Prediction of Grain Size in Cast Aluminum Alloys
Abstract
:1. Introduction
2. Empirical Model
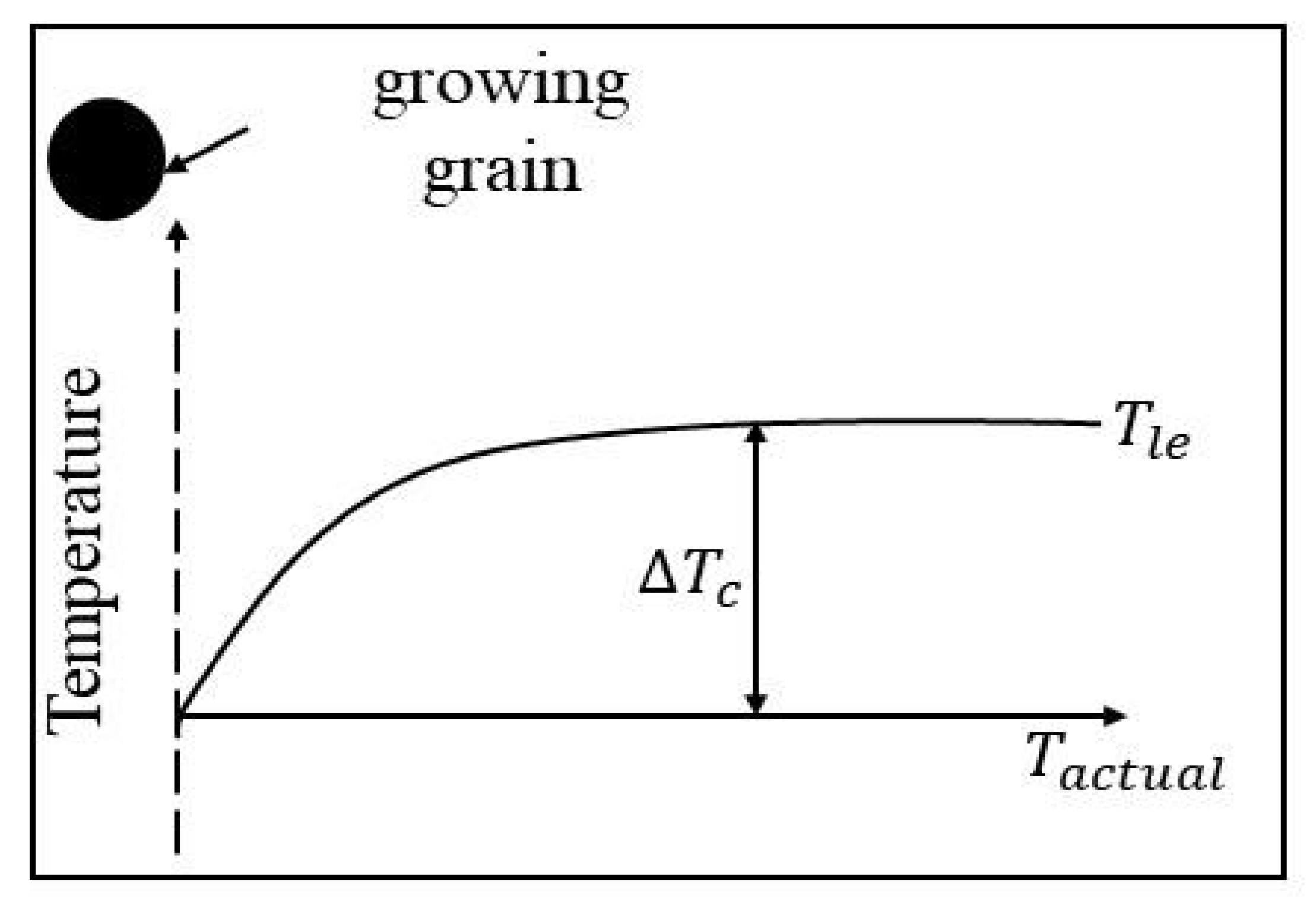
2.1. The Growth Restriction Factor
2.2. The Relative Grain Size Grain
2.3. The Semi-Empirical Model
3. Theoretical Model
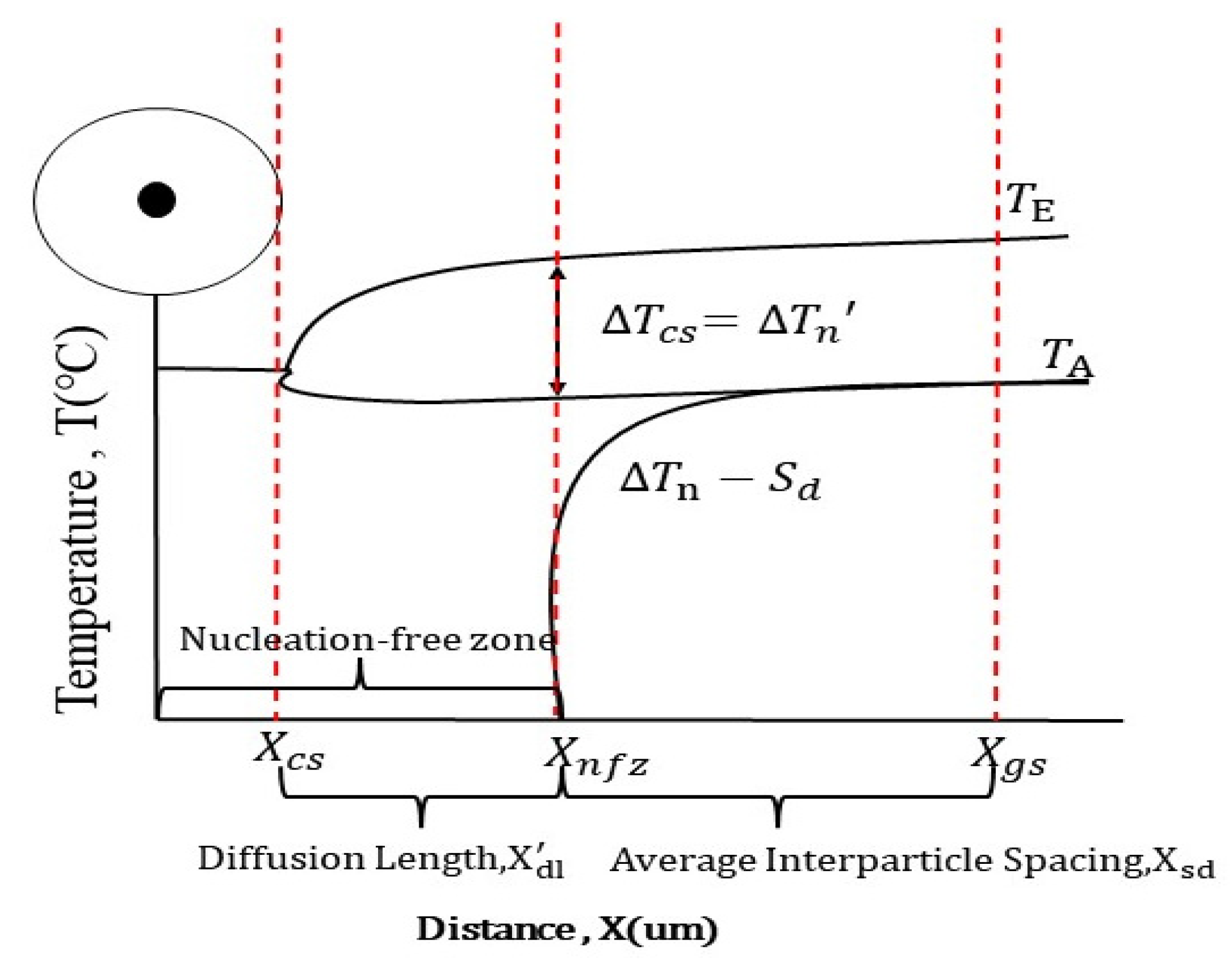
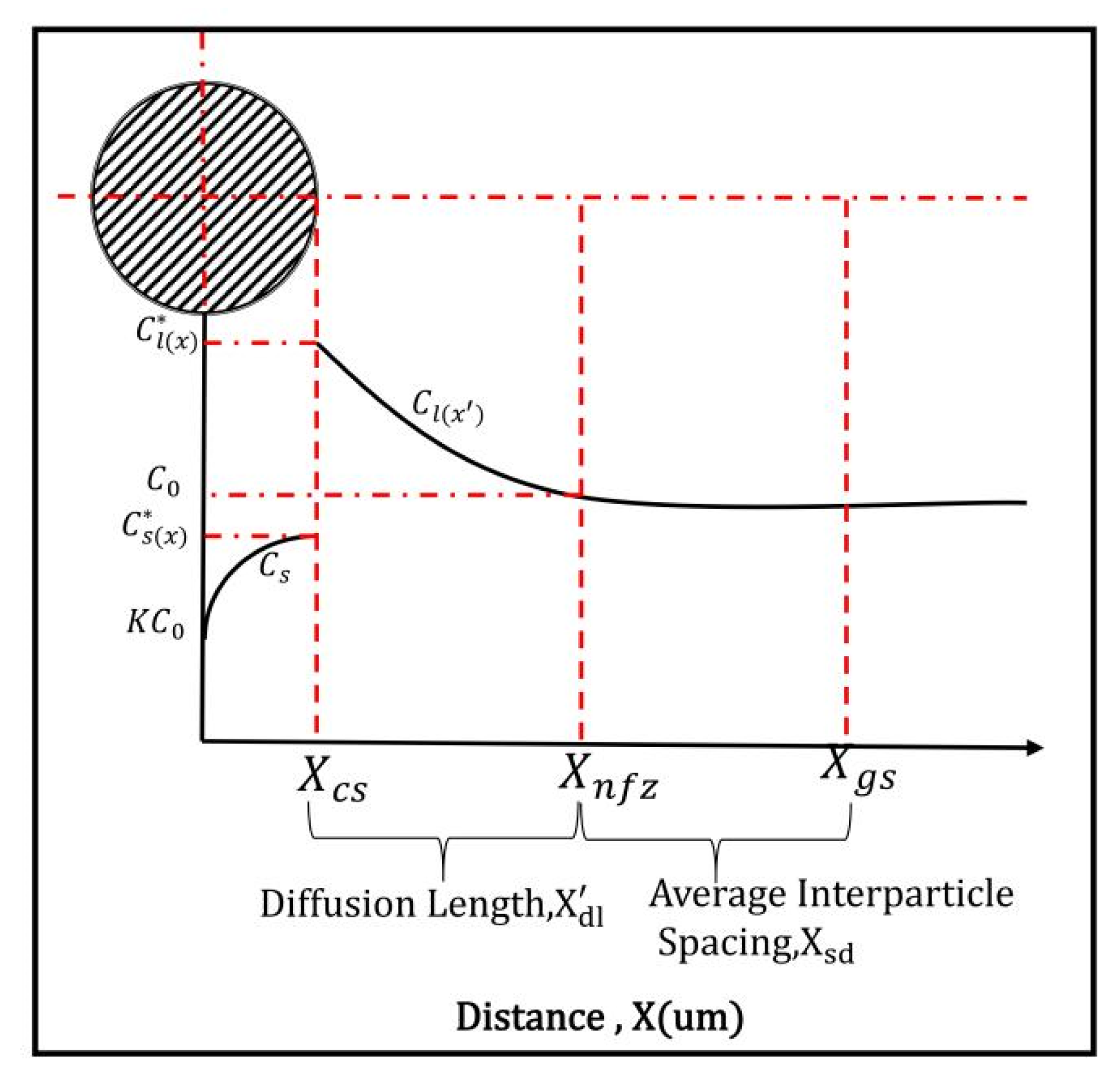
3.1. Free-form Nucleation Grain Size Model
3.2. Free-Growth Grain Model
4. Future Trends for Predicting Grain Size
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanty, P.; Gruzleski, J. Mechanism of grain refinement in aluminium. Acta Metall. Mater. 1995, 43, 2001–2012. [Google Scholar] [CrossRef]
- Cibula, A.; Ruddle, R. The effect of grain size on the tensile properties of high strength cast aluminum alloys. J. Inst. Met. 1949, 76, 1949–1950. [Google Scholar]
- Dahle, A.K.; Tondel, P.A.; Paradies, C.J.; Arnberg, L. Effect of grain refinement on the fluidity of two commercial Al-Si foundry alloys. Metall. Mater. Trans. A 1996, 27, 2305–2313. [Google Scholar] [CrossRef]
- Quested, T.; Greer, A. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Greer, A.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Qian, M.; Easton, M.A.; Cao, P.; Hildebrand, Z. Grain refinement of magnesium alloys. Metall. Mater. Trans. A 2005, 36, 1669–1679. [Google Scholar] [CrossRef]
- Peng, C.; Qian, M.; Stjohn, D.H. Effect of Iron on Grain Refinement of High-Purity Mg–Al Alloys. Scr. Mater. 2004, 51, 125–129. [Google Scholar]
- McCartney, D.G. Grain refining of aluminium and its alloys using inoculants. Int. Mater. Rev. 1989, 34, 247–260. [Google Scholar] [CrossRef]
- Ramirez, A.; Qian, M.; Davis, B.; Wilks, T.; StJohn, D.H. Potency of High-Intensity Ultrasonic Treatment for Grain Refinement of Magnesium Alloys. Scr. Mater. 2008, 59, 19–22. [Google Scholar] [CrossRef]
