Electrical and Structural Properties of CVD-Graphene Oxidized Using KMnO4/H2SO4 Solution
Abstract
:1. Introduction
2. Materials and Methods
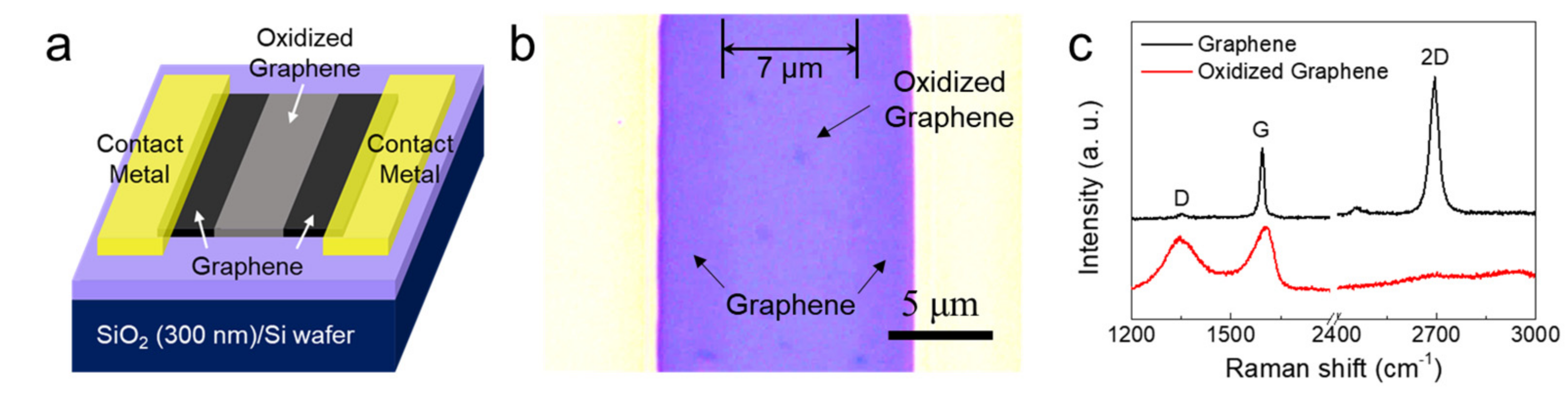
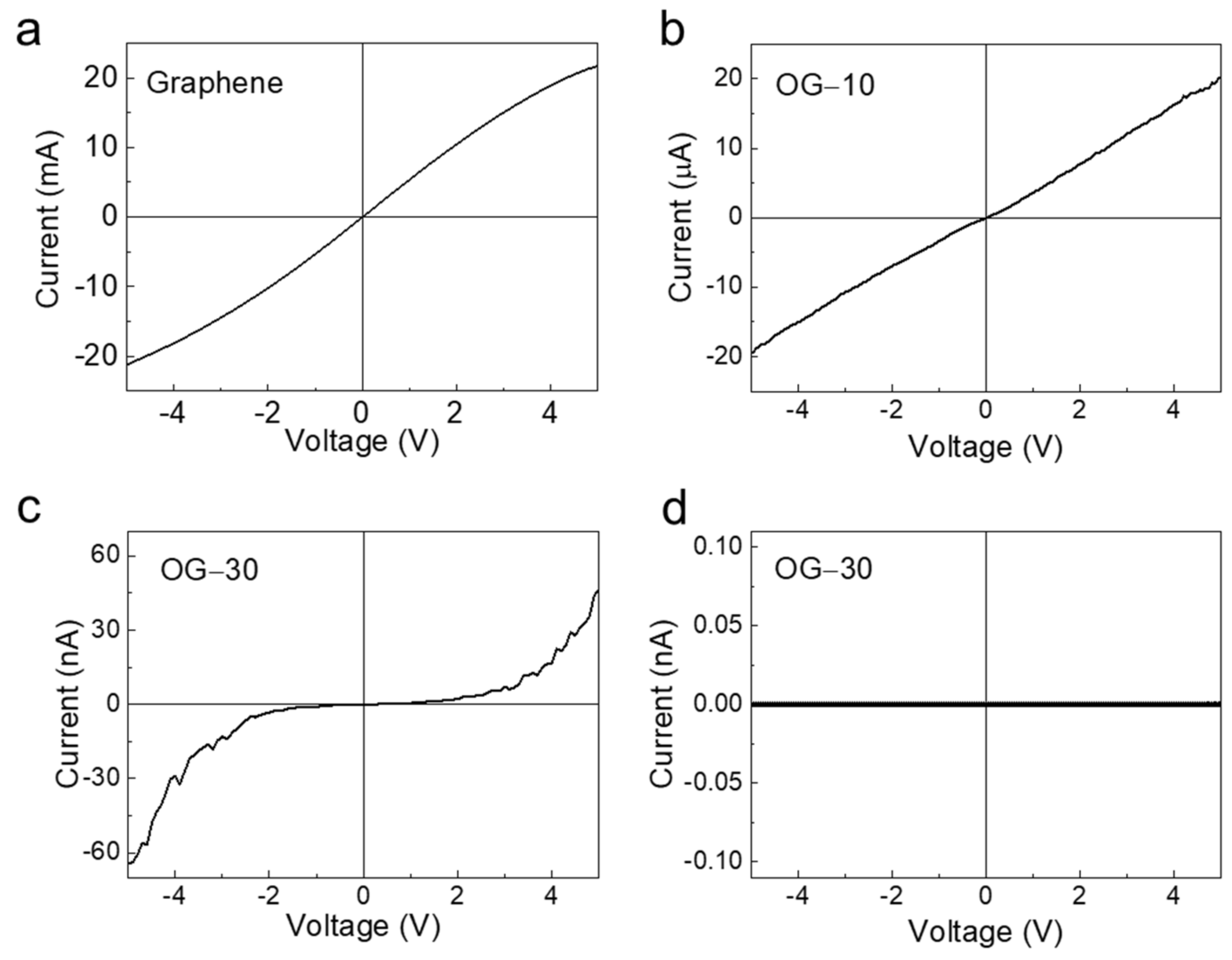
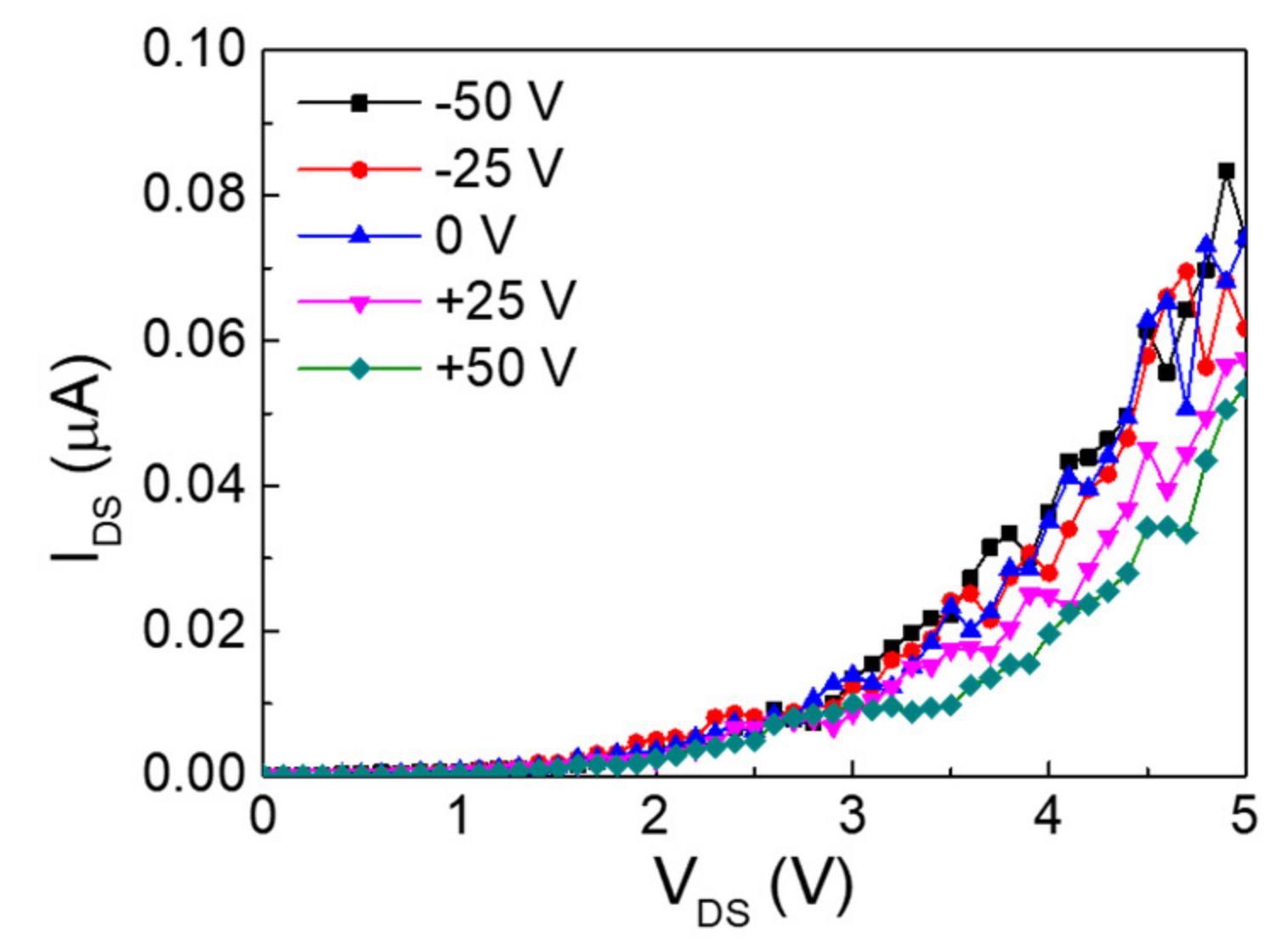
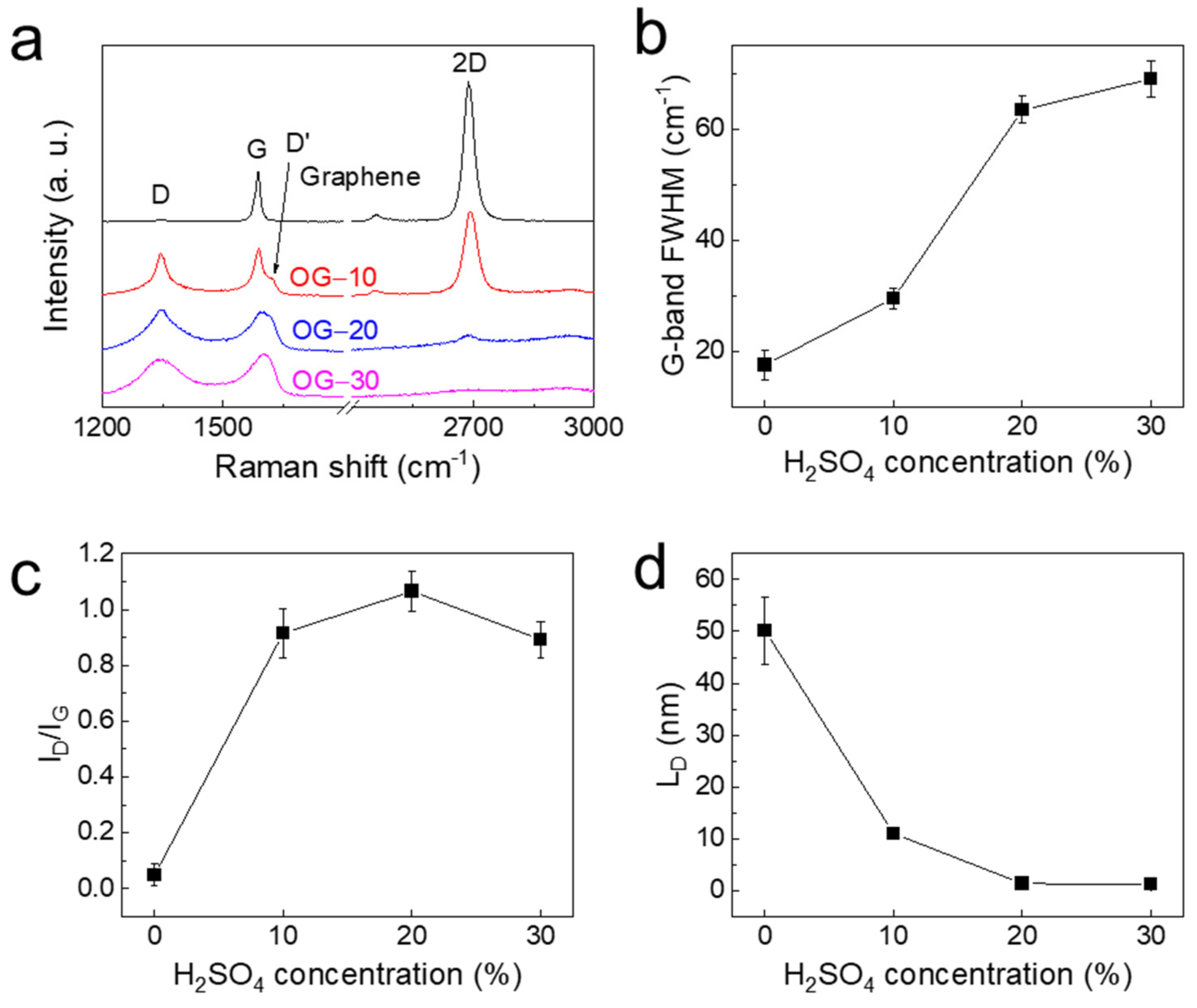
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Jin, M.; Jeong, H.-K.; Yu, W.J.; Bae, D.J.; Kang, B.R.; Lee, Y.H. Graphene oxide thin film field effect transistors without reduction. J. Phys. D. Appl. Phys. 2009, 42, 135109. [Google Scholar] [CrossRef]
- Eda, G.; Ball, J.; Mattevi, C.; Acik, M.; Artiglia, L.; Granozzi, G.; Chabal, Y.; Anthopoulos, T.D.; Chhowalla, M. Partially oxidized graphene as a precursor to graphene. J. Mater. Chem. 2011, 21, 11217–11223. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Suhaimin, N.S.; Hanifah, M.F.R.; Azhar, M.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N.; et al. The evolution of oxygen-functional groups of graphene oxide as a function of oxidation degree. Mater. Chem. Phys. 2022, 278, 125629. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Il Song, Y.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-C.; Lee, H.U.; Lee, K.; Kim, B.; Lee, S.Y.; Choi, M.-H.; Farooq, W.; Choi, J.S.; Park, J.-Y.; Lee, J.; et al. Aminoclay-conjugated TiO2 synthesis for simultaneous harvesting and wet-disruption of oleaginous Chlorella sp. Chem. Eng. J. 2014, 245, 143–149. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, W.J.; Liu, L.; Cheng, R.; Chen, Y.; Huang, X.; Liu, Y.; Wang, Y.; Huang, Y.; Duan, X. Chemical vapour deposition growth of large single crystals of monolayer and bilayer graphene. Nat. Commun. 2013, 4, 2096. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Lin, J.; Peng, Z.; Sun, Z.; Zhu, Y.; Li, L.; Xiang, C.; Samuel, E.L.; Kittrell, C.; Tour, J.M. Toward the synthesis of wafer-scale single-crystal graphene on copper foils. ACS Nano 2012, 6, 9110–9117. [Google Scholar] [CrossRef]
- Islam, A.E.; Kim, S.S.; Rao, R.; Ngo, Y.; Jiang, J.; Nikolaev, P.; Naik, R.; Pachter, R.; Boeckl, J.; Maruyama, B. Photo-thermal oxidation of single layer graphene. RSC Adv. 2016, 6, 42545–42553. