from Svalbard, Norway. Extremophiles 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Wunderlin, T.; Ferrari, B.; Power, M. Global and local-scale variation in bacterial community structure of snow from the Swiss and Australian Alps. FEMS Microbiol. Ecol. 2016, 92, fiw132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yang, G.; Wang, Y.; Hou, S. Abundance and community of snow bacteria from three glaciers in the Tibetan Plateau. J. Environ. Sci. 2010, 22, 1418–1424. [Google Scholar] [CrossRef]
- Møller, A.K.; Søborg, D.A.; Al-soud, W.A.; Sørensen, S.J.; Kroer, N. Bacterial community structure in High-Arctic snow and freshwater as revealed by pyrosequencing of 16S rRNA genes and cultivation. Polar Res. 2013, 32, 17390. [Google Scholar] [CrossRef]
- Lopatina, A.; Krylenkov, V.; Severinov, K. Activity and bacterial diversity of snow around Russian Antarctic stations. Res. Microbiol. 2013, 164, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Maccario, L.; Vogel, T.M.; Larose, C. Potential drivers of microbial community structure and function in Arctic spring snow. Front. Microbiol. 2014, 5, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, L.; Lo Giudice, A.; Mysara, M.; Monsieurs, P.; Raffa, C.; Leys, N.; Amalfitano, S.; Van Houdt, R. Snow surface microbiome on the High Antarctic Plateau (DOME C). PLoS ONE 2014, 9, e104505. [Google Scholar] [CrossRef] [PubMed]
- Larose, C.; Prestat, E.; Cecillon, S.; Berger, S.; Malandain, C.; Lyon, D.; Ferrari, C.; Schneider, D.; Dommergue, A.; Vogel, T.M. Interactions between snow chemistry, mercury inputs and microbial population dynamics in an Arctic snowpack. PLoS ONE 2013, 8, e79972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, T.; Miyamoto, K.; Ushida, K.; Agata, K.; Okada, N.; Kohshima, S. Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains. Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl. Environ. Microbiol. 2005, 71, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.-R.; Shang, T.-C.; Chen, Y.; Yao, T.-D. Deposition and postdeposition mechanisms as possible drivers of microbial population variability in glacier ice. FEMS Microbiol. Ecol. 2009, 70, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Cowan, D.A.; Tow, L.A. Endangered Antarctic environments. Annu. Rev. Microbiol. 2004, 58, 649–690. [Google Scholar] [CrossRef] [Green Version]
- Euzebi, J.P. List of Bacterial Names with Standing in Nomenclature: A folder available on the internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, H. Cryoprotectants and ice-binding proteins. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 229–246. [Google Scholar]
- Bouvet, V.; Ben, R.N. Antifreeze glycoproteins: Structure, conformation, and biological applications. Cell Biochem. Biophys. 2003, 39, 133–144. [Google Scholar] [CrossRef]
- Wilson, S.L.; Walker, V.K. Selection of low-temperature resistance in bacteria and potential applications. Environ. Technol. 2010, 31, 943–956. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeast from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Mason, O.U.; Nakagawa, T.; Rosner, M.; Van Nostrand, J.D.; Zhou, J.; Maruyama, A.; Fisk, M.R.; Giovanonni, S.J. First Investigation of the Microbiology of the Deepest Layer of Ocean Crust. PLoS ONE 2010, 5, e15399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef] [Green Version]
- Kirchman, D.L.; Meon, B.; Ducklow, H.W.; Carlson, C.A.; Hansell, D.A.; Steward, G.F. Glucose fluxes and concentrations of dissolved combined neutral sugars (polysaccharides) in the Ross Sea and Polar Front Zone, Antarctica. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 4179–4197. [Google Scholar] [CrossRef]