- Qian, M.; Cao, P. Discussions on grain refinement of magnesium alloys by carbon inoculation. Scr. Mater. 2005, 52, 415–419. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. Grain refinement of aluminum alloys: Part I. the nucleant and solute paradigms—A review of the literature. Metall. Mater. Trans. A 1999, 30, 1613–1623. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, T.; Ju, W.; Shi, S. Materials discovery and design using machine learning. J. Mater. 2017, 3, 159–177. [Google Scholar] [CrossRef]
- Agrawal, A.; Parijat, D.D.; Ahmet, C.; Gautham, P.B.; Alok, N.C.; Surya, R.K. Exploration of data science techniques to predict fatigue strength of steel from composition and processing parameters. Integr. Mater. Manuf. Innov. 2014, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Wicker, J.G.; Cooper, R.I. Will it crystallise? Predicting crystallinity of molecular materials. CrystEngComm 2015, 17, 1927–1934. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xia, M.S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing. Acta Mater. 2009, 57, 4891–4901. [Google Scholar] [CrossRef]
- Tiller, W.; Jackson, K.A.; Rutter, J.W.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Maxwell, I.; Hellawell, A. A simple model for grain refinement during solidification. Acta Metall. 1975, 23, 229–237. [Google Scholar] [CrossRef]
- Li, S.; Tang, B.; Zeng, D.B. Effects and mechanism of Ca on refinement of AZ91D alloy. J. Alloys Compd. 2007, 437, 317–321. [Google Scholar] [CrossRef]
- Cheng, S.L.; Yang, G.C.; Fan, J.F.; Li, Y.J.; Zhou, Y.H. Effect of Ca and Y Additions on Oxidation Behavior of Az91 Alloy at Elevated Temperatures. Trans. Nonferrous Met. Soc. China. 2009, 19, 299–304. [Google Scholar] [CrossRef]
- Masoudpanah, S.; Mahmudi, R. Effects of rare-earth elements and Ca additions on the microstructure and mechanical properties of AZ31 magnesium alloy processed by ECAP. Mater. Sci. Eng. A 2009, 526, 22–30. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. Grain refinement of aluminum alloys: Part II. Confirmation of, and a mechanism for, the solute paradigm. Metall. Mater. Trans. A 1999, 30, 1625–1633. [Google Scholar] [CrossRef]
