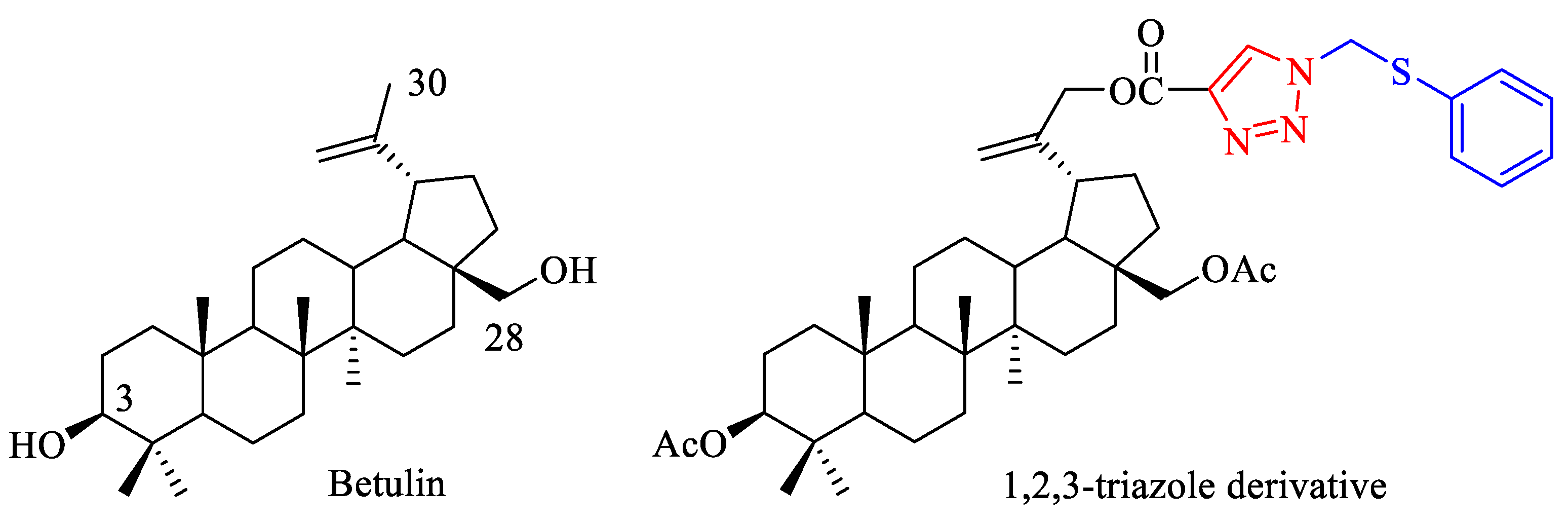
Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
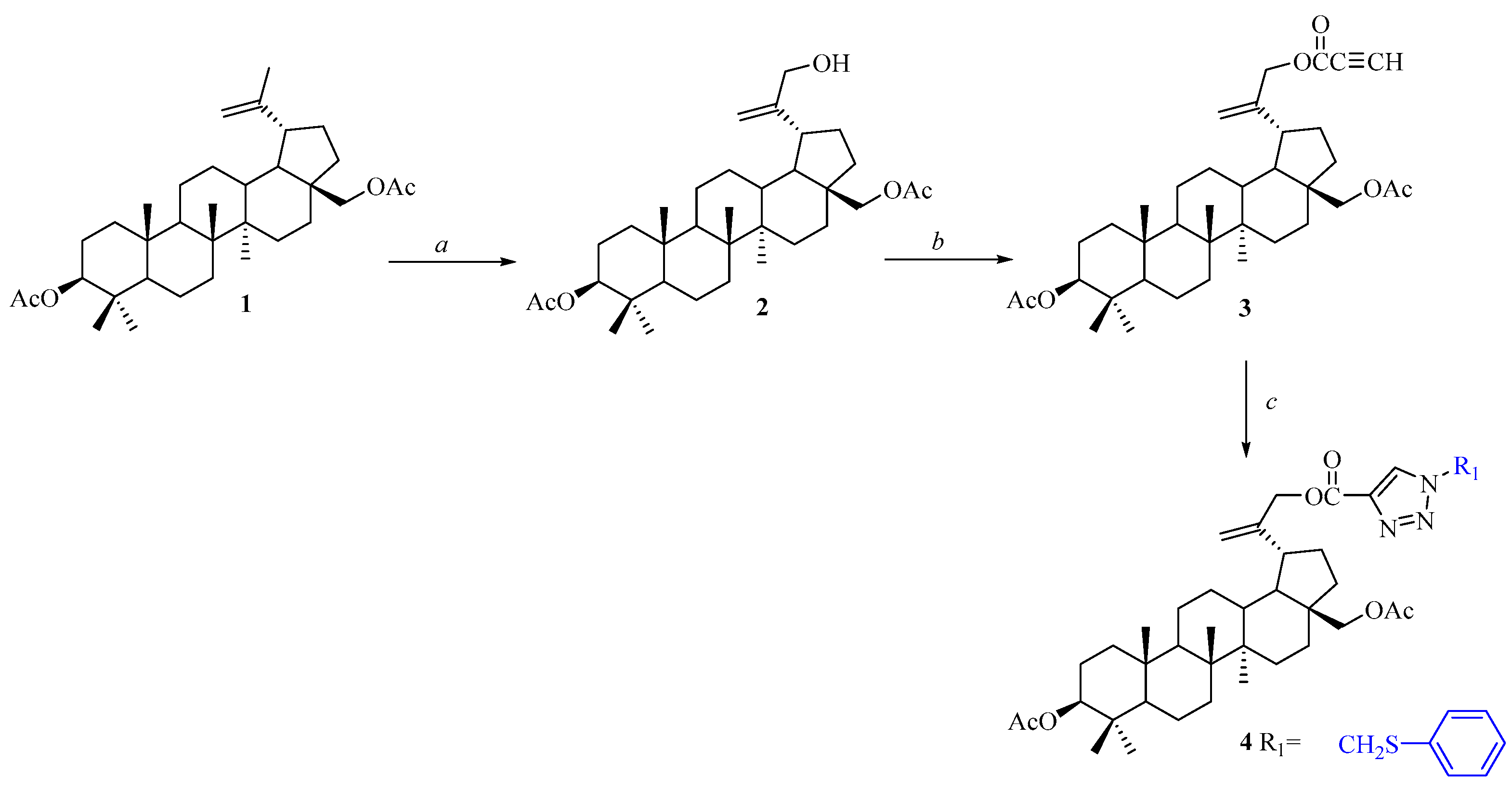
2.2. Synthesis of 3,28-O,O′-diacetyl-30-(1-phenylthiomethyl-1H-1,2,3-triazol-4-yl)carbonylbetulin 4
2.3. Determination of Crystal Structure
2.3.1. X-ray Diffraction Experiment
2.3.2. Refinement
2.4. Hirshfeld Surface Analysis
2.5. Computational Details
3. Results and Discussion
3.1. Synthesis of Compound 4
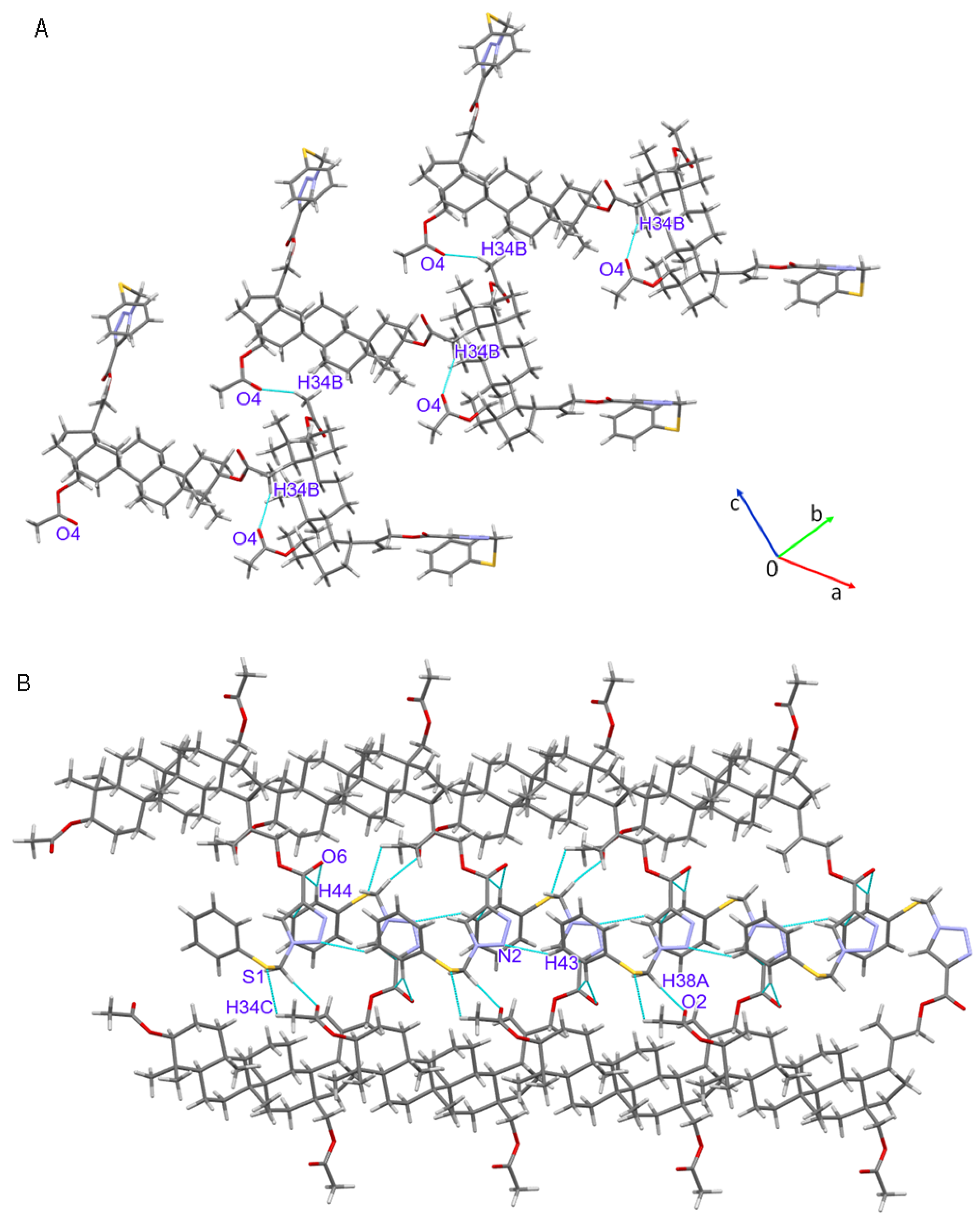
3.2. Crystal Structure of Compound 4
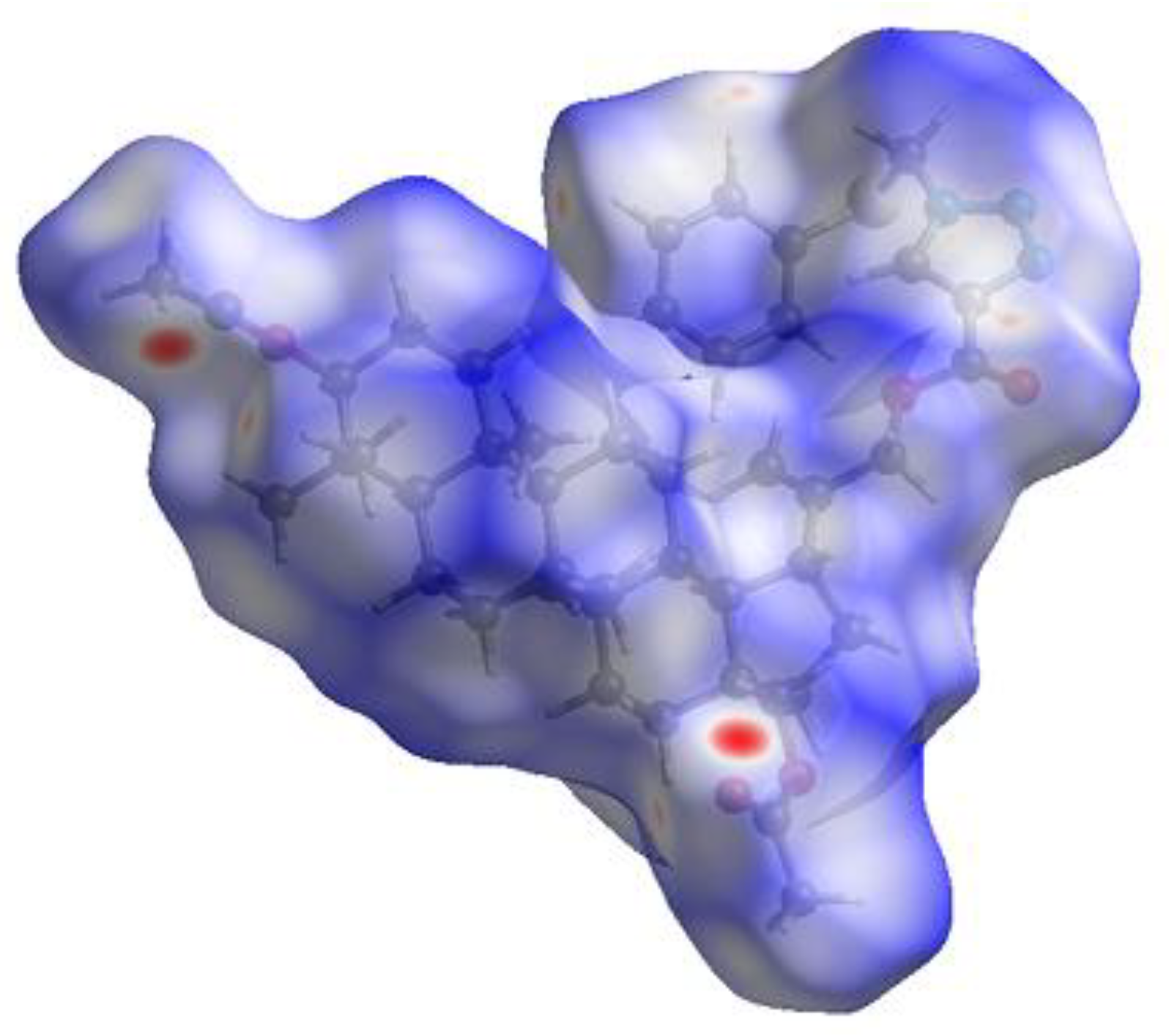
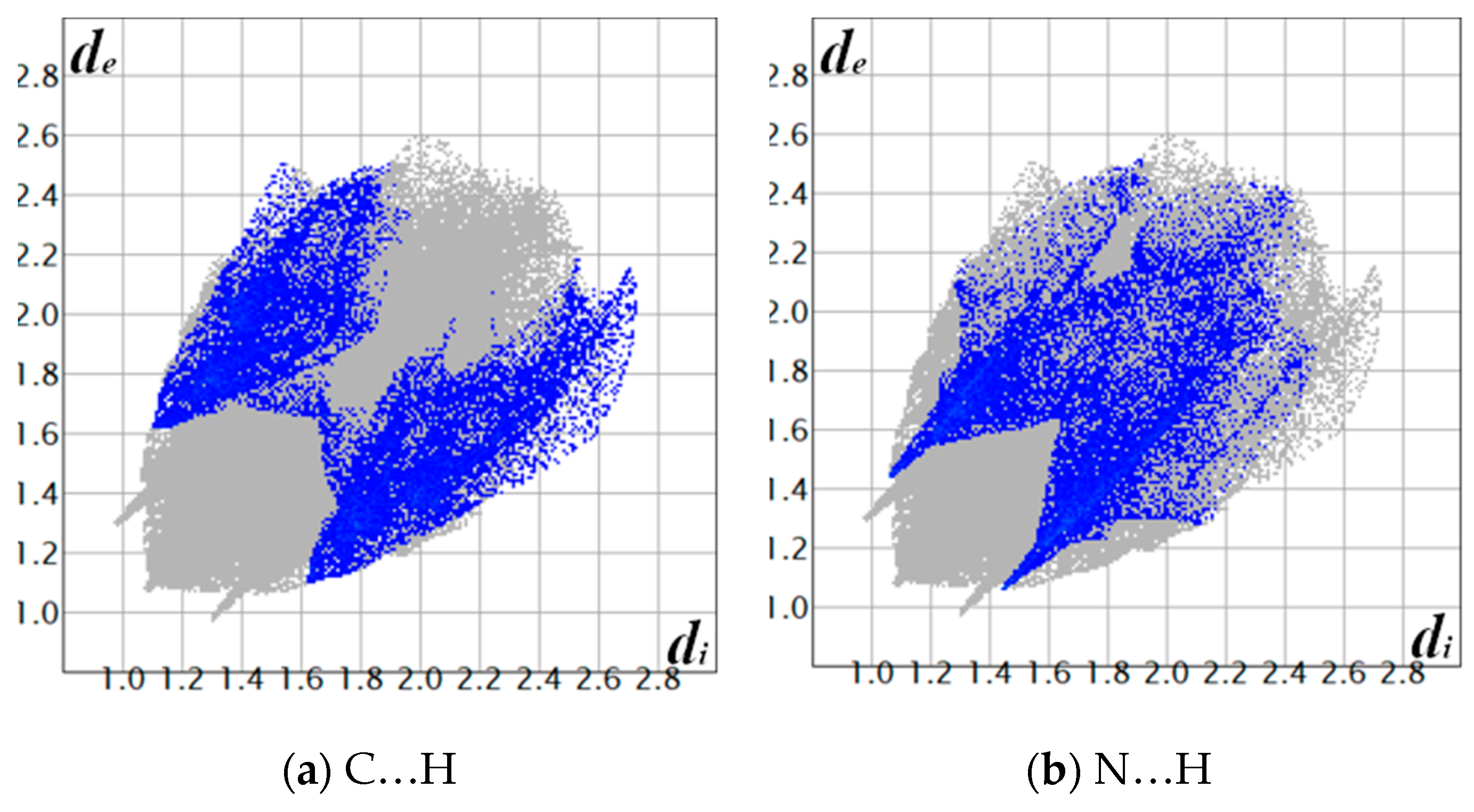
3.3. Hirshfeld Surface
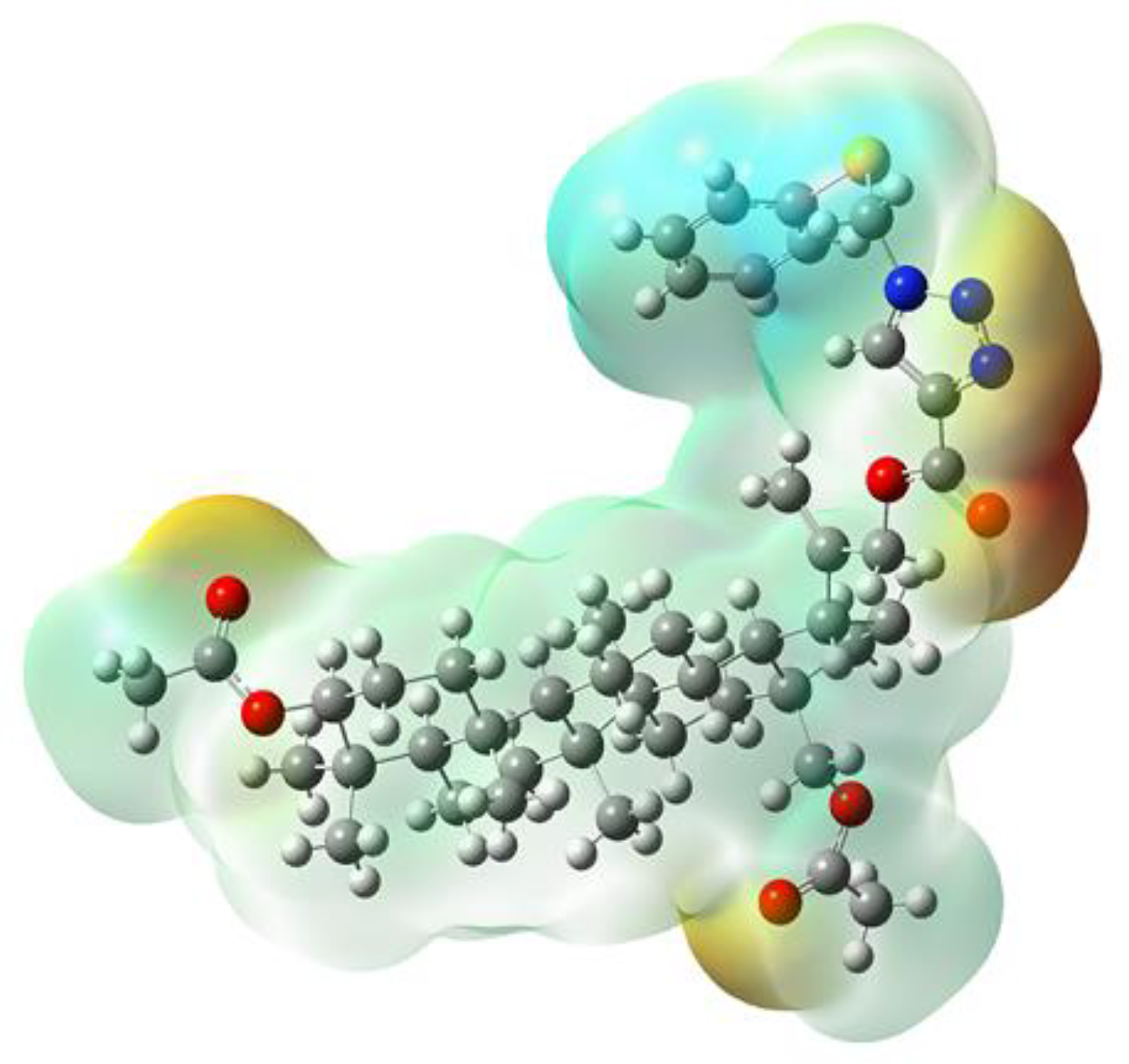
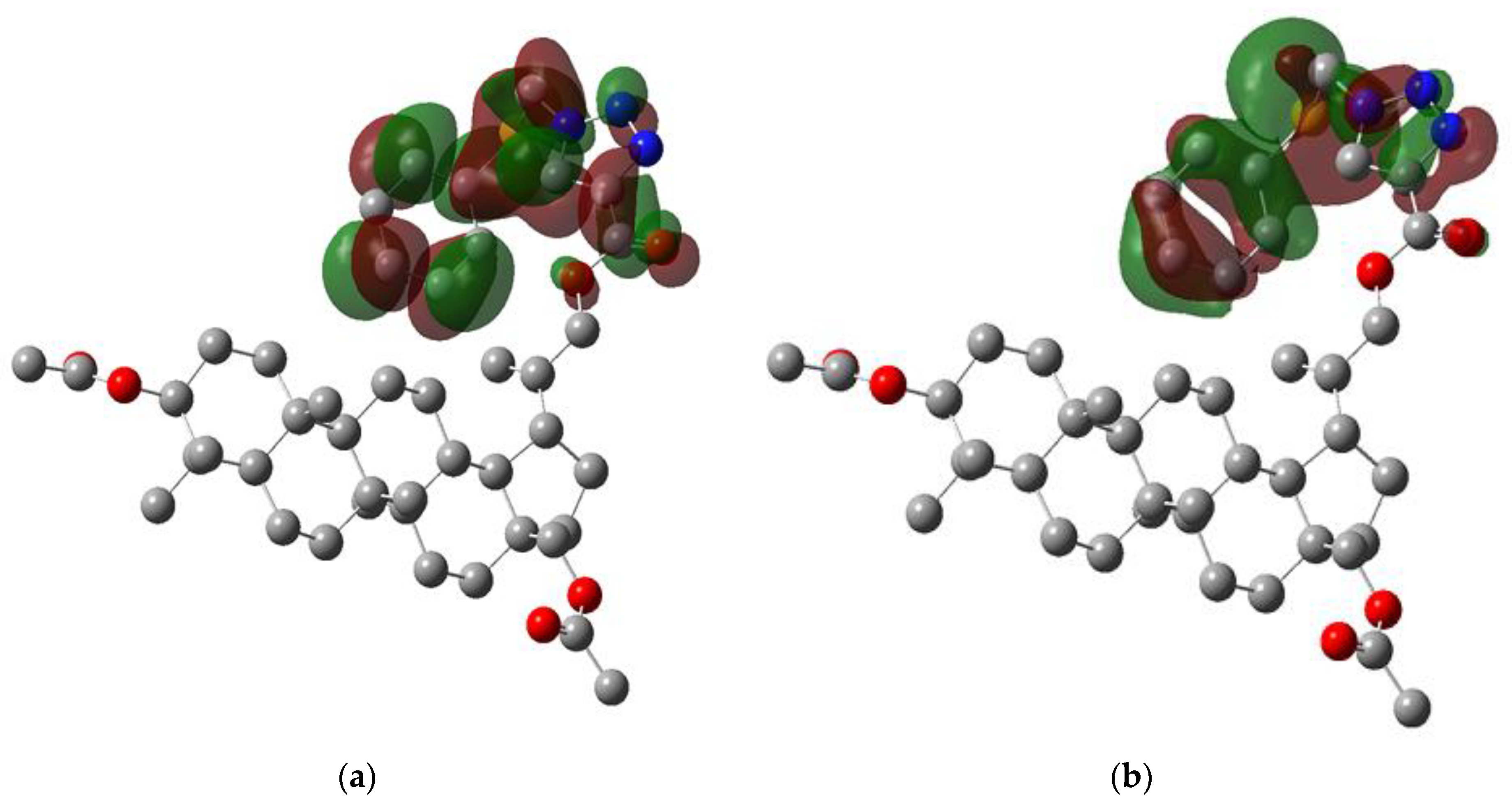
3.4. Molecular Electrostatic Potential Analysis
3.5. Molecular Properties of 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409–107447. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.H.; Septama, A.W.; Ahmad, W.A.N.W.; Suppian, R. Immunomodulatory effects and structure-activity relationship of botanical pentacyclic triterpenes: A review. Chin. Herb. Med. 2020, 12, 118–124. [Google Scholar] [CrossRef]