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, L.-P.; Pei, S.; Du, J.; Su, Y.; Ren, W.; Cheng, H.-M. Tuning the electrical and optical properties of graphene by ozone treatment for patterning monolithic transparent electrodes. ACS Nano 2013, 7, 4233–4241. [Google Scholar] [CrossRef]
- Aria, A.I.; Gani, A.W.; Gharib, M. Effect of dry oxidation on the energy gap and chemical composition of CVD graphene on nickel. Appl. Surf. Sci. 2014, 293, 1–11. [Google Scholar] [CrossRef]
- Mao, H.; Wang, R.; Zhong, J.; Zhong, S.; Chen, W. Mildly O2 plasma treated CVD graphene as a promising platform for molecular sensing. Carbon N. Y. 2014, 76, 212–219. [Google Scholar] [CrossRef]
- Liu, L.; Ryu, S.; Tomasik, M.R.; Stolyarova, E.; Jung, N.; Hybertsen, M.S.; Steigerwald, M.L.; Brus, L.E.; Flynn, G.W. Graphene oxidation: Thickness-dependent etching and strong chemical doping. Nano Lett. 2008, 8, 1965–1970. [Google Scholar] [CrossRef] [Green Version]
- Zandiatashbar, A.; Lee, G.-H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Jeon, D.-Y.; Chung, H.-J.; Woo, Y.; Shin, J.K.; Seo, S. The structural and electrical evolution of graphene by oxygen plasma-induced disorder. Nanotechnology 2009, 20, 375703. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Jia, S.; Sun, H.D.; Du, J.H.; Zhang, Z.K.; Zhang, D.D.; Ma, L.P.; Chen, J.S.; Ma, D.G.; Cheng, H.M.; Ren, W.C. Graphene oxide/graphene vertical heterostructure electrodes for highly efficient and flexible organic light emitting diodes. Nanoscale 2016, 8, 10714–10723. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sprinkle, M.; Li, X.; Ming, F.; Berger, C.; de Heer, W.A. Epitaxial-graphene/graphene-oxide junction: An essential step towards epitaxial graphene electronics. Phys. Rev. Lett. 2008, 101, 26801. [Google Scholar] [CrossRef] [Green Version]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; La Magna, A.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Agnello, S. Effect of air on oxygen p-doped graphene on SiO2. Phys. Status Solidi 2016, 213, 2341–2344. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon N. Y. 