Optical Temperature Sensor Capabilities of the Green Upconverted Luminescence of Er3+ in La3NbO7 Ceramic Powders
Abstract
:1. Introduction
2. Materials and Methods
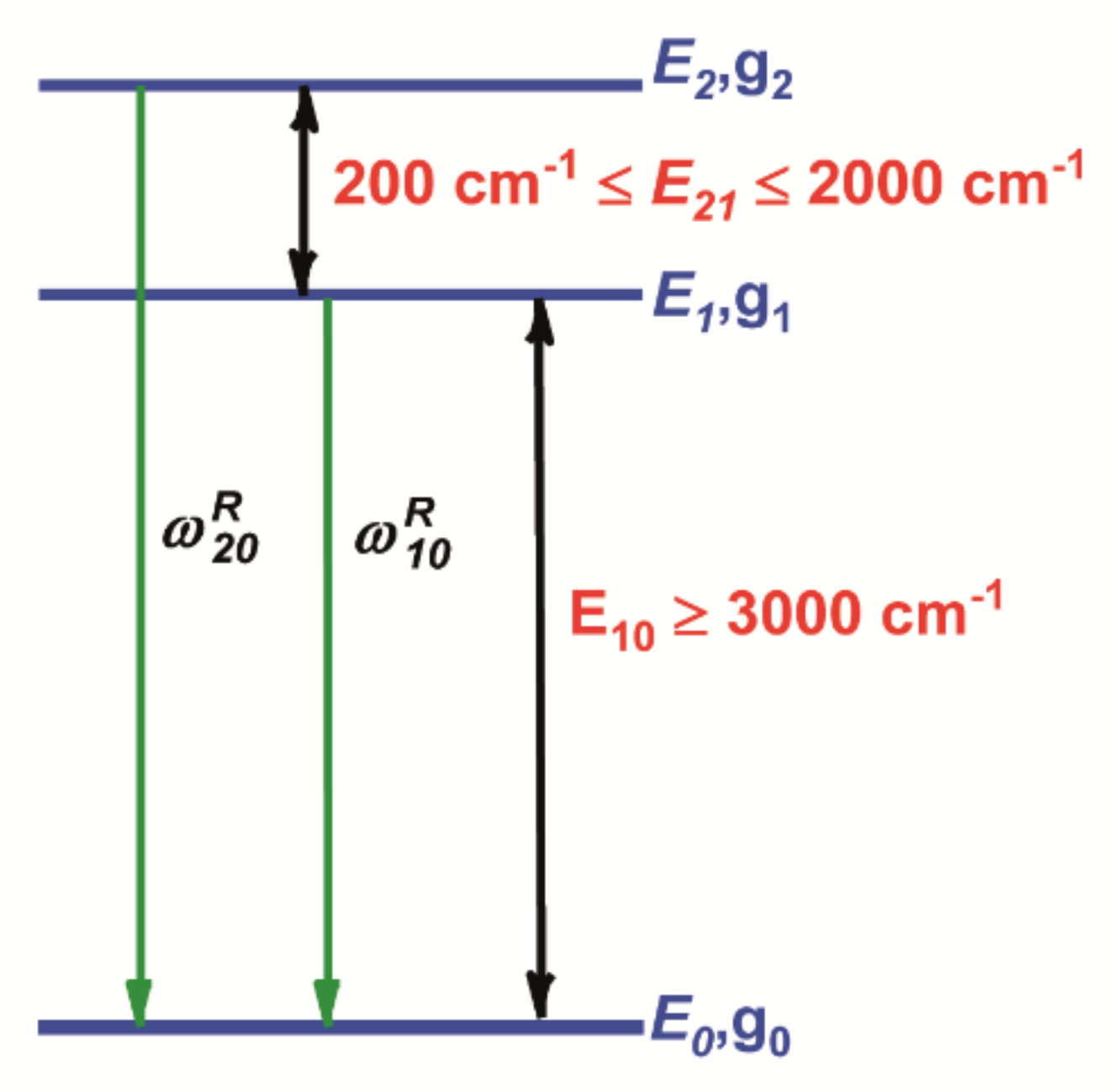
Fluorescence Intensity Ratio (FIR) Technique
3. Results and Discussion
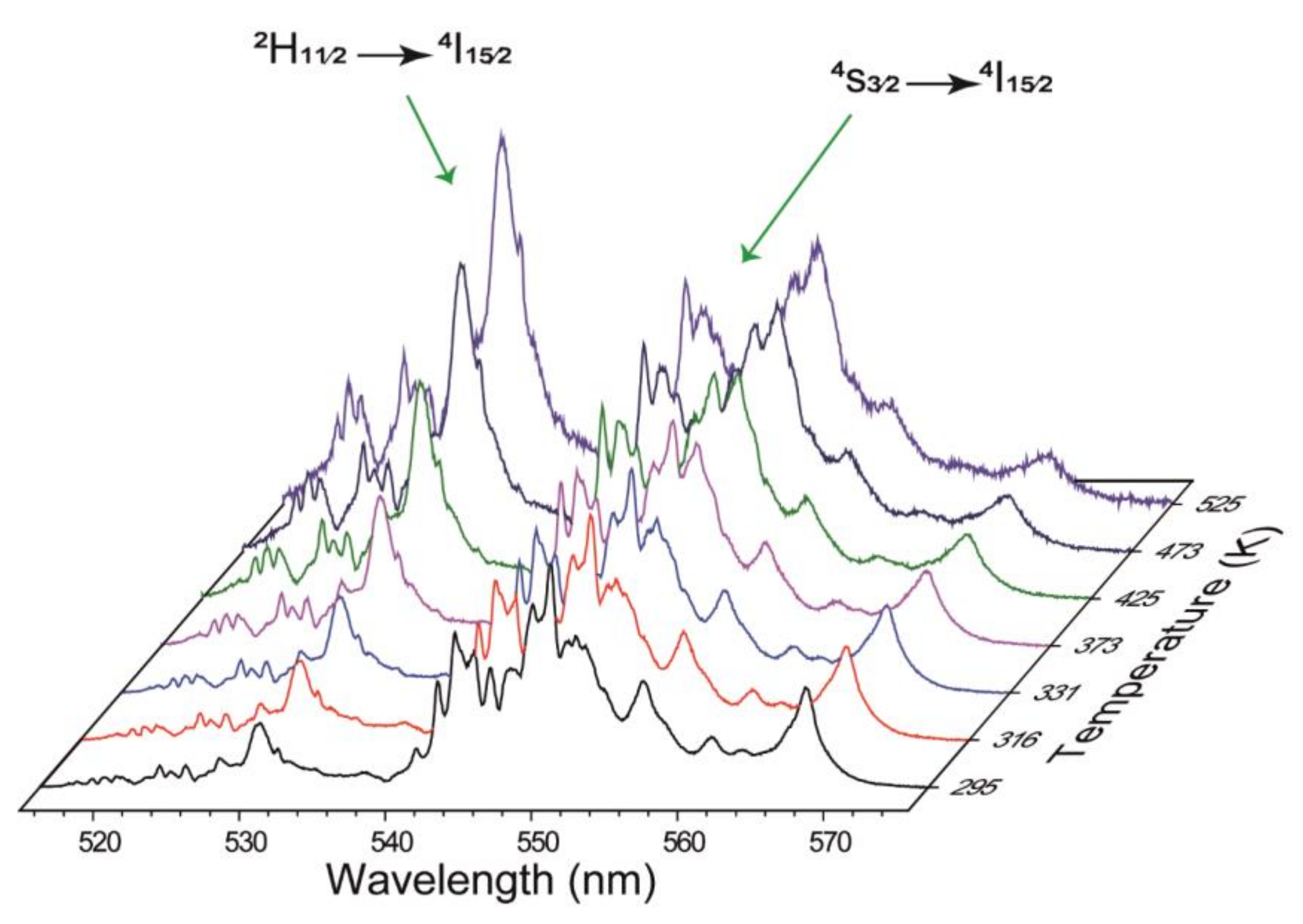
3.1. Luminescence
3.2. Optical Sensor Calibration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Cui, Y.; Hu, Y.; Zheng, L.; Zhang, Z.; Cao, W. Optical temperature sensing in Er3+-Yb3+ codoped CaWO4 and the laser induced heating effect on the luminescence intensity saturation. J. Alloy. Compd. 2017, 726, 547–555. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale using lanthanide-containing organic-inorganic hybrid materials. J. Lumin. 2013, 133, 230–232. [Google Scholar] [CrossRef] [Green Version]
- McLaurin, E.; Bradshaw, L.R.; Gamelin, D.R. Dual-Emitting Nanoscale Temperature Sensors. Chem. Mater. 2013, 25, 1283–1292. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarron, A.; de la Fuente, A.J.; Sanz-Rodriguez, F.; Maestro, L.M.; Rodriguez, E.M.; Jaque, D.; García Solé, J.; Capobianco, J.A. Temperature sensing using fluorescent nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef]
- Sun, M.; He, Q.; Kuang, X.; Zhang, Q.; Ye, S.; Huang, B. Probing oxide-ion conduction in low-temperature SOFCs. Nano Energy 2018, 50, 88–96. [Google Scholar] [CrossRef]
- Dos Santos, P.V.; De Araujo, M.T.; Gouveia-Neto, A.S.; Medeiros Neto, J.A.; Sombra, A.S.B. Optical temperature sensing using upconversion fluorescence emission in Er3+/Yb3+-codoped chalcogenide glass. Appl. Phys. Lett. 1998, 73, 578–580. [Google Scholar] [CrossRef]
- Kusama, H.; Sovers, O.J.; Yoshioka, T. Line Shift Method for Phosphor Temperature Measurements. Jpn. J. Appl. Phys. 1976, 15, 2349–2358. [Google Scholar] [CrossRef]
- Wade, S.; Collins, S.F.; Baxter, G. Fluorescence intensity ratio technique for optical fiber point temperature sensing. J. Appl. Phys. 2003, 94, 4743–4756. [Google Scholar] [CrossRef]
- Alencar, M.A.R.C.; Maciel, G.S.; de Araújo, C.B.; Patra, A. Er3+-doped BaTiO3 nanocrystals for thermometry: Influence of nanoenvironment on the sensitivity of a fluorescence based temperature sensor. Appl. Phys. Lett. 2004, 84, 4753–4755. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Rodriguez-Mendoza, U.; Lalla, E.; Lavín, V. Temperature sensor based on the Er3+ green upconverted emission in a fluorotellurite glass. Sens. Actuators B Chem. 2011, 158, 208–213. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Lozano-Gorrín, A.D.; Martín, I.R.; Rodríguez-Mendoza, U.R.; Lavín, V. Comparison of the sensitivity as optical temperature sensor of nano-perovskite doped with Nd3+ ions in the first and second biological windows. Sens. Actuators B Chem. 2018, 255, 970–976. [Google Scholar] [CrossRef]
- Hernández-Rodriguez, M.A.; Lozano-Gorrín, A.D.; Lavín, V.; Rodríguez-Mendoza, U.R.; Martín, I.R.; Manjón, F.J. Analysis of the upconversion emission of yttrium orthoaluminate nano-perovskite co-doped with Er3+/Yb3+ions for thermal sensing applications. J. Lumin. 2018, 202, 316–321. [Google Scholar] [CrossRef]
- Runowski, M.; Bartkowiak, A.; Majewska, M.; Martin, I.R.; Lis, S. Upconverting lanthanide doped fluoride NaLuF4:Yb3+-Er3+-Ho3+-optical sensor for multi-range fluorescence intensity ratio (FIR) thermometry in visible and NIR regions. J. Lumin. 2018, 201, 104–109. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Lozano-Gorrín, A.D.; Lavín, V.; Rodríguez-Mendoza, U.R.; Martín, I.R. Yttrium orthoaluminate nanoperovskite doped with Tm3+ ions as upconversion optical temperature sensor in the near-infrared region. Opt. Express 2017, 25, 27845–27856. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, L.; de Sousa-Vieira, L.; Hernández-Rodriguez, M.; Lozano-Gorrín, A.; Lavín, V.; Rodríguez-Mendoza, U.; González-Platas, J.; Ríos, S.; Martín, I. Nanoperovskite doped with Yb3+ and Tm3+ ions used as an optical upconversion temperature sensor. Opt. Mater. 2018, 83, 187–191. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Lozano-Gorrín, A.; Martín, I.; Rodríguez-Mendoza, U.; Lavín, V. Spectroscopic properties of Nd3+ ions in YAP nano-perovskites. J. Lumin. 2017, 188, 204–208. [Google Scholar] [CrossRef]
- Lozano-Gorrín, A.D.; Rodríguez-Mendoza, U.R.; Venkatramu, V.; Monteseguro, V.; Hernández-Rodríguez, M.A.; Martín, I.R.; Lavín, V. Lanthanide-doped Y3Ga5O12 garnets for nanoheating and nanothermometry in the first biological window. Opt. Mater. 2018, 84, 46–51. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Muñoz-Santiuste, J.E.; Lavín, V.; Lozano-Gorrín, A.D.; Rodríguez-Hernández, P.; Muñoz, A.; Venkatramu, V.; Martín, I.R.; Rodríguez-Mendoza, U.R. High pressure luminescence of Nd3+ in YAlO3 perovskite nanocrystals: A crystal-field analysis. J. Chem. Phys. 2018, 148, 044201. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Rodríguez-Mendoza, U.; Lavín, V.; Muñoz-Santiuste, J.; Martín, I.; Lozano-Gorrín, A. High pressure sensitivity of anti-Stokes fluorescence in Nd3+ doped yttrium orthoaluminate nano-perovskites. J. Lumin. 2018, 196, 20–24. [Google Scholar] [CrossRef]
