Spectroscopic Characterization, Thermogravimetry and Biological Studies of Ru(III), Pt(IV), Au(III) Complexes with Sulfamethoxazole Drug Ligand
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals and Equipment
2.2. Method of Synthesis
2.3. Antimicrobial Assay
2.4. Estimation of Cytotoxic Impacts of Particular Chemical Complex
3. Results and Discussion
3.1. Conductance and Microanalytical Studies
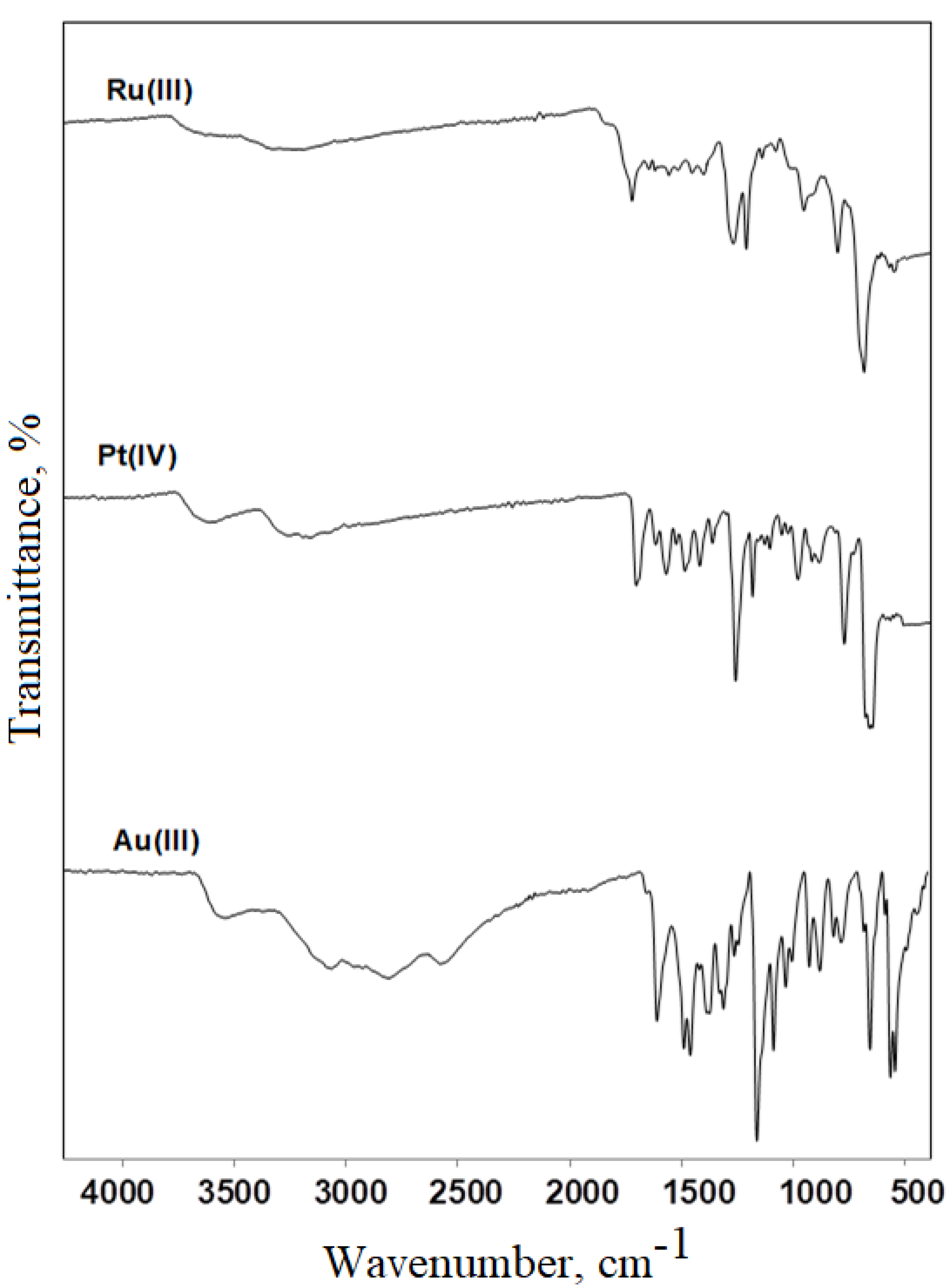
3.2. FT-IR Spectral Studies
3.3. Electronic Spectra
3.4. 1H NMR Spectra
3.5. Thermal Analysis
3.6. Morphological Studies
3.7. Biological Activity
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alias, M.F.; Abdul-Hassan, M.A. Synthesis and Characterization of Some Metal Complexes with their Sulfamethoxazole and 4,4′ -dimethyl-2,2′ -bipyridyl and study Cytotoxic Effect on Hep-2 Cell Line. Baghdad Sci. J. 2015, 12, 740–752. [Google Scholar]
- Zahid, H.; Chohan Hazoor, A.; Shad, N.F.H. Synthesis, Characterization and Biological Properties of Sulfonamide Derived Compounds and Their Transition Metal Complexes. Appl. Organ Metal. Chem. 2009, 23, 319–328. [Google Scholar]
- Chamundeeswari, S.V.; Samuel, E.J.J.; Sundaraganesan, N. Molecular structure, vibrational spectra, NMR and UV spectral analysis of sulfamethoxazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 1–10. [Google Scholar] [CrossRef]
- Hammoudeh, D.I.; Zhao, Y.; White, S.W.; Lee, R.E. Replacing sulfa drugs with novel DHPS inhibitors. Future Med. Chem. 2013, 5, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C.T. Anticancer and Antiviral Sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(vinylpyridine) as Basic Catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Nunes, J.H.B.; de Paiva, R.E.F.; Cuin, A.; Lustri, W.R.; Corbi, P.P. Silver complexes with sulfathiazole and sulfamethoxazole: Synthesis, spectroscopic characterization, crystal structure and antibacterial assays. Polyhedron 2015, 85, 437–444. [Google Scholar] [CrossRef]
- Sulfamethoxazole; Drug Bank: Alberta, AB, Canada, 2015.
- Toth, J.E.; Grindey, G.B.; Ehlhardt, W.J.; Ray, J.E.; Boder, G.B.; Bewley, J.R.; Klingerman, K.K.; Gates, S.B.; Rinzel, S.M.; Schultz, R.M.; et al. Sulfonimidamide analogs of oncolytic sulfonylureas. J. Med. Chem. 1997, 40, 1018. [Google Scholar] [CrossRef]
- Medina, J.C.; Roche, D.; Shan, B.; Learned, R.M.; Frankmoelle, W.P.; Clark, D.L.; Rosen, T.; Jaen, J.C. Novel halogenated sulfonamides inhibit the growth of multidrug resistant MCF-7/ADR cancer cells. Med. Chem. Lett. 1999, 9, 1843. [Google Scholar] [CrossRef]
- Yoshino, H.; Ueda, N.; Niijima, J.; Sugumi, H.; Kotake, Y.; Koyanagi, N.; Yoshimatsu, K.; Asada, M.; Watanabe, T. Novel sulfonamides as potential, systemically active antitumor agents. J. Med. Chem. 1992, 35, 2496. [Google Scholar] [CrossRef] [PubMed]
