Investigation of Magnetic Entropy Change in Intermetallic Compounds SmNi3−xFex Based on Maxwell Relation and Phenomenological Model
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
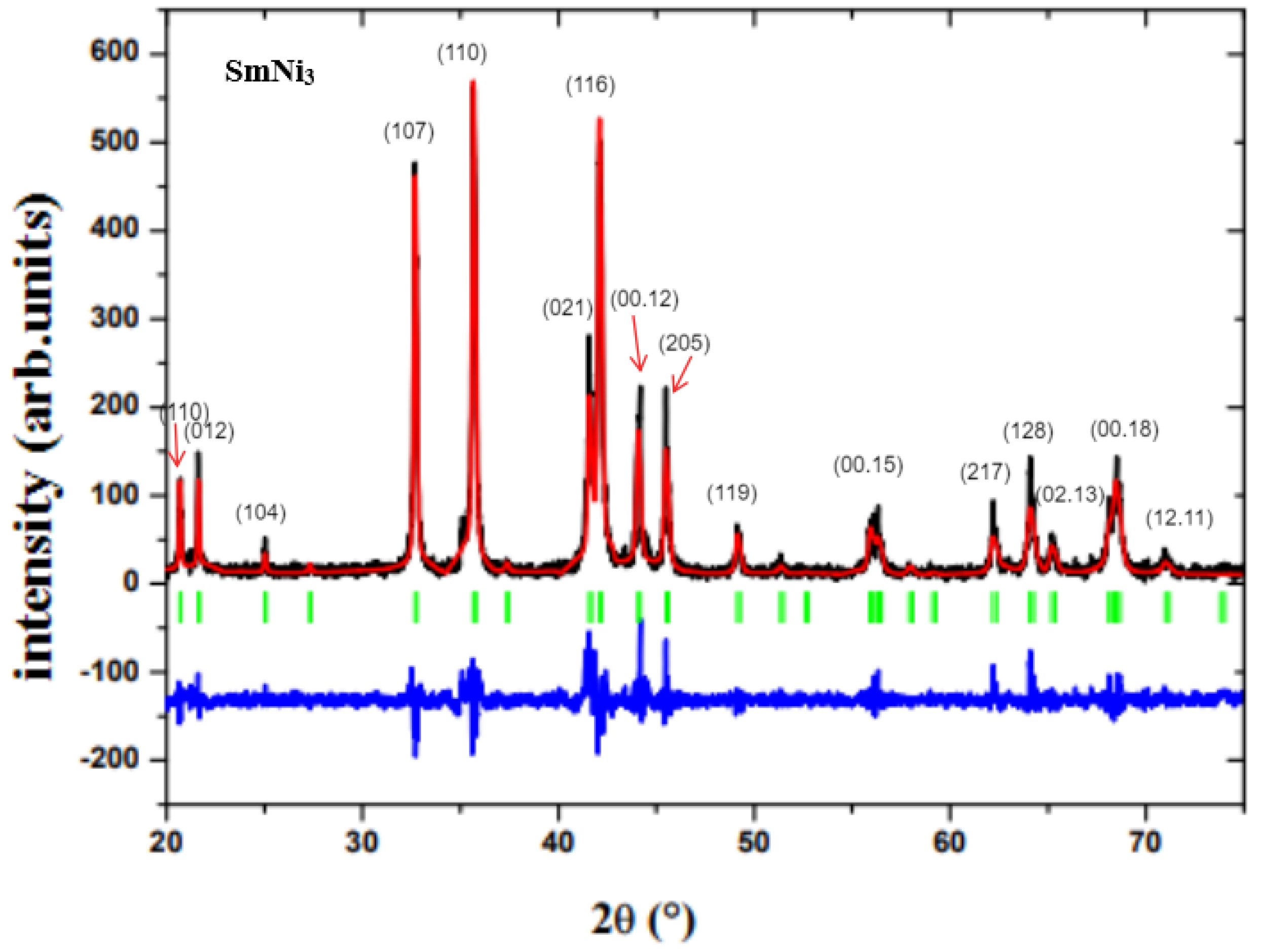
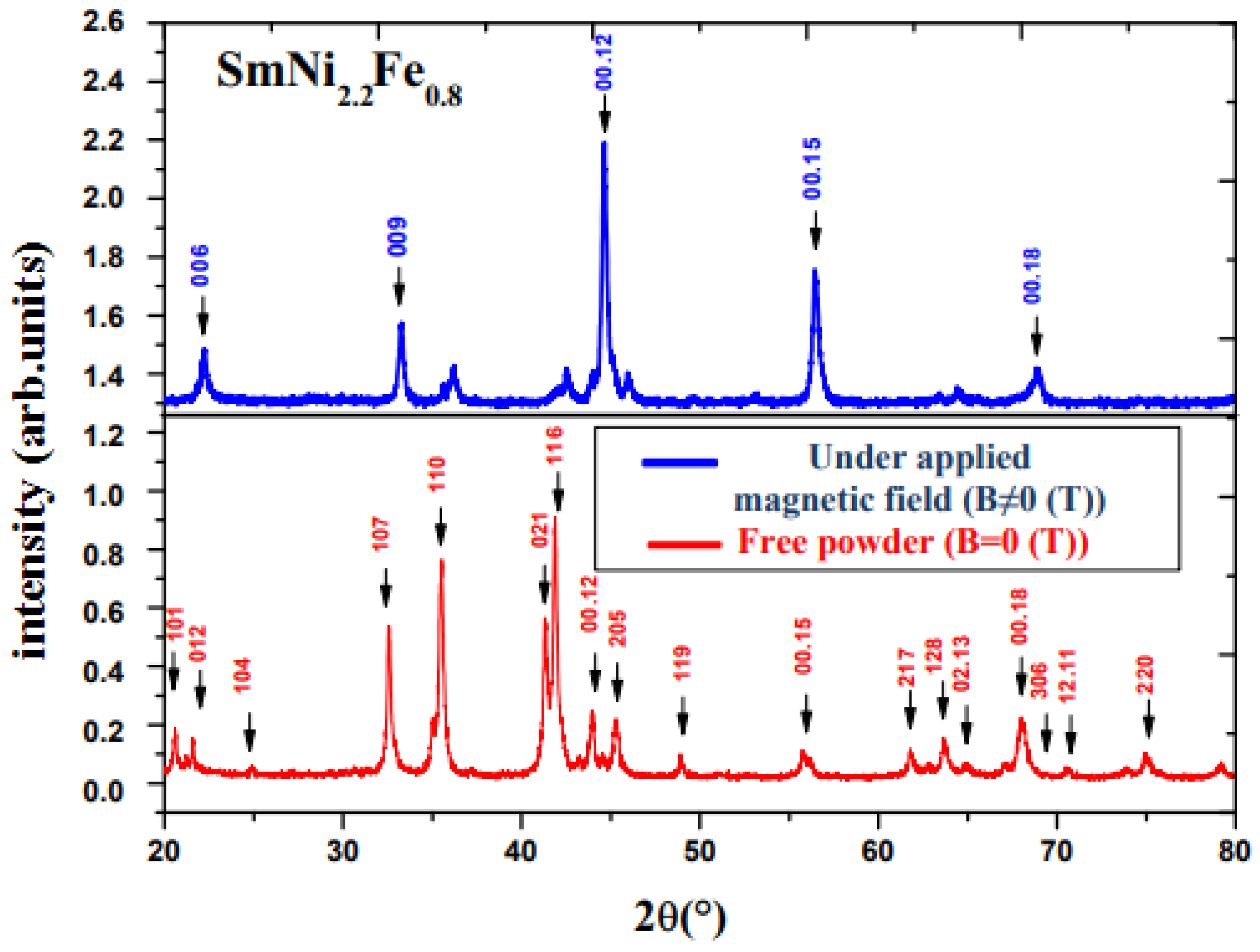
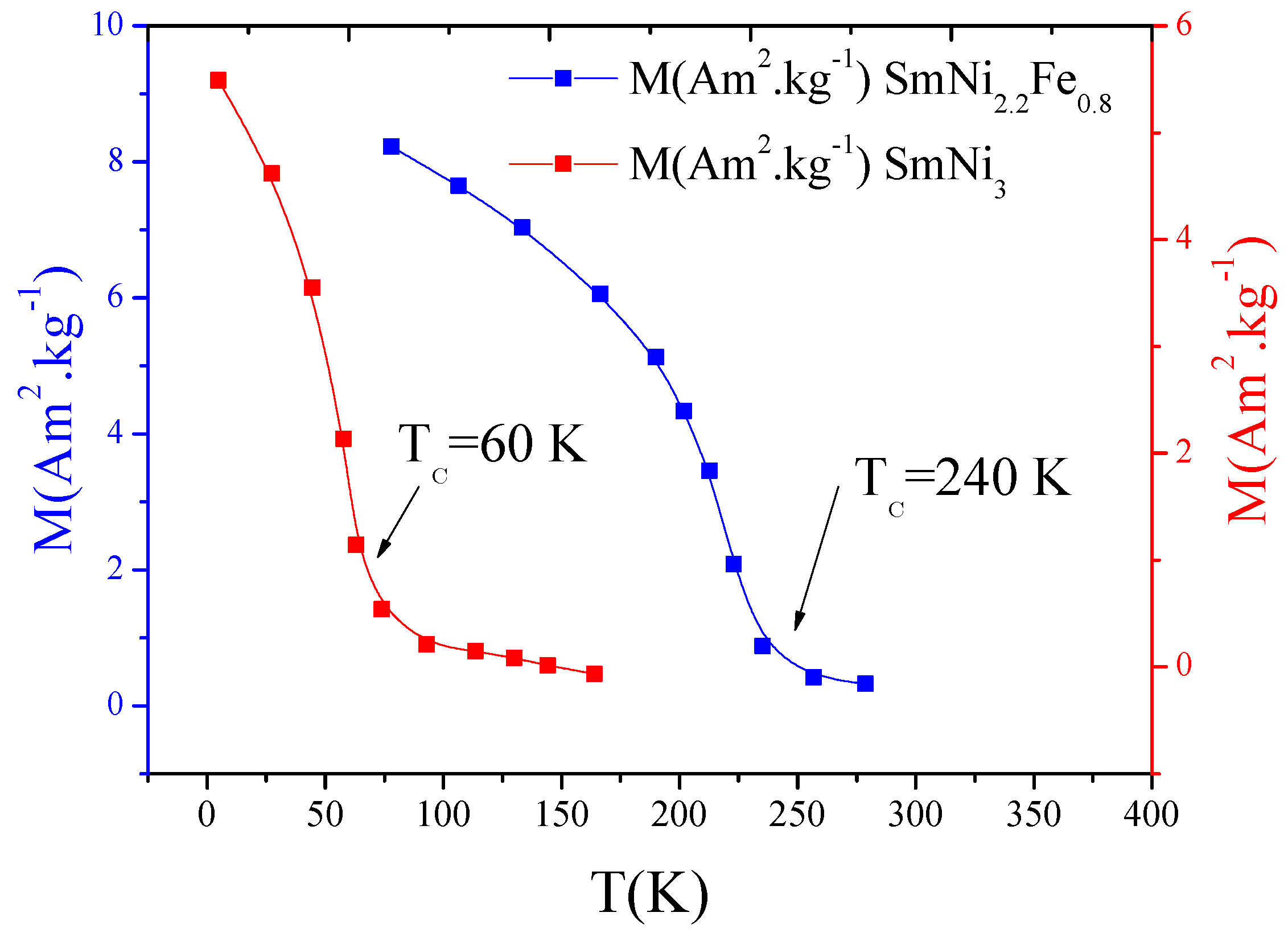