- Cai, L.; Nino, J.C. Structure and dielectric properties of Ln 3NbO7 (Ln = Nd, Gd, Dy, Er, Yb and Y). J. Eur. Ceram. Soc. 2007, 27, 3971–3976. [Google Scholar] [CrossRef]
- Abe, R.; Higashi, M.; Sayama, K.; Abe, Y.; Sugihara, H. Photocatalytic Activity of R3MO7 and R2Ti2O7 (R = Y, Gd, La; M = Nb, Ta) for Water Splitting into H2 and O2. J. Phys. Chem. B 2006, 110, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Wakeshima, M.; Nishimine, H.; Hinatsu, Y. Crystal structures and magnetic properties of rare earth tantalates RE3TaO7(RE = rare earths). J. Phys. Condens. Matter 2004, 16, 4103–4120. [Google Scholar] [CrossRef]
- Lia, Y.; Xub, Q.; Dai, L. A Calcination Technology for Ultrafine La3NbO7 Powder Prepared by Sol-Gel Process. Adv. Mater. Res. 2012, 412, 271–274. [Google Scholar] [CrossRef]
- Preux, N.; Rolle, A.; Merlin, C.; Benamira, M.; Malys, M.; Estournes, C.; Rubbens, A.; Vannier, R.-N. La3TaO7 derivatives with Weberite structure type: Possible electrolytes for solid oxide fuel cells and high temperature electrolysers. Comptes Rendus Chim. 2010, 13, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Toyoura, K.; Nakamura, A.; Matsunaga, K. First-principles analysis on proton diffusivity in La3NbO7. Solid State Ionics 2014, 262, 472–475. [Google Scholar] [CrossRef]
- Cai, L.; Nino, J.C. Complex ceramic structures. I. Weberites. Acta Cryst. B 2009, 65, 269–290. [Google Scholar] [CrossRef]
- Egaña, A.; Cantelar, E.; Tardío, M.; Muñoz Santiuste, J.E. Synthesis and luminescence properties of Er3+ doped La3NbO7 ceramic powder. Opt. Mater. 2019, 97, 109393. [Google Scholar] [CrossRef]
- Dai, L.; Xu, Q.; Zhu, S.Z.; Liu, L. Preparation of Ultra–Fine La3NbO7 Powder by Solid State Reaction. Key Eng. Mater. 2012, 512–515, 158–161. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Rodriguez-Mendoza, U.; Gonzalez, P.H.; Martin, I.R.; Lavin, V. Role of the host matrix on the thermal sensitivity of Er3+ luminescence in optical temperature sensors. Sens. Actuators B Chem. 2012, 174, 176–186. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Rodriguez-Mendoza, U.; Martin, I.R.; Lalla, E.; Lavin, V. Effects of Er3+ concentration on thermal sensitivity in optical temperature fluorotellurite glass sensors. Sens. Actuators B Chem. 2013, 176, 1167–1175. [Google Scholar] [CrossRef]