- Lauritano, C.; Rizzo, C.; Lo Giudice, A.; Saggiomo, M. Physiological and Molecular Responses to Main Environmental Stressors of Microalgae and Bacteria in Polar Marine Environments. Microorganisms 2020, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Giudice, A.L. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2017, 84, e01624-17. [Google Scholar] [CrossRef] [Green Version]
- Methé, B.A.; Nelson, K.E.; Deming, J.W.; Momen, B.; Melamud, E.; Zhang, X.; Moult, J.; Madupu, R.; Nelson, W.C.; Dodson, R.J.; et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 2005, 102, 10913–10918. [Google Scholar] [CrossRef] [Green Version]
- Hünken, M.; Harder, J.; Kirst, G.O. Epiphytic bacteria on the Antarctic ice diatom Amphiprora kuerathii Manguin cleave hydrogen peroxide produced during algal photosynthesis. Plant Biol. 2008, 10, 519–526. [Google Scholar] [CrossRef]
- Nichols, C.M.; Bowman, J.P.; Guezennec, J. Effects of incubation temperature on growth and production of exopolysaccharides by an Antarctic sea ice bacterium grown in batch culture. Appl. Environ. Microbiol. 2005, 71, 3519–3523. [Google Scholar] [CrossRef] [Green Version]
- Christensen, B.E.; Kjosbakken, J.; Smidsrød, O. Partial chemical and physical characterization of two extracellular polysaccharides produced by marine, periphytic Pseudomonas sp. strain NCMB 2021. Appl. Environ. Microbiol. 1985, 50, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Mykytczuk, N.C.S.; Lawrence, J.R.; Omelon, C.R.; Southam, G.; Whyte, L.G. Microscopic characterization of the bacterial cell envelope of Planococcus halocryophilus Or1 during subzero growth at −15 °C. Polar Biol. 2016, 39, 701–712. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial extracellular polymeric substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Whitfield, C. Bacterial extracellular polysaccharides. Can. J. Microbiol. 1988, 34, 415–420. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Nicolaus, B.; Kambourova, M.; Öner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Corsaro, M.M.; Lanzetta, R.; Parrilli, E.; Parrilli, M.; Tutino, M.L.; Ummarino, S. Influence of Growth Temperature on Lipid and Phosphate Contents of Surface Polysaccharides from the Antarctic Bacterium Pseudoalteromonas haloplanktis TAC 125. J. Bacteriol. 2004, 186, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Yim, J.-H. Cryoprotective properties of exopolysaccharide (P-21653) produced by the Antarctic bacterium, Pseudoalteromonas arctica KOPRI 21653. J. Microbiol. 2007, 45, 510–514. [Google Scholar] [PubMed]
- Mancuso Nichols, C.A.; Garon, S.; Bowman, J.P.; Raguenes, G.; Guezennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 2004, 96, 1057–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Giudice, A.L. Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ. Sci. Pollut. Res. 2017, 25, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Finore, I.; Di Marco, G.; Michaud, L.; Giudice, A.L. Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct. Sci. 2019, 31, 69–79. [Google Scholar] [CrossRef]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of cryoprotectant extracellular polysaccharide substance (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef]
- Liu, S.-B.; Chen, X.-L.; He, H.-L.; Zhang, X.-Y.; Xie, B.-B.; Yu, Y.; Chen, B.; Zhou, B.-C.; Zhang, Y.-Z. Structure and Ecological Roles of a Novel Exopolysaccharide from the Arctic Sea Ice Bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2012, 79, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, B.-G.; Park, H.J.; Yim, J.H. Cryoprotective Properties and Preliminary Characterization of Exopolysaccharide (P-ArcPo 15) Produced by the Arctic Bacterium Pseudoalteromonas elyakovii ArcPo 15. Prep. Biochem. Biotechnol. 2016, 46, 261–266. [Google Scholar] [CrossRef]