- Grishko, V.V.; Tolmacheva, I.A.; Nebogatikov, V.O.; Galaiko, N.V.; Nazarov, A.V.; Dmitriev, M.V.; Ivshina, I.B. Preparation of novel ring-A fused azole derivatives of betulin and evaluation of their cytotoxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Smirnova, I.; Tret’yakova, E.; Csuk, R.; Hoenke, S.; Fischer, L. Cytotoxic potential of a-azepano- and 3-amino-3,4-seco-triterpenoids. Int. J. Mol. Sci. 2021, 22, 1714. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Orzechowska, B.; Chrobak, E.; Boryczka, S. Novel betulin dicarboxylic acid ester derivatives as potent antiviral agents: Design, synthesis, biological evaluation, structure-activity relationship and in-silico study. Eur. J. Med. Chem. 2021, 225, 113738–113750. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, A.Ç.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef]
- Myszka, H.; Grzywacz, D.; Zdrowowicz, M.; Spisz, P.; Butowska, K.; Rak, J.; Piosik, J.; Jaśkiewicz, M.; Kamysz, W.; Liberek, B. Design, synthesis and biological evaluation of betulin-3-yl 2-amino-2-deoxy-β-D-glycopyranosides. Bioorg. Chem. 2020, 96, 103568–103574. [Google Scholar] [CrossRef]
- Kazakova, O.; Lopatina, T.; Giniyatullina, G.; Mioc, M.; Soica, C. Antimycobacterial activity of azepanobetulin and its derivative: In vitro, in vivo, ADMET and docking studies. Bioorg. Chem. 2020, 104, 104209–104217. [Google Scholar] [CrossRef]
