Abstract
Kenoargentotetrahedrite-(Fe) is observed as greenish-grey anhedral grains, 50–150 μm in size, in association with galena, sphalerite and chalcopyrite in the Bajiazi Pb-Zn deposit of magmatic-hydrothermal type, Liaoning, China. The empirical formula from electron microprobe analyses is Ag5.50Cu4.17Fe1.75Zn0.31Sb3.96As0.04S12.08, corresponding to the ideal formula Ag6Cu4Fe2Sb4S12. The crystal structure of kenoargentotetrahedrite-(Fe) has been determined and refined by single-crystal X-ray diffraction with R1 = 0.0192 for 1866 (404 unique) reflections. It is cubic, space group I3m with unit cell parameters a = 10.4928(2) Å, V = 1155.26(7) Å3 and Z = 2. The structure of kenoargentotetrahedrite-(Fe) is characterized by a poor occupancy of 0.05 of the octahedral S(2) site with the S(2)-M(2) bonding length of 1.9994(8) Å. The six Ag atoms at M(2) around S(2) form an octahedron cluster (Ag6)4+ with the valence state of +4 and Ag-Ag distance of 2.8276(1) Å. The structure is identical to that by Rozhdestvenskaya et al., being composed of a collapsed sodalite-like framework of corner-connected M(1)S4 tetrahedron forming cages containing M(2)6-octahedron cluster, encircled by four SbS3 trigonal pyramids. It is related to the tetrahedrite group minerals with the existence of the (Ag6)4+ cluster replacing the S(2)-centered Ag6 octahedron according to the substitution mechanism 6M(2)Ag+ + S(2)S2−=M(2)(Ag6)4+ + S(2) S.
1. Introduction
Tetrahedrite, as a common sulfur salt mineral in nature, plays an important role in the study of the distribution of metallogenic elements. Theoretically, the standard structural chemical formula of tetrahedrite can be written as M(2)6M(1)6X(3)4S(1)12S(2) (Z = 2) [1]. The crystal structure of tetrahedrite was proposed by Machatschki [2,3], and the ideal chemical formula was Cu3SbS3. It was not until 1964 that a more precise crystal structure of tetrahedrite was given by a scholar [4]. Tetrahedrite is cubic, with space group symmetry I3m. The crystal structure could be described as a sulfidic sodalite-like architecture with Laves truncated tetrahedra holes [5]. The tetrahedrite can convert thermal energy into electricity because of its thermoelectric properties, and it contains many trace elements in nature, which may improve its thermoelectric properties.
The kenoargentotetrahedrite belongs to a mineral species of the tetrahedrite group. If the A in the tetrahedrite (M(2)A6M(1)(B4C2)X(3)D4S(1)Y12S(2)Z) is Ag+, that is, the chemical formula is Ag6Cu4Fe2Sb4S13, it can be called argentotetrahedrite. If A6 is (Ag6)4+ clusters and Z is a vacancy, that is, the chemical formula is Ag6Cu4Fe2Sb4S12, it can be called kenoargentotetrahedrite.
The definition and nomenclature of kenoargentotetrahedrite are highly controversial. Since the mid-1980s, the discussion of related terms has been uninterrupted, and it is often confused with silver-rich tetrahedrite and argentotetrahedrite. It took a long and complex process from the first proposal of the name of kenoargentotetrahedrite to the final determination of its formal official name [1]. The earliest name of kenoargentotetrahedrite was “Freibergite”, which was proposed by Kenngott when studying the silver-rich tetrahedrite from Freiberg in 1853 [6], but later researchers found that the “Freibergite” here is not a kenoargentotetrahedrite, but an argentotetrahedrite (Ag5.74Cu4.61Fe2.12Zn0.30Sb4.00S13.06) in the process of recalculating its chemical composition. Similarly, Peterson and Miller studied natural samples from Keno Hill, Yukon(Canada), and found the existence of argentotetrahedrite in 1986 [7]. The name used at that time was also “Freibergite”, which was confirmed by Welch et al. [8]. In 1993, Rozhdestvenskaya et al. reported the crystal structure of kenoargentotetrahedrite for the first time and found that when most or all of the copper at the M(2) site was replaced by silver, the S(2) site of the octahedral coordination would be vacant [9]. It was proposed that the ideal chemical formula of kenoargentotetrahedrite was Ag6(Cu4Fe2)Sb4S12, which was completely different from argentotetrahedrite. In 2018, Welch et al. refined the crystal structure of kenoargentotetrahedrite with Fe and Zn as divalent cations [8]. The name “Freibergite” was used for the kenoargentotetrahedrite in the above research work, but they are actually two different types of minerals, which would cause some confusion for future research. Therefore, in 2020, Cristian Biagioni et al. summarized the systematic nomenclature and classification of the tetrahedrite group minerals and named the minerals with the chemical formula Ag6(Cu4Me2)Sb4S13-x as “Freibergite”, which had the unknown occupancy rate of the S(2) site [1]. When x was equal to 0, the chemical formula was Ag6(Cu4Me2)Sb4S13, the mineral species was named “argentotetrahedrite-(Me)”. When x was equal to 1, the chemical formula was Ag6(Cu4Me2)Sb4S12, and the mineral species was named “kenoargentotetrahedrite-(Me)”, if Fe was the main component of Me, the mineral species could be precisely named “kenoargentotetrahedrite-(Fe)”, and this paper focuses on the study of this mineral. If Zn was the main component of Me, the mineral species could be precisely named “kenoargentotetrahedrite-(Zn)”. In the formal official name of “kenoargentotetrahedrite-(Fe)”, the prefix “keno-” means that the S(2) site is empty, the prefix “argento-” represents that the mineral is silver-rich, and the suffix “-(Fe)” indicates that the divalent cations at M(1) site are mainly Fe.