- Kawahara, H. The structures and functions of ice crystal-controlling proteins from bacteria. J. Biosci. Bioeng. 2002, 94, 492–496. [Google Scholar] [CrossRef]
- Scotter, A.J.; Marshall, C.B.; Graham, L.A.; Gilbert, J.A.; Garnham, C.P.; Davies, P.L. The basis for hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef]
- Ben, R.N. Antifreeze glycoproteins—Preventing the growth of ice. ChemBioChem 2001, 2, 161–166. [Google Scholar] [CrossRef]
- Graether, S.P.; Jia, Z. Modeling Pseudomonas syringae icenucleation protein as a β-helical protein. Biophys. J. 2001, 80, 1169–1173. [Google Scholar] [CrossRef] [Green Version]
- Muryoi, N.; Sato, M.; Kaneko, S.; Kawahara, H.; Obata, H.; Yaish, M.W.F.; Griffith, M.; Glick, B.R. Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant Scientifica 17 growth-promoting rhizobacterium Pseudomonas putida GR12-2. J. Bacteriol. 2004, 186, 5661–5671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, N.; Liu, X.Y.; Hew, C.L. Aggregation of antifreeze protein and impact on antifreeze activity. J. Phys. Chem. B 2006, 110, 20562–20567. [Google Scholar] [CrossRef] [PubMed]
- Lorv, J.S.H.; Rose, D.R.; Glick, B.R. Bacterial Ice Crystal Controlling Proteins. Scientifica 2014, 2014, 976895. [Google Scholar] [CrossRef] [Green Version]
- Garnham, C.P.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Novel dimeric β-helical model of an ice nucleation protein with bridged active sites. BMC Struct. Biol. 2011, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, J.A.; Hill, P.J.; Dodd, C.E.R.; Laybourn-Parry, J. Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 2004, 150, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Kondo, H.; Hanada, Y.; Sugimoto, H.; Hoshino, T.; Garnham, C.P.; Davies, P.L.; Tsuda, S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA 2012, 109, 9360–9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, Y.; Graham, L.A.; Mok, Y.F.; Bar, M.; Davies, P.L.; Braslavsky, I. Superheating of ice crystals in antifreeze protein solutions. Proc. Natl. Acad. Sci. USA 2010, 107, 5423–5428. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.; Davies, P.; Laybourn-Parry, J. A hyperactive, Ca2+—Dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol. Lett. 2005, 145, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Raymond, J.A.; Fritsen, C.; Shen, K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 2007, 61, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Garnham, C.P.; Gilbert, J.A.; Hartman, C.P.; Campbell, R.L.; Laybourn-Parry, J.; Davies, P.L. A Ca2+—Dependent bacterial antifreeze protein domain has a novel –helical ice–binding fold. Biochem. J. 2008, 411, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, H.; Lee, J.H.; Lee, S.G.; Kim, H.J. Crystallization and preliminary X-ray crystallographic analysis of an ice-binding protein (FfIBP) from Flavobacterium frigoris PS1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 806–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.O.; Brown, A.; Middleton, A.J.; Tomczak, M.M.; Walker, V.K.; Davies, P.L. Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis. Cryobiology 2010, 61, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Vance, T.D.R.; Olijve, L.L.C.; Campbell, R.L.; Voets, I.K.; Davies, P.L.; Guo, S. Ca2+—Stabilized adhesin helps an Antarctic bacterium reach out and bind ice. Biosci. Rep. 2014, 34, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Iwanaka, Y.; Higa, S.; Muryoi, N.; Sato, M.; Honda, M.; Omura, H.; Obata, H. A novel, intracellular antifreeze protein in an Antarctic bacterium, Flavobacterium xanthum. Cryoletters 2007, 28, 39–49. [Google Scholar] [PubMed]