- StJohn, D.; Qian, M.; Easton, M.A.; Cao, P. The Interdependence Theory: The relationship between grain formation and nucleant selection. Acta Mater. 2011, 59, 4907–4921. [Google Scholar] [CrossRef]
- Lu, H.; Wang, L.C.; Kung, S.K. Grain refining in A356 alloys. J. Chin. Foundrym Assoc. 1981, 29, 10–18. [Google Scholar]
- Spittle, J.; Sadli, S. Effect of alloy variables on grain refinement of binary aluminium alloys with Al–Ti–B. Mater. Sci. Technol. 1995, 11, 533–537. [Google Scholar] [CrossRef]
- Desnain, P.H.; Fautrelle, Y.; Meyer, J.L.; Riquet, J.P.; Durand, F. Prediction of Equiaxed Grain Density in Multicomponent Alloys, Stirred Electromagnetically. Acta Metall. Mater. 1990, 38, 1513–1523. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. An analysis of the relationship between grain size, solute content, and the potency and number density of nucleant particles. Metall. Mater. Trans. A 2005, 36, 1911–1920. [Google Scholar] [CrossRef]
- Chai, G. Relation between grain size and coherency parameters in aluminium alloys. Mater. Sci. Technol. 1995, 11, 1099–1103. [Google Scholar] [CrossRef]
- Easton, M.; Davidson, C.; John, D.S. Effect of alloy composition on the dendrite arm spacing of multicomponent aluminum alloys. Metall. Mater. Trans. A 2010, 41, 1528–1538. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. Partitioning of titanium during solidification of aluminium alloys. Mater. Sci. Technol. 2000, 16, 993–1000. [Google Scholar] [CrossRef]
- Johnsson, M.; Backerud, L.; Sigworth, G.K. Study of the mechanism of grain refinement of aluminum after additions of Ti-and B-containing master alloys. Metall. Trans. A 1993, 24, 481–491. [Google Scholar] [CrossRef]
- Lee, Y.; Dahle, A.K.; Stjohn, D.H.; Hutt, J.E.C. The effect of grain refinement and silicon content on grain formation in hypoeutectic Al–Si alloys. Mater. Sci. Eng. A 1999, 259, 43–52. [Google Scholar] [CrossRef]