- Venkatramu, V.; León-Luis, S.F.; Rodríguez-Mendoza, U.R.; Monteseguro, V.; Manjón, F.J.; Lozano-Gorrín, A.D.; Valiente, R.; Navarro-Urrios, D.; Jayasankar, C.K.; Muñoz, A.; et al. Synthesis, structure and luminescence of Er3+-doped Y3Ga5O12 nano-garnets. J. Mater. Chem. 2012, 22, 13788–13799. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Milla, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.V.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Sung Lim, C.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Atuchin, V. Microwave sol–gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4: Er3+/Yb3+ phosphors with incommensurately modulated structure. J. Solid State Chem. 2015, 228, 160–166. [Google Scholar]
- Sung Lim, C.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Atuchin, V.V. Microwave synthesis and spectroscopic properties of ternary scheelite-type molybdate phosphors NaSrLa(MoO4)3:Er3+,Yb3+. J. Alloys Compd. 2017, 713, 156–163. [Google Scholar]
- Peacock, R.D. The Intensities of Lanthanide f-f Transitions. Struct. Bond. 1975, 22, 83–122. [Google Scholar]
- Zhao, H.; Zhang, Z.; Qin, F.; Li, L.; Zheng, Y.; Zhou, Y. Using the upconversion luminescence of the CaWO4 :Yb3+ − X3+ (X = Er/Ho/Tm) phosphors for ratiometric thermal sensing. J. Lumin. 2018, 202, 301–308. [Google Scholar]
- Soni, A.K.; Rai, V.K.; Mahata, M.K. Yb3+ sensitized Na2Y2B2O7: Er3+ phosphors in enhanced frequency upconversion, temperature sensing and field emission display. Mater. Res. Bull. 2017, 89, 116–124. [Google Scholar] [CrossRef]
- Liu, X.; Lei, R.; Huang, F.; Deng, D.; Wang, H.; Zhao, S.; Xu, S. Dependence of upconversion emission and optical temperature sensing behavior on excitation power in Er3+ /Yb3+ co-doped BaMoO 4 phosphors. J. Lumin. 2019, 210, 119–127. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, C.; Liu, Y.; Gao, G.; Liu, D.; Wang, T.; Liu, T.; Zhang, Y. Optical thermometry through infrared excited green upconversion of KLa(MoO4)2:Yb3+/Er3+ phosphor. J. Lumin. 2019, 207, 93–97. [Google Scholar] [CrossRef]
- Liu, G.; Wu, L.; Wei, X.; Zhang, D.; Hu, L. Investigation on laser-induced heating in NaYbF4:Er3+ for accurate photo-thermal conversion with temperature feedback. Opt. Commun. 2018, 426, 418–422. [Google Scholar] [CrossRef]