- Owa, T.; Yoshino, H.; Okauchi, T.; Yoshimatsu, K.; Ozawa, Y.; Sugi, N.H.; Nagasu, T.; Koyanagi, N.; Kitoh, K. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J. Med. Chem. 1999, 42, 3789. [Google Scholar] [CrossRef] [PubMed]
- Alias, M.F.; Hassan, M.M.A.; Khammas, S.J. Synthesis, characterization of some metal complexes with mixed ligands derived from sulfamethoxazole and 4, 4-dimethyl-2, 2-bipyridyl. Int. J. Sci. Res. 2015, 4, 2337–2342. [Google Scholar]
- Karthikeyan, G.; Mohanraj, K.; Elango, K.P.; Girishkumar, K. Synthesis and spectral characterization of lanthanide complexes with sulfamethoxazole and their antibacterial activity. Russ. J. Coord. Chem. 2006, 32, 380–385. [Google Scholar] [CrossRef]
- Mahind, L.H.; Waghmode, S.A.; Nawale, A.; Mane, V.B.; Dagade, S.P. Structural characterization of nanosized Fe2O3-CeO2 catalysts by XRD, EDX and TEM techniques. J. Pharm. Biosci. 2013, 5, 2–105. [Google Scholar]
- Mahmoud, W.H.; Mohamed, G.G.; Ele-Dessouky, M.I. Synthesis, Characterization and in vitro Biological Activity of Mixed Transition Metal Complexes of Lornoxicam with 1,10- phenanthroline. Int. J. Electrochem. Sci. 2014, 9, 1415–1438. [Google Scholar]
- Ade, S.B.; Kolhatkar, D.G.; Deshpande, M.N. Synthesis, characterization and biological activity of a schiff base derived from isatin and 2–amino, 4–methyl phenol and its transition metal complexes. Int. J. Pharma. Bio. Sci. 2012, 3, 350–356. [Google Scholar]
- Baenziger, N.C.; Struss, A.W. Crystal structure of 2-sulfanilamidopyrimidinesilver(I). Inorg. Chem. 1976, 15, 1807. [Google Scholar] [CrossRef]
- Cook, D.S.; Turner, M.F. Crystal and molecular structure of silver sulphadiazine (N1-pyrimidin-2-ylsulphanilamide). J. Chem. Soc. Perkin Trans. 1975, 2, 1021. [Google Scholar] [CrossRef]
- Baenziger, C.; Modak, S.L.; Fox, C.L., Jr. Diamminebis(2-sulfanilamidopyrimidinato)zinc(II), [Zn(C10H9N4O2S)2(NH3)2]. Acta Crystallogr. 1983, 39, 1620. [Google Scholar] [CrossRef]
- Brown, C.J.; Cook, D.S.; Sengier, L. Bis[N1-(2-pyrimidinyl)sulphanilamido]zinc–ammonia (1/2), [Zn(C10H9N4O2S)2].2NH3. Acta Crystallogr. 1985, 41, 718. [Google Scholar] [CrossRef]
- Weder, J.E.; Dillon, C.T.; Hambley, T.W. Copper complexes of nonsteroidal anti-inflammatory drugs: An Opportunity yet to be realized. Coord. Chem. Rev. 2002, 232, 95–126. [Google Scholar] [CrossRef]
- Thompson, K.H.; McNeill, J.H.; Orvig, C. Vanadium Compounds as Insulin Mimics. Chem. Rev. 1999, 99, 2561. [Google Scholar] [CrossRef]
- Shechter, Y.; Shisheva, A.; Gefel, D. Chemistry, Biochemistry, and Therapeutic Applications; Tracey, A.S., Crans, D.C., Eds.; Oxford University Press: New York, NY, USA, 1998; Volume 20. [Google Scholar]