This paper will be the first detailed research and report on the kenoargentotetrahedrite-(Fe) after its official name. In this paper, the chemical composition and crystal structure of kenoargentotrtrahedrite-(Fe) were accurately tested by advanced rock and mineral testing and analysis technology, and a more detailed chemical composition and crystal structure data filled some vacancies in the crystal database, which is helpful for the interpretation of its crystal structure.
2. Samples and Experimental Methods
The kenoargentotetrahedrite-(Fe) samples were collected from the Bajiazi magmatic-hydrothermal Pb-Zn deposits in Liaoning Province, China (30°40′ N, 119°10′ E). The polished sections of the kenoargentotetrahedrite-(Fe) samples were observed using a Leica DM2500p microscope manufactured by the Shimadzu company of Japan (Figure 1). The kenoargentotetrahedrite-(Fe) is anhedral granular, and its particle size is 50–150 μm. It is sporadically distributed in quartz, pyrosmalite-(Mn) and fluorite, which is associated with galena, sphalerite and chalcopyrite. In plane-polarized light, kenoargentotetrahedrite-(Fe) is greenish-grey, and its reflectivity is between galena and sphalerite.
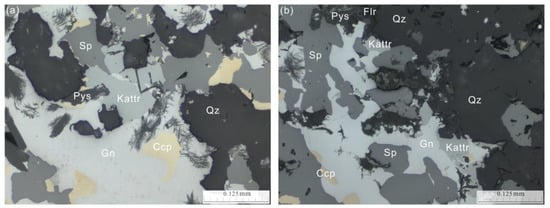
Figure 1.
Reflected-light photomicrographs of the polished sections which showing occurrences and mineral associations of kenoargentotetrahedrite-(Fe) (Kattr). (a) The greenish-grey grains of kenoargentotetrahedrite-(Fe) (Kattr) in plane-polarized light. (b) Anhedral grains of kenoargentotetrahedrite-(Fe) (Kattr) associated with galena (Gn), sphalerite (Sp) and chalcopyrite (Ccp) in quartz (Qz) and minor pyrosmalite-(Mn) (Pys) and fluorite (Flr).
The compositions of kenoargentotetrahedrite-(Fe) were analyzed with a Shimadzu-1720 electron probe microanalyzer (EPMA) at accelerating voltage 15 kV and beam size 1 μm. Pure materials of Ag and Cu, ZnS, FeS2, CdSe, Sb2S3, FeAsS and FeS2 were used as standards for the quantification of Ag(Lα), Cu(Kα), Zn(Kα), Fe(Kα), Cd(Lα), Sb(Lα), As(Lα) and S(Kα) using ZAF correction. The composition data listed in Table 1 were derived from analyzing nine positions on the kenoargentotetrahedrite-(Fe) sample.

Table 1.
Chemical data (wt. %) for kenoargentotetrahedrite-(Fe) (N = 9).