- Du, P.; Luo, L.; Li, W.; Yue, Q.; Chen, H. Optical temperature sensor based on upconversion emission in Er-doped ferroelectric 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3 ceramic. Appl. Phys. Lett. 2014, 104, 2–6. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Zhang, Z.; Shio, R.; Wu, Y.; Xiang, J.; Guo, C. Local Symmetric Distortion Boosted Photon Up-conversion and Thermometric Sensitivity in lanthanum Oxide Nanospheres. Nanoscale 2018, 10, 9245–9251. [Google Scholar] [CrossRef]
- Jin, Y.; Pang, T. Highly efficient green upconversion luminescence of ZnMoO4:Yb3+/Er3+/Li+ for accurate temperature sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 306–312. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Monteseguro, V.; Rodríguez-Mendoza, U.R.; Rathaiah, M.; Venkatramu, V.; Lozano-Gorrín, A.D.; Valiente, R.; Muñoz, A.; Lavín, V. Optical nanothermometer based on the calibration of the Stokes and upconverted green emissions of Er3+ ions in Y3Ga5O12 nano-garnets. RSC Adv. 2014, 4, 57691–57701. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qin, F.; Zhou, Y.; Zheng, Y.; Zhao, H.; Zhang, Z. Temperature sensing based on the 4F7/2/4S3/2−4I15/2 upconversion luminescence intensity ratio in NaYF4:Er3+/Yb3+ nanocrystals. J. Lumin. 2019, 206, 335–341. [Google Scholar] [CrossRef]
- Leal, J.J.; Narro-García, R.; Flores-De los Ríos, J.P.; Gutierrez-Mendez, N.; Ramos-Sánchez, V.H.; González-Castillo, J.R.; Rodríguez, E. Effect of TiO2 on the thermal and optical properties of Er3+/Yb3+ co-doped tellurite glasses for optical sensor. J. Lumin. 2019, 208, 342–349. [Google Scholar] [CrossRef]
- Du, P.; Luo, L.; Li, W.; Yue, Q. Upconversion emission in Er-doped and Er/Yb-codoped ferroelectric Na0.5Bi0.5TiO3 and its temperature sensing application. J. Alloys Compd. 2014, 116, 014102–014107. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X. Ultraviolet and infrared photon-excited synergistic effect in Er3+−doped YbF3+ phosphors. Opt. Lett. 2011, 36, 4353–4355. [Google Scholar] [CrossRef]


| Er3+ Doped and Er3+-Yb3+ Based Host Matrix | λexc (nm) | T Range (K) | FIR Equation | SREL (%K−1) at 303 K | Ref. |
|---|---|---|---|---|---|
| BZT-BCT: Er3+ ceramic | 980 | 200–443 | 9.97•exp(−1135/T) | 1.24 | [44] |
| Y2O3: Er3+-Yb3+ nanoparticles | 980 | 273–480 | 13.30•exp(−1131/T) | 1.23 | [45] |