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhang, M.; Yao, B.; Li, Y.; Huang, L.; Li, C.; Shi, G. Water-enhanced oxidation of graphite to graphene oxide with controlled species of oxygenated groups. Chem. Sci. 2016, 7, 1874–1881. [Google Scholar] [CrossRef] [Green Version]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 32183. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon N. Y. 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Hill, R.M. Hopping conduction in amorphous solids. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1971, 24, 1307–1325. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in non-crystalline materials. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1969, 19, 835–852. [Google Scholar] [CrossRef]
- Sun, Y.; Kirimoto, K.; Hattori, H.; Kitamura, Y.; Fan, E.; Onishi, K. Electric field and oxygen concentration-dependent transport properties of nano-graphene oxide. AIP Adv. 2019, 9, 95010. [Google Scholar] [CrossRef]

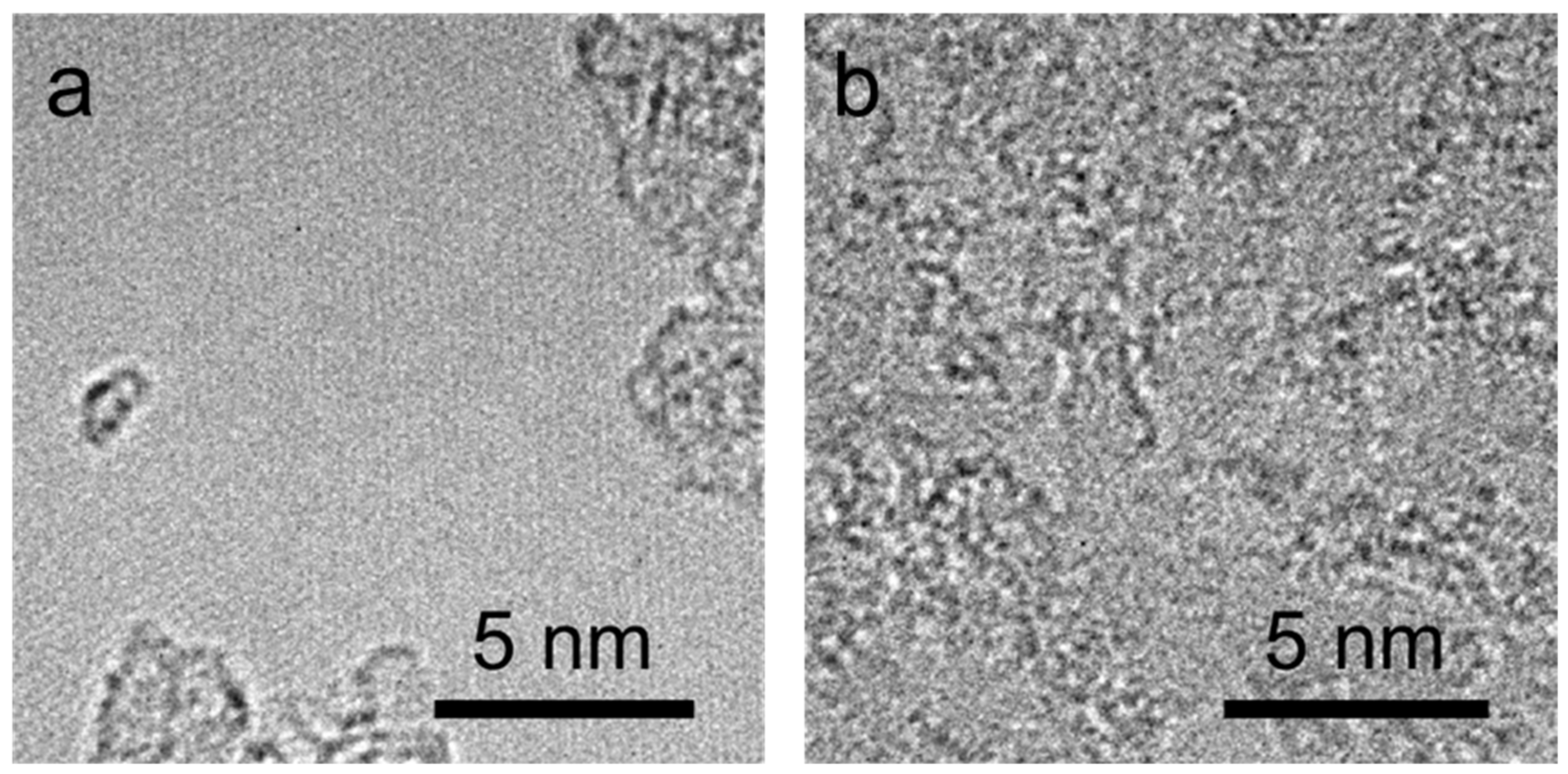
| Chemical Bonds | Graphene | OG-10 | OG-20 | OG-30 |
|---|---|---|---|---|