- Campos, M.M.A.; Gris, L.R.S. New gold(I) and silver(I) complexes of sulfamethoxazole: Synthesis, X-ray structural characterization and microbiological activities of triphenylphosphine(sulfamethoxazolato-N2) gold(I) and (sulfamethoxazolato)silver(I) Inorg. Chem. Commun. 2007, 10, 1083–1087. [Google Scholar]
- Garg, S.K.; Ghosh, S.S.; Mathur, V.S. Comparative pharmacokinetic study of four different sulfonamides in combination with trimethoprim in human volunteers. Int. J. Clin. Pharm. Therapy. Toxicol. 1986, 24, 23. [Google Scholar]
- Holm, R.H.; Connor, M.J.O. The Stereochemistry of Bis-Chelate Metal(II) Complexes. Prog. Inorg. Chem. 1971, 14, 263. [Google Scholar]
- Melagraki, G.; Afantitis, A.; Sarimveis, H.; Igglessi-Markopoulou, O.; Supuran, C.T. QSAR study on para-substituted aromatic sulfonamides as carbonic anhydrase II inhibitors using topological information indices. Med. Chem. 2006, 14, 1108–1114. [Google Scholar] [CrossRef]
- Adamu, U.A.; Magaji, B.; Mohammad, A.B.; Sani, M.M.; Adoram, N. Synthesis, Characterization and Antibacterial Study of Co (II) and Cu (II) Complexes of Sulfamethoxazole. AJARR 2020, 10, 38–43. [Google Scholar] [CrossRef]
- Mallikarjuna, N.M.; Keshavayya, J.; Maliyappa, M.R.; Shoukat Ali, R.A.; Venkatesh, T. Synthesis, characterization, thermal and biological evaluation of Cu (II), Co (II) and Ni (II) complexes of azo dye ligand containing sulfamethaxazole moiety. J. Mol. Struct. 2018, 1165, 28–36. [Google Scholar] [CrossRef]
- Machado, A.P.; Anza, M.; Fischman, O. Bacillus subtilis induces morphological changes in Fonsecaea pedrosoi in vitro resulting in more resistant fungal forms in vivo. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 592–598. [Google Scholar] [CrossRef][Green Version]
- Molina-Hernandez, J.B.; Aceto, A.; Bucciarelli, T.; Paludi, D.; Valbonetti, L.; Zilli, K.; Scotti, L.; Chaves-López, C. The membrane depolarization and increase intracellular calcium level produced by silver nanoclusters are responsible for bacterial death. Sci. Rep. 2021, 11, 21557. [Google Scholar] [CrossRef]
- Gasbarri, C.; Ronci, M.; Aceto, A.; Vasani, R.; Iezzi, G.; Florio, T.; Barbieri, F.; Angelini, G.; Scotti, L. Structure and Properties of Electrochemically Synthesized Silver Nanoparticles in Aqueous Solution by High-Resolution Techniques. Molecules 2021, 26, 5155. [Google Scholar] [CrossRef]
- Tweedy, B.G. Synthesis, Characterization and Antibacterial Activity of Mixed Ligand (HL) Complexes Mn(ll), Co(ll), Ni(ll), Zn(ll), Cd(ll) and Hg(ll) with Azide (N3-). Open J. Inorg. Chem. 1964, 55, 910–918. [Google Scholar]