- Oboh, M.; Govender, L.; Siwela, M.; Mkhwanazi, B.N. Anti-diabetic potential of plant-based pentacyclic triterpene derivatives: Progress made to improve efficacy and bioavailability. Molecules 2021, 26, 7243. [Google Scholar] [CrossRef]
- Buko, V.; Zavodnik, I.; Palecz, B.; Stepniak, A.; Kirko, S.; Shlyahtun, A.; Misiuk, W.; Belonovskaya, E.; Lukivskaya, O.; Naruta, E.; et al. Betulin/2-hydroxypropyl-β-cyclodextrin inclusion complex: Physicochemical characterization and hepatoprotective activity. J. Mol. Liq. 2020, 309, 113118–113127. [Google Scholar] [CrossRef]
- Li, J.; Jiang, B.; Chen, C.; Fan, B.; Huang, H.; Chen, G. Biotransformation of betulin by Mucor subtilissimus to discover anti-inflammatory derivatives. Phytochemistry 2019, 166, 112076–112081. [Google Scholar] [CrossRef]
- Kuczynska, K.; Bończak, B.; Rárová, L.; Kvasnicová, M.; Strnad, M.; Pakulski, Z.; Cmoch, P.; Fiałkowski, M. Synthesis and cytotoxic activity of 1,2,3-triazoles derived from 2,3-seco-dihydrobetulin via a click chemistry approach. J. Mol. Struct. 2022, 1250, 131751–131765. [Google Scholar] [CrossRef]
- Csuk, R.; Niesen-Barthel, A.; Schäfer, R.; Barthel, A.; Al-Harrasi, A. Synthesis and antitumor activity of ring A modified 11-keto-β-boswellic acid derivatives. Eur. J. Med. Chem. 2015, 92, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Pastuch-Gawołek, G.; Lalik, A.; Zawojak, M.; Boczek, S.; Krawczyk, M.; Erfurt, K. Glycoconjugation