| ZnMoO4:Yb3+-Er3+-Li+ | 980 | 300–400 | 14.27•exp(−1111/T) | 1.21 | [46] |
| NaYbF4: Er3+ | 980 | 293–593 | 10.78•exp(−1098/T) | 1.20 | [43] |
| La3-xNbO7: xEr3+ (x = 0.1) phosphor | 975 | 295–525 | 5.96•exp(−1097/T) | 1.19 | This work |
| KLa(MoO4)2: Yb3+-Er3+ phosphor | 980 | 303–423 | 21.95•exp(−1079/T) | 1.18 | [42] |
| YAP: Er3+-Yb3 nanoperovskites | 980 | 296–600 | 5.77•exp(−1073/T) | 1.17 | [13] |
| Y3Ga5O12: Er3+ nanoparticles | 488/800 | 300–335 | 11.04•exp(−1047/T) | 1.16 | [47] |
| NaYF4: Er3+-Yb3+ nanocrystals | 980 | 303–483 | 30.80•exp(−1034/T) | 1.13 | [48] |
| BaMoO4: Er3+-Yb3+ phosphors | 980 | 300–575 | 20.06•exp(−980/T) | 1.07 | [41] |
| Tellurite glass: Er3+-Yb3 (7.5 mol% TiO2) | 785 | 318–431 | 8.10•exp(−962/T) | 1.05 | [49] |
| CaWO4: Er3+-Yb3+ phosphors | 980 | 303–753 | 17.20•exp(−944/T) | 1.02 | [39] |
| Chalcogenide glass: Er3+-Yb3 | 1060 | 296–438 | 8.85•exp(−962/T) | 1.01 | [7] |
| Na0.5Bi0.5TiO3: Er3+-Yb3+ ceramic | 980 | 93–613 | 4.71•exp(−827/T) | 0.90 | [50] |
| Na2Y2B2O7: Er3+-Yb3+ phosphor | 980 | 300–613 | 27.60•exp(−766/T) | 0.83 | [40] |
| NaLuF4:Yb3+-Er3+-Ho3+ | 975 | 298–503 | 1.46•exp(−654/T) | 0.71 | [14] |
| Silicate glass: Er3+-Yb3+ | 980 | 296–723 | 3.65•exp(−593/T) | 0.64 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rodríguez, M.A.; Egaña, A.; Rodríguez-Mendoza, U.R.; Lavín, V.; Muñoz-Santiuste, J.E. Optical Temperature Sensor Capabilities of the Green Upconverted Luminescence of Er3+ in La3NbO7 Ceramic Powders. Crystals 2022, 12, 455. https://doi.org/10.3390/cryst12040455
Hernández-Rodríguez MA, Egaña A, Rodríguez-Mendoza UR, Lavín V, Muñoz-Santiuste JE. Optical Temperature Sensor Capabilities of the Green Upconverted Luminescence of Er3+ in La3NbO7 Ceramic Powders. Crystals. 2022; 12(4):455. https://doi.org/10.3390/cryst12040455
Chicago/Turabian StyleHernández-Rodríguez, Miguel A., Alvaro Egaña, Ulises R. Rodríguez-Mendoza, Victor Lavín, and Juan E. Muñoz-Santiuste. 2022. "Optical Temperature Sensor Capabilities of the Green Upconverted Luminescence of Er3+ in La3NbO7 Ceramic Powders" Crystals 12, no. 4: 455. https://doi.org/10.3390/cryst12040455
APA StyleHernández-Rodríguez, M. A., Egaña, A., Rodríguez-Mendoza, U. R., Lavín, V., & Muñoz-Santiuste, J. E. (2022). Optical Temperature Sensor Capabilities of the Green Upconverted Luminescence of Er3+ in La3NbO7 Ceramic Powders. Crystals, 12(4), 455. https://doi.org/10.3390/cryst12040455