- Lee, Y.; Dahle, A.K.; Stjohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Greer, A.L. Overview: Application of Heterogeneous Nucleation in Grain-Refining of Metals. J. Chem. Phys. 2016, 145, 211704. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Cao, P.; Easton, M.A.; Mcdonald, S.D.; Stjohn, D.H. An analytical model for constitutional supercooling-driven grain formation and grain size prediction. Acta Mater. 2010, 58, 3262–3270. [Google Scholar] [CrossRef]
- Dantzig, J.A.; Rappaz, M. Solidification, 2nd ed.; EPFL Press: Lausanne, Switzerland, 2016. [Google Scholar]
- Trivedi, R.; Kurz, W. Solidification microstructures: A conceptual approach. Acta Metall. Mater. 1994, 42, 15–23. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. Improved prediction of the grain size of aluminum alloys that includes the effect of cooling rate. Mater. Sci. Eng. A 2008, 486, 8–13. [Google Scholar] [CrossRef]
- Shu, D.; Sun, B.; Mi, J.; Grant, P.S. A quantitative study of solute diffusion field effects on heterogeneous nucleation and the grain size of alloys. Acta Mater. 2011, 59, 2135–2144. [Google Scholar] [CrossRef]
- Rappaz, M.; Thévoz, P. Solute diffusion model for equiaxed dendritic growth: Analytical solution. Acta Metall. 1987, 35, 2929–2933. [Google Scholar] [CrossRef]
- Aaron, H.B. Diffusion-limited phase transformations: A comparison and critical evaluation of the mathematical approximations. J. Appl. Phys. 1970, 41, 4404–4410. [Google Scholar] [CrossRef]
- Mueller, B.; Perepezko, J. The undercooling of aluminum. Metall. Trans. A 1991, 18, 1143–1150. [Google Scholar] [CrossRef]
- Men, H.; Jiang, B.; Fan, Z. Mechanisms of grain refinement by intensive shearing of AZ91 alloy melt. Acta Mater. 2010, 58, 6526–6534. [Google Scholar] [CrossRef] [Green Version]
- Günther, R.; Hartig, C.; Bormann, R. Grain refinement of AZ31 by (SiC) P: Theoretical calculation and experiment. Acta Mater. 2006, 54, 5591–5597. [Google Scholar] [CrossRef]
- Arnberg, L.; Bäckerud, L.; Klang, H. Production and properties of master alloys of Al–Ti–B type and their ability to grain refine aluminium. Metall. Technol. 1982, 9, 1–6. [Google Scholar] [CrossRef]