- Kesimli, B.; Topacli, A. Infrared studies on Co and Cd complexes of Sulfamethoxazole. Spectrochim. Acta Part A 2001, 57, 1031–1036. [Google Scholar] [CrossRef]
- El-Shwiniy, W.H.; Shehab, W.S.; Mohamed, S.F.; Ibrahium, H.G. Synthesis and cytotoxic evaluation of some substituted pyrazole zirconium (IV) complexes and their biological assay. Appl. Organometal. Chem. 2018, 32, 4503. [Google Scholar] [CrossRef]
- Chavda, B.R.; Socha, B.N.; Pandya, S.B.; Chaudhary, K.P.; Padariya, T.J.; Alalawy, M.D.; Patel, M.K.; Dubey, R.P.; Patel, U.H. Coordination behavior of dinuclear silver complex of sulfamethoxazole with solvent molecule having static rotational disorder: Spectroscopic characterization, crystal structure, Hirshfeld surface and antimicrobial activity. J. Mol. Struct. 2021, 1228, 129777. [Google Scholar] [CrossRef]
- Torre, M.; Calvo, S.; Pardo, H.; Mombru, A.W. Synthesis, spectroscopic characterization and crystal structure of disulfamethoxazole diaquo Ni(II) monohydrate. J. Coord. Chem. 2005, 58, 513–520. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Maheswaran, S.; Ramesh, R. Synthesis, Spectra, Redox property and catalytic activity of ruthenium Schiff base complexes. Indian J. Chem. 2005, 44, 705. [Google Scholar]
- Lever, A.B.P. Electronic spectra of dn ions. In Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- El-Shwiniy, W.H.; Abbass, L.M.; Sadeek, S.A.; Zordok, W.A. Synthesis, Structure, DFT, and Biological Activity of Metal Complexes of Norfloxacin and Metformin Mixed Ligand. Russ. J. Gen. Chem. 2020, 90, 483. [Google Scholar] [CrossRef]
- El-Shwiniy, W.H.; Gamil, M.A.; Sadeek, S.A.; Zordok, W.A.; El-farargy, A.F. Ligational, density functional theory, and biological studies on some new Schiff base 2-(2-hydroxyphenylimine) benzoic acid (L) metal complexes. Appl. Organometal. Chem. 2020, 34, 5696. [Google Scholar]
- Sadeek, S.A.; Abd El-Hamid, S.M.; El-Shwiniy, W.H. Synthesis, spectroscopic characterization, thermal stability and biological studies of mixed ligand complexes of gemifloxacin drug and 2,2′-bipyridine with some transition metals. Res. Chem. Intermed. 2016, 42, 3183. [Google Scholar] [CrossRef]
- Dhanaraj, C.J.; Nair, M.S. Synthesis, characterization, and antimicrobial studies of some Schiff-base metal(II) complexes. J. Coord. Chem. 2009, 62, 4018. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Sharaby, C.M. Metal complexes of Schiff base derived from sulphametrole and o-vanilin: Synthesis, spectral, thermal characterization and biological activity. Spectrochim. Acta A 2007, 66, 949–958. [Google Scholar] [CrossRef]






| Type of Analysis | Models |
|---|---|
| Analyses of the elements | Perkin Elmer CHN 2400 |
| Conductance | Jenway 4010 conductivity meter |
| FTIR spectra | Bruker FTIR Spectrophotometer |