- Yamashita, Y.; Nakamura, N.; Omiya, K.; Nishikawa, K.; Kawahara, H.; Obata, H. Identification of an antifreeze lipoprotein from Moraxella sp. of Antarctic origin. Biosci. Biotechnol. Biochem. 2002, 66, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Duman, J.G.; Olsen, T.M. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology 1993, 30, 322–328. [Google Scholar] [CrossRef]
- Wilson, S.L.; Grogan, P.; Walker, V.K. Prospecting for ice association: Characterization of freeze-thaw selected enrichment cultures from latitudinally distant soils. Can. J. Microbiol. 2012, 58, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Griffith, M.; Pasternak, J.J.; Glick, B.R. Low temperature growth, freezing survival, and production of antifreeze protein by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 1995, 41, 776–784. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular characterization of an ice nucleation protein variant (InaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int. J. Biol. Sci. 2012, 8, 1097. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Augustin, S.; Clauss, T.; Wex, H.; Šantl-Temkiv, T.; Voigtländer, J.; Niedermeier, D.; Stratmann, F. Immersion freezing of ice nucleation active protein complexes. Atmos. Chem. Phys. 2013, 13, 5751–5766. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.A.; Arellano, F.; Kozloff, L.M. Components of ice nucleation structures of bacteria. J. Bacteriol. 1991, 173, 6515–6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, H.; Nakano, Y.; Omiya, K.; Muryoi, N.; Nishikawa, J.; Obata, H. Production of two types of ice crystalcontrolling proteins in Antarctic bacterium. J. Biosci. Bioeng. 2004, 98, 220–223. [Google Scholar] [CrossRef]
- Wilson, S.L.; Kelley, D.L.; Walker, V.K. Ice-active characteristics of soil bacteria selected by ice-affinity. Environ. Microbiol. 2006, 8, 1816–1824. [Google Scholar] [CrossRef]
- Guo, S.; Garnham, C.P.; Whitney, J.C.; Graham, L.A.; Davies, P.L. Re-evaluation of a bacterial antifreeze protein as an adhesin with ice-binding activity. PLoS ONE 2012, 7, e48805. [Google Scholar] [CrossRef] [Green Version]
- Kozloff, L.M.; Turner, M.A.; Arellano, F.; Lute, M. Phosphatidylinositol, a phospholipid of ice-nucleating bacteria. J. Bacteriol. 1991, 173, 2053–2060. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, H.; Mano, Y.; Obata, H. Purification and characterization of extracellular ice-nucleating matter from Erwinia uredovora KUIN-3. Biosci. Biotechnol. Biochem. 1993, 57, 1429–1432. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, S.; Kamishima, H.; Tamiya, E.; Karube, I. Spontaneous release of outer-membrane vesicles by Erwinia Carotovora. Microbios 1992, 72, 167–173. [Google Scholar]
- Obata, H.; Tanaka, T.; Kawahara, H.; Tokuyama, T. Properties of cell-free ice nuclei from ice nucleation-active Pseudomonas fluorescens KUIN-1. J. Ferment. Bioeng. 1993, 76, 19–24. [Google Scholar] [CrossRef]
- Muryoi, N.; Matsukawa, K.; Yamade, K.; Kawahara, H.; Obata, H. Purification and properties of an ice-nucleating protein from an ice-nucleating bacterium, Pantoea ananatis KUIN-3. J. Biosci. Bioeng. 2003, 95, 157–163. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, C.; Lo Giudice, A. Life from a Snowflake: Diversity and Adaptation of Cold-Loving Bacteria among Ice Crystals. Crystals 2022, 12, 312. https://doi.org/10.3390/cryst12030312
Rizzo C, Lo Giudice A. Life from a Snowflake: Diversity and Adaptation of Cold-Loving Bacteria among Ice Crystals. Crystals. 2022; 12(3):312. https://doi.org/10.3390/cryst12030312
Chicago/Turabian StyleRizzo, Carmen, and Angelina Lo Giudice. 2022. "Life from a Snowflake: Diversity and Adaptation of Cold-Loving Bacteria among Ice Crystals" Crystals 12, no. 3: 312. https://doi.org/10.3390/cryst12030312