- Kailkhura, B.; Gallagher, B.; Kim, S.; Hiszpanski, A.; Han, Y.J. Reliable and explainable machine-learning methods for accelerated material discovery. Comput. Mater. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Meredig, B.; Agrawal, A.; Kirklin, S.; Saal, J.E. Combinatorial screening for new materials in unconstrained composition space with machine learning. Phys. Rev. B 2014, 89, 094104. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Sheng, V.S.; Wang, Z.J.; Ho, D.; Li, S. Incremental learning for ν-support vector regression. Neural Netw. 2015, 67, 140–150. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Padula, D.; Simpson, J.D.; Troisi, A. Combining Electronic and Structural Features in Machine Learning Models to Predict Organic Solar Cells Properties. Mater. Horiz. 2019, 6, 343–349. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, Z.; Balachandran, P.V.; Xue, D.Q.; Lookman, T. Accelerated discovery of large electrostrains in BaTiO3-based piezoelectrics using active learning. Adv. Mater. 2018, 30, 1702884. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Zhang, Z.; Huang, Z.; Song, D.; Jia, Y.; Zhou, N.; Wang, K.; Zheng, K.; Du, H. Prediction of Grain Size in Cast Aluminum Alloys. Crystals 2022, 12, 474. https://doi.org/10.3390/cryst12040474
Ma S, Zhang Z, Huang Z, Song D, Jia Y, Zhou N, Wang K, Zheng K, Du H. Prediction of Grain Size in Cast Aluminum Alloys. Crystals. 2022; 12(4):474. https://doi.org/10.3390/cryst12040474
Chicago/Turabian StyleMa, Shuai, Zhibo Zhang, Zhuming Huang, Dongfu Song, Yiwang Jia, Nan Zhou, Kai Wang, Kaihong Zheng, and Huijing Du. 2022. "Prediction of Grain Size in Cast Aluminum Alloys" Crystals 12, no. 4: 474. https://doi.org/10.3390/cryst12040474
APA StyleMa, S., Zhang, Z., Huang, Z., Song, D., Jia, Y., Zhou, N., Wang, K., Zheng, K., & Du, H. (2022). Prediction of Grain Size in Cast Aluminum Alloys. Crystals, 12(4), 474. https://doi.org/10.3390/cryst12040474