The single-crystal diffraction data were collected using a Rigaku XtaLAB Synergy-DW diffractometer with microfocus sealed Mo anode tubes at 50 kV and 1 mA. The specific experimental steps are as follows: (a) Under the stereoscope, the kenoargentotetrahedrite-(Fe) particle was picked out with a diamond knife, loaded into a 100 μm glass loop, and fixed with crystal oil. (b) The glass loop loaded with the kenoargentotetrahedrite-(Fe) particle was mounted on the sample, and the positions were adjusted in two vertical directions of 0° and 90° to ensure the crystal grain was in the center of the X-ray. (c) The pre-experiment was performed on the sample to obtain multiple crystal diffraction pictures. (d) According to the crystal size and X-ray tube intensity collected from the pre-experiment, the exposure time per frame was 20 s for the sample from Bajiazi to gather hundreds of diffraction data.
The experimental data were treated with CrysAlisPro, and all reflections were indexed on the basis of a cubic unit cell (Table 2). The intensity data were corrected for X-ray absorption using the Rigaku program ABSPACK. The systematic absence of reflections is suggestive of the space group I3m. The crystal structure was then solved for the space group I3m with SHELXT and refined with SHELXL [10,11], both included in the freeware Olex2 [12]. The structure model and site labels of Cristian Biagioni et al. were adopted (Table 3) [1]. The positions of atoms and anisotropic displacement parameters were refined with full occupancies for X(3) and S(1), free occupancies for S(2), manual occupancies for Ag and Cu at M(2) sites and manual occupancies for Cu, Fe and Zn at M(1) sites. The structures were illustrated with the freeware VESTA [13].

Table 2.
Summary of crystal data and refinement.

Table 3.
Fractional atomic coordinates and displacement parameters (Å2) of atoms in kenoargentotetrahedrite-(Fe) from Bajiazi.
3. Results and Discussion
The earlier investigators usually calculated the formula unit of the tetrahedrite family mineral on the basis of S = 13 to obtain the empirical chemical formula [14,15]. Nevertheless, the miscalculation of the formula unit will be caused by using 13 S atoms as the standard for the kenoargentotetrahedrite-(Fe), which only contains 12 S atoms. In this paper, 4 atoms(As + Sb) are used as the standard to calculate the chemical formula. From the data in Table 1, it is not difficult to find that the chemical composition of kenoargentotetrahedrite-(Fe) is relatively stable and evenly distributed, on the other hand, the content of some metal elements(Ag, Cu, Fe and Zn) changes slightly, which is mainly caused by isomorphic replacement. Based on the composition data of kenoargentotetrahedrite-(Fe), it can be concluded that its empirical chemical formula is Ag5.50Cu4.17Fe1.75Zn0.31Sb3.96As0.04S12.08, the corresponding ideal chemical formula is Ag6Cu4(Fe,Zn)2Sb4S12. On account of the existence of some trace elements that are lower than the detection limit of the EPMA, the total metal atoms number is 11.73 instead of 12 in the formula unit. The kenoargentotetrahedrite-(Fe) is obviously different from argentotetrahedrite-(Fe) (Ag6Cu4(Fe,Zn)2Sb4S13) because of the unequal S atoms number. They belong to the same mineral family but different mineral species.
The cell parameters and the structural chemical formula of kenoargentotetrahedrite-(Fe) are calculated by refining the data obtained by the single-crystal diffraction technique. The kenoargentotetrahedrite-(Fe) is cubic with a I3m space group and unit cell parameters a = 10.4928(2) Å, V = 1155.26(7) Å3 and Z = 2. Its structural chemical formula is Ag5.70Cu4.26Fe1.74Zn0.30Sb4S12.05. Due to the widespread existence of isomorphism between metal cations in the kenoargentotetrahedrite-(Fe), the different elements may appear at the same site, and the same elements may appear at the different sites. Moreover, the natural sample has a large number of trace elements. Therefore, the chemical formula obtained by single-crystal diffraction cannot be completely consistent with the ideal chemical formula. According to the above data, it can be directly seen that the relationship between the cell parameters and Ag content [16,17] in kenoargentotetrahedrite-(Fe) just falls in the “freibergite trend” region [8,9,18,19,20,21], which was summarized by Cristian Biagioni et al. [1], illustrating the objective existence of the “freibergite trend”. Comparing the cell parameters of kenoargentotetrahedrite-(Fe) in this paper with those obtained by Welch et al. [8] and Rozhdestvenskaya et al. [9], it is not difficult to find that the unit cell parameters of the three studies are almost the same, while the 2θ range tested in this paper is wider, ranging from 5.49° to 67.058°, and the collected diffraction data are more detailed. More importantly, the R1 value calculated in this study, which represents that the quality of refinement is 0.0187, is smaller than the other two. It shows that the crystal structure solved in this paper is more accurate.