of betulin derivatives using copper-catalyzed 1,3-dipolar azido-alkyne cycloaddition reaction and a preliminary assay of cytotoxicity of the obtained compounds. Molecules 2020, 25, 6019. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2021, 212, 113069–113100. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700–111736. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Deigner, H.P. The potential of click reactions for the synthesis of bioactive triterpenes. Bioorg. Med. Chem. Lett. 2019, 29, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Snyder, J.K. Preparation of a 24-nor-1,4-dien-3-one triterpene derivative from betulin: A new route to 24-nortriterpene analogues. J. Org. Chem. 2002, 67, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Chung, B.Y.; Bentley, M.D.; Alford, A.R. Colorado potato beetle antifeedants by simple modification of the birch bark triterpene betulin. J. Agric. Food Chem. 1995, 43, 2513–2516. [Google Scholar] [CrossRef]
- Chrobak, E.; Bębenek, E.; Marciniec, K.; Kadela-Tomanek, M.; Siudak, S.; Latocha, M.; Boryczka, S. New 30-substituted derivatives of pentacyclic triterpenes: Preparation, biological activity, and molecular docking study. J. Mol. Struct. 2021, 1226, 129394–129404. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.38.41q. Rigaku Oxford Diffraction. 2015. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 21 November 2021).
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Grimwood, D.J.; McKinnon, J.; Jayatilaka, D.; Spackman, M. CrystalExplorer 3.0.; University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef] [Green Version]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Latocha, M.; Boryczka, S. Novel triazoles of 3-acetylbetulin and betulone as anticancer agents. Med. Chem. Res. 2018, 27, 2051–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, I.E.; Choudhary, M.I.; Ali, S.; Anjum, S. Atta-ur-Rahman. 20(29)-Lupene-3β,28β-diacetate. Acta Crystallogr. 2006, E62, o1352–o1354. [Google Scholar]
- Spackman, M.; McKinnon, J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Thilak Kumar, R. Structural, spectral, thermodynamical, NLO, HOMO, LUMO and NBO analysis of fluconazole. Spectrochim. Acta A Mol. Biomol. 2015, 150, 974–991. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Akman, F.; Vasilieva, N.Y.; Issaoui, N.; Malyar, Y.N.; Kondrasenko, A.A.; Borovkova, V.S.; Miroshnikova, A.V.; Kazachenko, A.S.; Al-Dossary, O.; et al. Catalytic sulfation of betulin with sulfamic acid: Experiment and DFT calculation. Int. J. Mol. Sci. 2022, 23, 1602. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Boryczka, S. Lipophilicity, pharmacokinetic properties, and molecular docking study on SARS-CoV-2 target for betulin triazole derivatives with attached 1,4-quinone. Pharmaceutics 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.K.; Bhatt, A.K.; Dash, D.; Sharma, N. DFT calculations on molecular structures, HOMO–LUMO study, reactivity descriptors and spectral analyses of newly synthesized diorganotin(IV) 2-chloridophenylacetohydroxamate complexes. J. Comput. Chem. 2019, 40, 2354–2363. [Google Scholar] [CrossRef]
- Govindarajan, M.; Karabacak, M.; Periandy, S.; Tanuja, D. Spectroscopic (FT-IR, FT-Raman, UV and NMR) investigation and NLO, HOMO–LUMO, NBO analysis of organic 2,4,5-trichloroaniline. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Heredia, C.L.; Ferraresi-Curotto, V.; López, M.B. Characterization of Ptn (n = 2–12) clusters through global reactivity descriptors and vibrational spectroscopy, a theoretical study. Comput. Mater. Sci. 2012, 53, 18–24. [Google Scholar] [CrossRef]
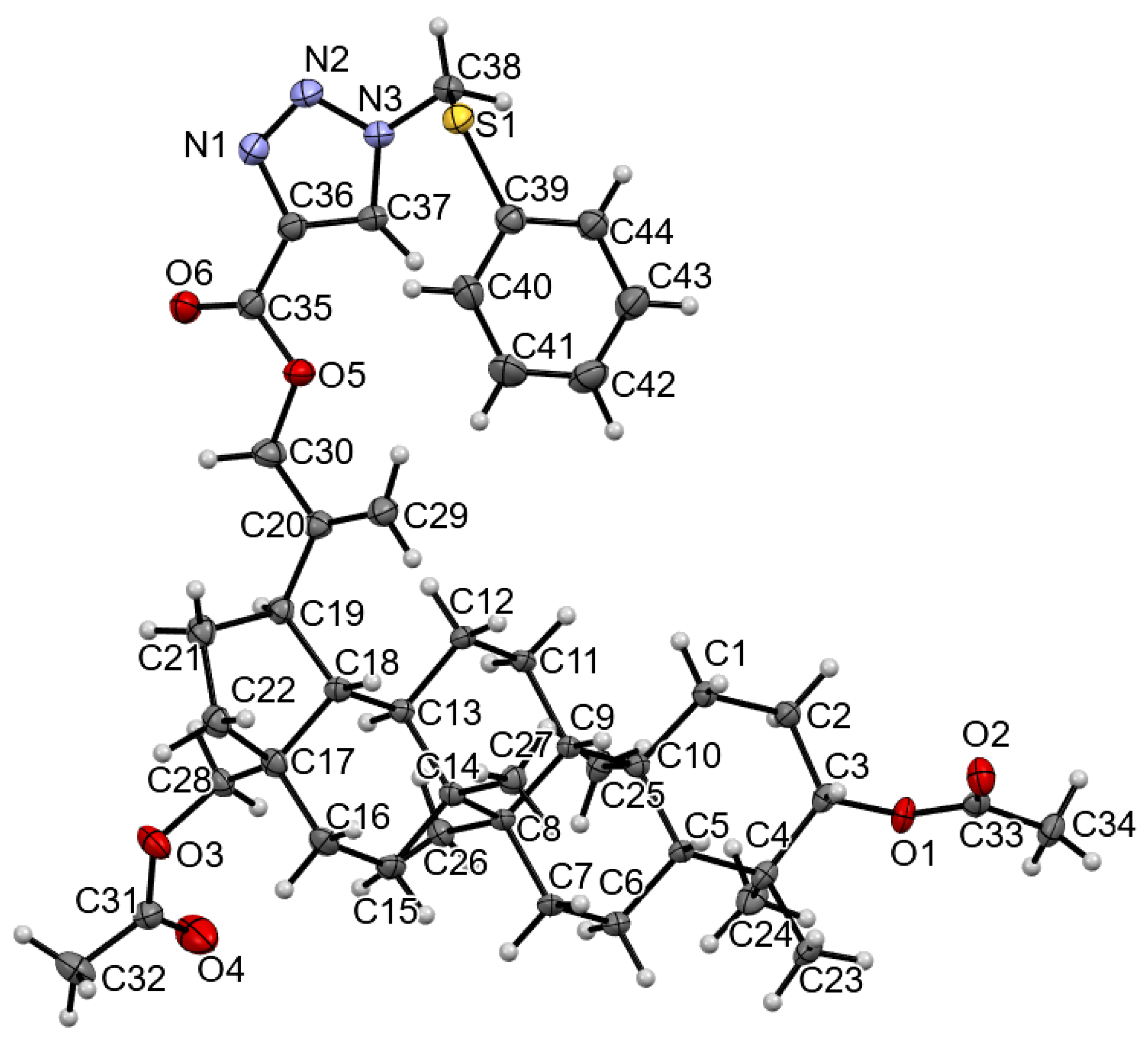

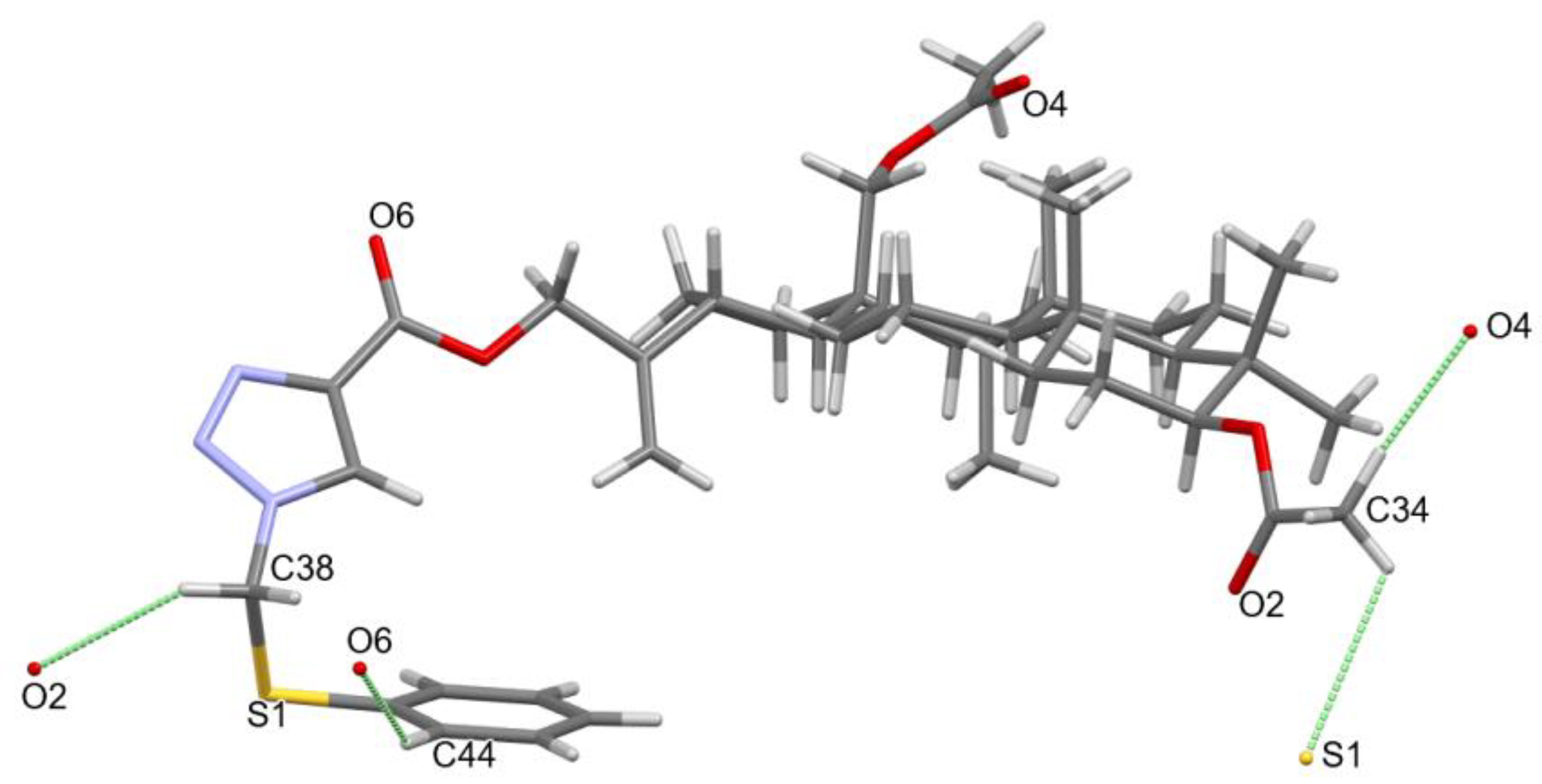

| Compound | 4 |
|---|---|
| CCDC deposition number | 2,153,148 |
| Chemical formula | C44H61N3O6S |
| Mr | 760.01 |