| Raman laser | Bruker FT Raman with laser 50 mW |
| 1H NMR spectra | Varian Mercury VX-300 NMR spectrometer, 300 MHz |
| Electronic spectra | UV2 Unicam UV/Vis Spectrophotometer |
| Magnetic moment | Balance of Magnetic Susceptibility |
| SEM | Quanta FEG 250 equipment |
| XRD | X ‘Pert PRO PANanalytical, with copper target |
| TEM | JEOL 100 s microscopy |
| Complex (MF) Mwt. | Yield% | mp/°C | Color | Magnetic Moment (BM) | Conductance (ohm−1·cm2·mol−1) | Element | Calc. | Found |
|---|---|---|---|---|---|---|---|---|
| (C10H11N3O3S) (smx) (253) | - | 140 | White | Diamagnetic | 0 | %C %H | 47.43 4.34 | 46.21 3.22 |
| %N | 16.60 | 15.36 | ||||||
| %M | - | - | ||||||
| %S | - | - | ||||||
| %Cl | - | - | ||||||
| [Ru(C10H11N3O3S)(H2O)2Cl2]Cl (496.74) | 85 | 337 | Dark green | 1.65 | 46.80 | %C %H | 24.18 3.04 | 24.34 3.11 |
| %N | 8.46 | 8.79 | ||||||
| %M | 20.35 | 20.14 | ||||||
| %S | 6.46 | 6.22 | ||||||
| %Cl | 21.41 | 21.01 | ||||||
| [Pt(C10H11N3O3S)(H2O)Cl3] 572.73 | 90 | 258 | Brown | Diamagnetic | 0.200 | %C %H | 20.97 2.29 | 20.23 2.32 |
| %N | 7.34 | 7.25 | ||||||
| %M | 34.06 | 34.03 | ||||||
| %S | 5.60 | 5.58 | ||||||
| %Cl | 18.57 | 18.00 | ||||||
| [Au(C10H11N3O3S)Cl2]Cl·H2O 574.62 | 88 | 216 | Pale yellow | Diamagnetic | 47.60 | %C %H | 20.90 2.28 | 20.54 2.25 |
| %N | 7.31 | 7.46 | ||||||
| %M | 34.28 | 34.45 | ||||||
| %S | 5.58 | 5.45 | ||||||
| %Cl | 18.51 | 18.51 |
| IR Frequencies | Assignments | |||
|---|---|---|---|---|
| SMX | Au(III) | Pt(IV) | Ru(III) | |
| 3466 | 3549 | 3508 | 3495 | νas(NH2), aniline |
| 3377 | 3357 | - | - | νs(NH2), aniline |
| 3298 | - | 3156 | 3211 | ν(-NH), sulfonamide |
| 3143 | - | 3094 | 3193 | ν(C-H), isoxazole ring |
| 2989 | 2956 | 2968 | - | ν(CH3) |
| 1621 | 1608 | 1606 | 1693 | ν(C=O) isoxazole ring |
| 1596 | 1487 | 1520 | 1594 | ν(C=N) |
| 1503 | 1462 | 1470 | 1495 | isoxazole ring vibrations |
| 1383 | 1386 | 1392 | 1390 | νas(SO2); asymmetric |
| 1266 | 1262 | 1266 | 1277 | νs(C-N) sulfonamide |
| 1091 | 1089 | 1089 | 1083 | νs(SO2); symmetric |
| 927 | 929 | 933 | 914 | ν(S-N) |
| 884 | 883 | 887 | 894 | ν(C-H) isoxazole ring |
| 831 | 821 | 828 | 828 | ᵹ(C-H) |
| 684 | 657 | 677 | 677 | ν(C-S) |
| 575 | 566 | 578 | 562 | ν(M-O) |
| 426 | 414 | 412 | 420 | ν(M-N) |
| Assignments (nm) | SMX | SMX Complex with | ||
|---|---|---|---|---|
| Ru(III) | Pt(IV) | Au(III) | ||
| n-π* transitions | 366 | 378 | 376 | 386 |
| π-π* transitions | 298,322 | 338 | 338 | 336 |
| d–d transitions | - | 572 | 570 | - |
| Ligand–metal charge transfer | - | 396 | 418 | 400 |
| A | B | C | Assignments |
|---|---|---|---|
| 2.25, 2.50 | 2.21, 2.49 | 2.26, 2.49 | δ H, (s,3H,-CH3) |
| - | 3.95 | 3.95 | δ H, (s,2H,H2O) |