Due to the widespread existence of isomorphism between metal cations in the kenoargentotetrahedrite-(Fe), the different elements may appear at the same site in its crystal structure, especially at the M(1) and M(2) sites. Combined with the elements’ data measured by the electron probe, it can be known that Cu, Fe and Zn atoms are distributed at the M(1) site, Ag and Cu atoms are distributed at the M(2) site, and Sb atoms are fully distributed at the X(3) site. The fact that Cu atoms are distributed at two different sites in the crystal structure cannot be concluded from the electron microprobe data and can only be summarized from the data of single-crystal diffraction. In the process of solving and refining the crystal structure of kenoargentotetrahedrite-(Fe), the M(1) and M(2) sites of atoms are refined with fully free occupancies firstly, but the R1 values obtained by this method are relatively large. In order to reduce the R1 value and improve the accuracy of crystal structure refinement, the elements at the M(1) and M(2) sites are accurately assigned with the manual occupancies. Through continuous attempts, the occupancy rate of each element at the M(1) and M(2) site is finally determined with the R1 = 0.0198 at this moment. The As atoms are also added to the X(3) site in the structure refinement process, but this cannot reduce the R1 value. The specific values can be observed in Table 3, and the crystal structure can be seen in Figure 2a. Cu atoms are distributed at both M(1) and M(2) sites, the occupancy rate of Cu at the M(1) site is 0.66, and the occupancy rate of Cu at the M(2) site is 0.05. The S(2) site is refined with the free occupancies, and the occupancy rate of S at the S(2) site is 0.05 with R1 = 0.0192, which is smaller. It illustrates that the crystal structure of kenoargentotetrahedrite-(Fe) with the vacancy S(2) site is more accurate (Figure 2c).
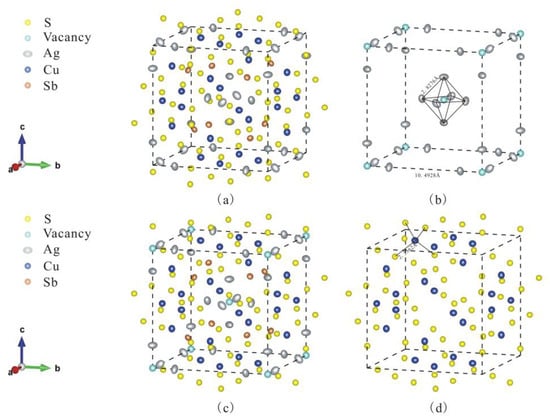
Figure 2.
The crystal structure of kenoargentotetrahedrite-(Fe), showing the sites and the anisotropic displacement ellipsoids in the unit cell: (a) without the vacancy of the S(2) sites; (b) the bond length of Ag-Ag is 2.8277 Å, and the distance of two vacancy sites is 10.4928 Å; (c) with the vacancy of the S(2) sites; (d) the tetrahedron is composed of Cu and S atoms and the distance between the Cu atom and the adjacent S atoms.
Based on the crystal structure diagram (Figure 2 and Figure 3), the coordination polyhedron formed between each metal atom and S atoms can be intuitively discovered. Among them, the coordination tetrahedron centered on the metal atoms is formed by metal atoms (Cu, Fe and Zn) at the M(1) site and S atoms, and the coordination number of metal atoms is 4; the triangular plane with the metal atoms as the center, two S atoms and one S atoms vacancy as the vertex is composed of metal atoms (Ag and Cu) at the M(2) site and S atoms, and the coordination number of metal atoms is 3; the triangular pyramid with the metal atom as the vertex is made up of Sb atoms at the X(3) site and S atoms, and the coordination number of Sb atoms is 3. Combined with the bond lengths (Table 4) between the metal atom and S atom in each coordination polyhedron, the valence state of each element can be calculated using the bond valence sum formula [22], in which the valence of Cu at the M(1) site is +1, the valence of Fe and Zn at the M(1) site is +2, the valence of Sb at the X(3) site is +3, while the valence of Ag at the M(2) site is not +1. In argentotetrahedrite, the valence of the Ag atom at the M(2) site is +1, and the lengths of the Ag-Ag bond is 3.24 Å [8]. In kenoargentotetrahedrite-(Fe), due to the empty of S atom at the center point, the distance of the Ag-Ag bond is only 2.8277(11) Å, which is shorter than that in argentotetrahedrite. It indicated that Ag and Ag are connected by metal bonds, and six Ag atoms form an octahedron cluster. According to the law of conservation of electricity valence, the valence state of the octahedron cluster is +4, and it can be written as (Ag6)4+.