| Solvent | CH3CN |
| Crystal system, space group | orthorhombic; P212121 |
| Temperature (K) | 100 |
| a, b, c (Å) | 9.4860(10); 13.9440(2); 30.2347(4) |
| α, β, γ [°] | 90; 90; 90 |
| V(Å)3 | 3999.23(9) |
| Z | 4 |
| Z′ | 1 |
| Dcalc (g/cm3) | 1.262 |
| Radiation type | Cu Kα |
| µ (mm−1) | 1.13 |
| Crystal size (mm3) | 0.02 × 0.04 × 0.32 |
| Rint | 0.0304 |
| Rsigma | 0.0226 |
| No. reflns. (5.8° ≤ 2θ ≤ 145.0°) | 19,516 |
| Unigue reflns. | 7268 |
| λ (Å) | 1.5418 |
| GOOF (F2) | 1.053 |
| θ range for data collection (◦) | 2.9 to 72.5 |
| R1 | 0.0383 |
| wR2 | 0.1038 |
| Nr | D-H…A | D-H [Å] | H…A [Å] | D…A [Å] | <(DHA) | Symmetry Codes |
|---|---|---|---|---|---|---|
| 1 | C34-H34B…O4 | 0.98 | 2.39 | 3.305(4) | 155.5 | −x + 1, y + 1/2, −z + 1/2 |
| 2 | C34-H34C…S1 | 0.98 | 2.98 | 3.517(3) | 116.0 | x − 3/2, −y + 5/2, −z |
| 3 | C38-H38A…O2 | 0.99 | 2.59 | 3.299(4) | 128.6 | x + 3/2, −y + 5/2, −z |
| 4 | C44-H44…O6 | 0.95 | 2.65 | 3.460(4) | 143.2 | x − 1/2, −y + 5/2, −z |
| 5 | C43-H43…N2 | 0.95 | 2.63 | 3.450(4) | 144.7 | −1 + x,y,z |
| Contacts | Contribution (%) |
|---|---|
| C…H | 8.0 |
| N…H | 7.5 |
| O…H | 16.5 |
| S…H | 3.9 |
| S…O | 0.3 |
| N…C | 0.8 |
| C…C | 0.2 |
| Parameters | 6-311G+(d,p) |
|---|---|
| Compound 4 | |
| SCF Energy (kcal/mol) | −74,208.4052 |