| Graphitic carbon (%) | 80.38 | 77.06 | 62.59 | 54.33 |
| Oxidized carbon (%) | 19.62 | 22.94 | 37.41 | 45.67 |
| IOC/IGC | 0.24 | 0.30 | 0.60 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-S.; Im, K.-S.; Lee, T.-K.; Choi, Y.-J.; An, S.-J. Electrical and Structural Properties of CVD-Graphene Oxidized Using KMnO4/H2SO4 Solution. Crystals 2022, 12, 439. https://doi.org/10.3390/cryst12040439
Choi J-S, Im K-S, Lee T-K, Choi Y-J, An S-J. Electrical and Structural Properties of CVD-Graphene Oxidized Using KMnO4/H2SO4 Solution. Crystals. 2022; 12(4):439. https://doi.org/10.3390/cryst12040439
Chicago/Turabian StyleChoi, Jin-Seok, Ki-Sik Im, Tae-Kyun Lee, Yeo-Jin Choi, and Sung-Jin An. 2022. "Electrical and Structural Properties of CVD-Graphene Oxidized Using KMnO4/H2SO4 Solution" Crystals 12, no. 4: 439. https://doi.org/10.3390/cryst12040439
APA StyleChoi, J.-S., Im, K.-S., Lee, T.-K., Choi, Y.-J., & An, S.-J. (2022). Electrical and Structural Properties of CVD-Graphene Oxidized Using KMnO4/H2SO4 Solution. Crystals, 12(4), 439. https://doi.org/10.3390/cryst12040439