| 6.32 | 6.56 | 6.62 | δ H, (m,2H,NH2) |
| 7.50 | 7.41–7.74 | 7.90 | δ H, (m,5H,Ar-CH) |
| 10.99 | 11.50 | 11.10 | δ H, (s,H,SO2-NH) |
| Compounds (M. F) M.wt | Decomposition | Weight Loss (%) | Tmax (°C) | Lost Species | |
|---|---|---|---|---|---|
| Found | Calc. | ||||
| Sulfamethoxazole (smx) 253 (C10H11N3O3S) | First step Total loss Residue | 77.87 77.77 22.13 | 78.85 78.85 21.15 | 113, 266, 378 | 3C2H2 + CH4 + HCN + NO2 + NO S + 2C |
| [Ru(C10H11N3O3S)(H2O)2Cl2]Cl (1) 496.74 (RuC10H15Cl3N3O5S) | First step Second step Total loss Residue | 7.15 65.90 73.05 26.95 | 7.25 65.96 73.22 26.78 | 169 312, 402 | 2H2O 5C2H2 + HCl + Cl2 + 1.5N2 + SO RuO2 |
| [Pt(C10H11N3O3S)(H2O)Cl3] (2) 572.73 (PtC10H13Cl3N3O4S) | First step Second step Total loss Residue | 3.10 57.00 60.10 39.90 | 3.14 57.21 60.35 39.65 | 175 226, 287 | H2O 5C2H2 + 3HCl + 1.5N2 + SO2 PtO2 |
| [Au(C10H11N3O3S)Cl2]Cl·H2O (3) 574.62 (AuC10H13Cl3N3O4S) | First step Second step Total loss Residue | 3.11 62.79 65.90 34.10 | 3.13 62.60 65.73 34.27 | 100 242, 476 | H2O 5C2H2 + 3HCl + N2O + NO + SO2 Au |
| Compounds | Microbial Species | |||
|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| SMX | 10 ± 0.2 | 15 ± 0.11 | 9 ± 0.03 | 6 ± 0.22 |
| Ru(III)-SMX | 9NS ± 0.11 | 11NS ± 0.01 | 9NS ± 0.02 | 10+1 ± 0.33 |
| Pt(IV)-SMX | 19+2 ± 0.5 | 19+1 ± 0.11 | 17+2 ± 0.6 | 17+3 ± 0.22 |
| Au(III)-SMX | 12+1 ± 0.1 | 15NS ± 0.2 | 13+1 ± 0.1 | 13+2 ± 0.02 |
| Control (DMSO) | 0 | 0 | 0 | 0 |
| Ampicillin | 26 ± 0.3 | 21 ± 0.02 | 25 ± 0.11 | 26 ± 0.05 |
| Sample Code | IC50 Values (µg/mL) | |
|---|---|---|
| HepG-2 | MCF-7 | |
| Au(III)-SMX | 30 ± 2.1 | 41 ± 2.6 |
| Ru(III)-SMX | 110 ± 8.2 | 124 ± 9.7 |
| Pt(IV)-SMX | 70.9 ± 5.7 | 81 ± 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alosaimi, E.H. Spectroscopic Characterization, Thermogravimetry and Biological Studies of Ru(III), Pt(IV), Au(III) Complexes with Sulfamethoxazole Drug Ligand. Crystals 2022, 12, 340. https://doi.org/10.3390/cryst12030340
Alosaimi EH. Spectroscopic Characterization, Thermogravimetry and Biological Studies of Ru(III), Pt(IV), Au(III) Complexes with Sulfamethoxazole Drug Ligand. Crystals. 2022; 12(3):340. https://doi.org/10.3390/cryst12030340
Chicago/Turabian StyleAlosaimi, Eid H. 2022. "Spectroscopic Characterization, Thermogravimetry and Biological Studies of Ru(III), Pt(IV), Au(III) Complexes with Sulfamethoxazole Drug Ligand" Crystals 12, no. 3: 340. https://doi.org/10.3390/cryst12030340
APA StyleAlosaimi, E. H. (2022). Spectroscopic Characterization, Thermogravimetry and Biological Studies of Ru(III), Pt(IV), Au(III) Complexes with Sulfamethoxazole Drug Ligand. Crystals, 12(3), 340. https://doi.org/10.3390/cryst12030340