| Field independent dipole moment (Debye) | |
| μx | −4.4518 |
| μy | −1.8292 |
| μz | 3.4166 |
| μtotal | 5.9024 |
| Fourier molecular orbital energies (eV) | |
| EHOMO | −6.900 |
| ELUMO | −1.412 |
| ΔELUMO-EHOMO | 5.488 |
| Global reactivity descriptors (eV) | |
| Ionization potential (I) | 6.901 |
| Electron affinity (A) | 1.416 |
| Hardness (η) | 2.742 |
| Chemical potential (μ) | −4.158 |
| Electronegativity (ϰ) | 4.158 |
| Electrophilicity index (ω) | 3.152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Jastrzębska, M.; Książek, M. Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene. Crystals 2022, 12, 422. https://doi.org/10.3390/cryst12030422
Bębenek E, Kadela-Tomanek M, Chrobak E, Jastrzębska M, Książek M. Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene. Crystals. 2022; 12(3):422. https://doi.org/10.3390/cryst12030422
Chicago/Turabian StyleBębenek, Ewa, Monika Kadela-Tomanek, Elwira Chrobak, Maria Jastrzębska, and Maria Książek. 2022. "Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene" Crystals 12, no. 3: 422. https://doi.org/10.3390/cryst12030422
APA StyleBębenek, E., Kadela-Tomanek, M., Chrobak, E., Jastrzębska, M., & Książek, M. (2022). Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene. Crystals, 12(3), 422. https://doi.org/10.3390/cryst12030422