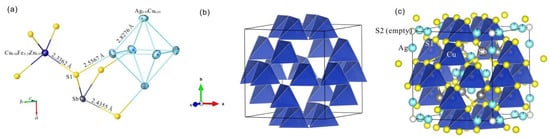
Figure 3.
The crystal structure of kenoargentotetrahedrite-(Fe): (a) showing anisotropic ellipsoids, connectivity and bonding lengths of atoms; (b) showing the cage within the cube formed from the distribution of M(1)S4 tetrahedra on the six faces of the unit-cell cube; (c) showing the distribution of SbS3 pyramid and Ag6 cluster in the cage of M(1)S4 tetrahedra, with the S(2) site being empty.

Table 4.
Bond lengths and bond valence sum (v.u.) of atoms in kenoargentotetrahedrite-(Fe).
The crystal structure of kenoargentotetrahedrite-(Fe) can be explained from two different models, which are the coordination polyhedron model and the space-filling model (Figure 3). In the literature, the crystal structure of tetrahedrite (and those of its isotypes) is an example of a sulfidic sodalite-like (SOD) framework, with cavities that can be described as Laves truncated tetrahedra [5]. Using the coordination polyhedron model, the crystal structure of kenoargentotetrahedrite-(Fe) can be interpreted as follows: it is made up of a collapsed sodalite-like framework of corner-connected M(1)S4 tetrahedron with cages containing a M(2)6-octahedron cluster, encircled by four SbS3 trigonal pyramids. The chemical formula of kenoargentotetrahedrite-(Fe) is Ag6Cu4(Fe,Zn)2Sb4S12, and the chemical formula of argentotetrahedrite-(Fe) is Ag6Cu4(Fe,Zn)2Sb4S13. The S(2) site of the kenoargentotetrahedrite-(Fe) is empty in the crystal structure, which is different from the argentotetrahedrite-(Fe). The valence of Ag in argentotetrahedrite-(Fe) is +1, and the valence of Ag6 in kenoargentotetrahedrite-(Fe) is +4. Therefore, the kenoargentotetrahedrite-(Fe) has a close relation with the tetrahedrite group minerals, with the existence of the (Ag6)4+ cluster replacing the S(2)-centered Ag6 octahedron, according to the substitution mechanism 6M(2)Ag+ + S(2)S2− = M(2)(Ag6)4+ + S(2)□ [8,9]. Applying the space-filling model, the crystal structure of kenoargentotetrahedrite-(Fe) can be clarified as follows: the structure is composed of sulfur layers perpendicular to the three coordinate axes with interstices occupied by a large number of metal atoms. Among them, most of the Cu atoms and all the Fe, Zn atoms are distributed on the surface of the unit cell with the coordination number of 4, which form a M(1)S4 tetrahedron; the Sb atoms are distributed in the body of the unit cell with the coordination number of 3, which form SbS3 trigonal pyramids; the Ag and Cu atoms with the coordination number of 3 (one of them is almost empty) form a M(2)S3 triangular plane.
4. Conclusions
From this single-crystal X-ray diffraction study of samples from Bajiazi magmatic-hydrothermal Pb-Zn deposits, we confirmed the cubic structure of kenoargentotetrahedrite-(Fe), the occupancy rate of S at the S(2) site is 0.05 rather than 0, which is refined with R1 = 0.0192, and six Ag atoms form an octahedron cluster with the valence state of +4, which can be written as (Ag6)4+. We also proposed two different structure models: (a) Using the coordination polyhedron model, the structure is made up of a collapsed sodalite-like framework of corner-connected M(1)S4 tetrahedron with cages containing M(2)6-octahedron cluster, encircled by four SbS3 trigonal pyramids. (b) Applying the space-filling model, the structure is composed of sulfur layers perpendicular to the three coordinate axes with interstices occupied by a large number of metal atoms.
The kenoargentotetrahedrite-(Fe) is an important metal sulfide mineral owing to its thermoelectric properties, and it has been reported for the first time in China. It has been identified that the mode of occurrence of Ag is an independent mineral called kenoargentotetrahedrite-(Fe), which provides a new idea for mineral exploration in this mining area and a new challenge for mineral processing technology.
Author Contributions
Conceptualization, A.L. and X.G.; Data curation, Z.S. and X.G.; Formal analysis, Z.S.; Funding acquisition, X.G.; Investigation, Z.S.; Methodology, Z.S.; Resources, X.G.; Software, C.S.; Writing—original draft, Z.S.; Writing—review & editing, C.S., A.L. and X.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research and the APC were funded by the National Natural Science Foundation of China (Grant No.41172042, 42072054 to X.G.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the research of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biagioni, C.; George, L.L.; Cook, N.J.; Makovicky, E.; Moëlo, Y.; Pasero, M.; Sejkora, J.; Stanley, C.J.; Welch, M.D.; Bosi, F. The tetrahedrite group: Nomenclature and classification. Am. Mineral. 2020, 105, 109–122. [Google Scholar] [CrossRef]
- Machatschki, F. Formel und Kristallstruktur des tetraedrites. Norsk Geologisk Tidsskrift 1928, 10, 23–32. [Google Scholar]
- Machatschki, F. Präzisionsmessungen der Gitterkonstanten verschiedener Fahlerze. Formel und struktur derselben. Zeitschrift für Kristallographie 1928, 68, 204–222. [Google Scholar] [CrossRef]
- Wuensch, B.J. The crystal structure of tetrahedrite, Cu12Sb4S13. Zeitschrift für Kristallographie 1964, 119, 437–453. [Google Scholar] [CrossRef]
- Johnson, N.E.; Craig, J.R.; Rimstidt, J.D. Crystal chemistry of tetrahedrite. Am. Mineral. 1988, 73, 389–397. [Google Scholar]
- Kenngott, A. Das Mohs’sche Mineralsystem, dem Gegenwärtigen Standpuncte der Wissenschaft Gemäss Bearbeitet; Gerol & Sohn: Vienna, Austria, 1853; 164p. [Google Scholar]
- Peterson, R.C.; Miller, I. Crystal structure and cation distribution in freibergite and tetrahedrite. Mineral. Mag. 1986, 50, 717–721. [Google Scholar] [CrossRef]
- Welch, M.D.; Stanley, C.J.; Spratt, J.; Mills, S.J. Rozhdestvenskayaite Ag10Zn2Sb4S13 and argentotetrahedrite Ag6Cu4(Fe2+,Zn)2Sb4S13: Two Ag-dominant members of the tetrahedrite group. Eur. J. Mineral. 2018, 30, 1163–1172. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Zayakina, N.V.; Samusikov, V.P. Crystal structure features of minerals from a series of tetrahedrite-freibergite. Mineral. Zhurnal 1993, 15, 9–17. [Google Scholar]
- Sheldrick, G.M. SHEXLT-integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Chvileva, T.N.; Borodaev, Y.S.; Vinogradova, R.A.; Kononov, O.V. The influence of bismuth on optical properties of gray copper. Doklady Akademii Nauk SSSR 1986, 290, 1475–1478. [Google Scholar]
- Zhdanov, Y.Y.; Amuzinskiy, V.A.; Andrianov, N.G. A natural variety of high-silver fahlerz with a large unit-cell parameter. Doklady Akademii Nauk SSSR 1992, 327A, 134–138. [Google Scholar]
- Samusikov, V.P.; Zayakina, N.V.; Leskova, N.V. Relation between unit cell of fahlores and Ag-concentration. Doklady Akademii Nauk SSSR 1988, 299, 468–471. [Google Scholar]
- Balitskaya, O.V.; Mozgova, N.N.; Borodaev, Y.S.; Efimova, A.V.; Tsepin, A.I. Evolution of the unit-cell parameter of fahlores with their silver content. Izvestiya Akademii Nauk SSSR 1989, 9, 112–120. [Google Scholar]
- Kalbskopf, R. Strukturverfeinerung des Freibergits. Tschermaks Mineral. Petrogr. Mitt. 1972, 18, 147–155. [Google Scholar] [CrossRef]
- Riley, J.F. The tetrahedrite–freibergite series, with reference to the Mount Isa Pb-Zn-Ag orebody. Miner. Depos. 1974, 9, 117–124. [Google Scholar] [CrossRef]
- Sugaki, A.; Shima, H.; Kitakaze, A. Experimental Study of Argentian Tetrahedrite. In Scientific Reports of the Tohoku University; 2nd Series; Tohoku University: Sendai, Japan, 1975; “Prof. T. Takéuchi Memorial” Volume; pp. 63–72. [Google Scholar]
- Pattrick, R.A.D.; Hall, A.J. Silver substitution into synthetic zinc, cadmium, and iron tetrahedrites. Mineral. Mag. 1983, 47, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).