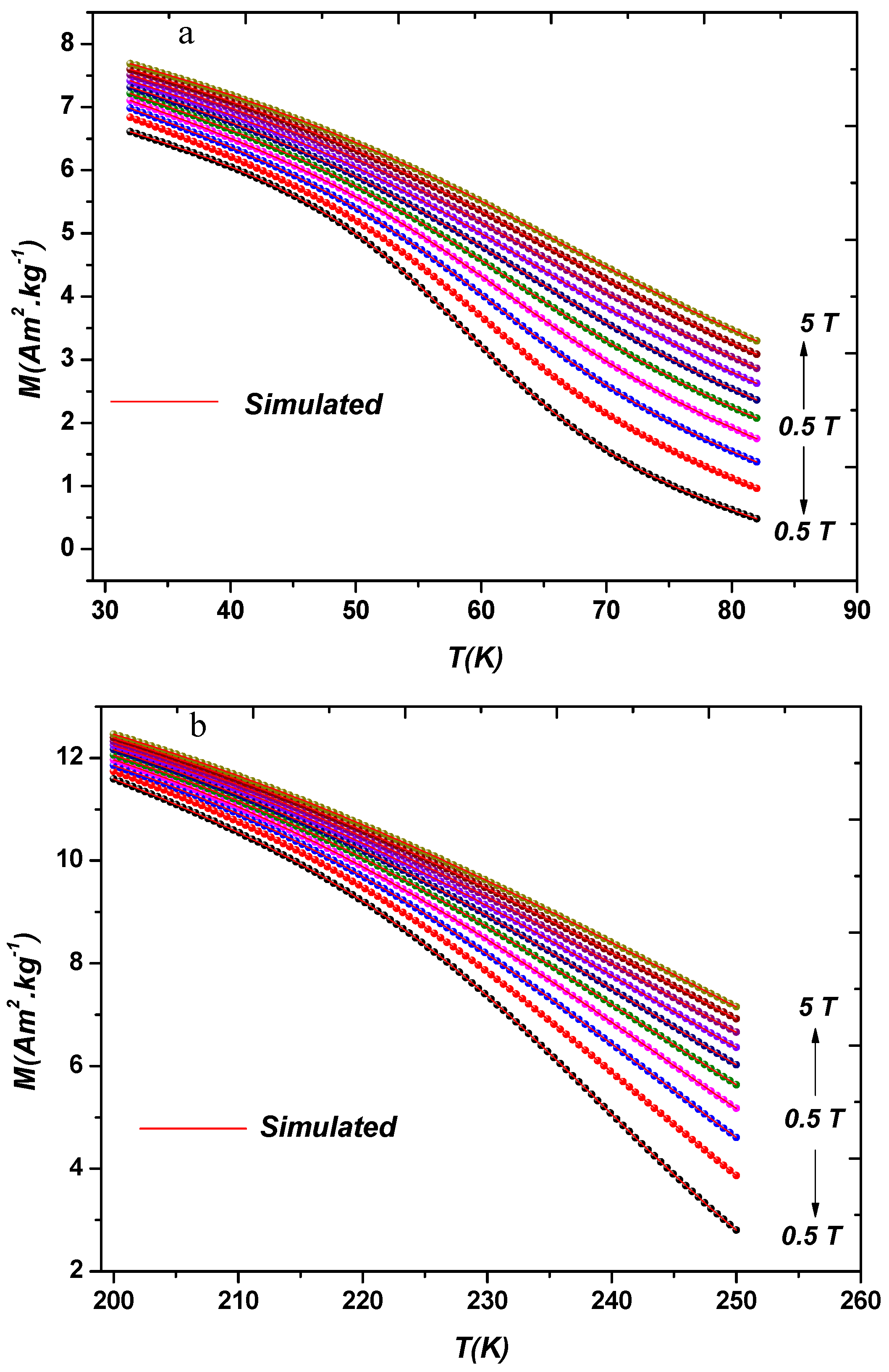
3.1. Structure and Magnetic Properties
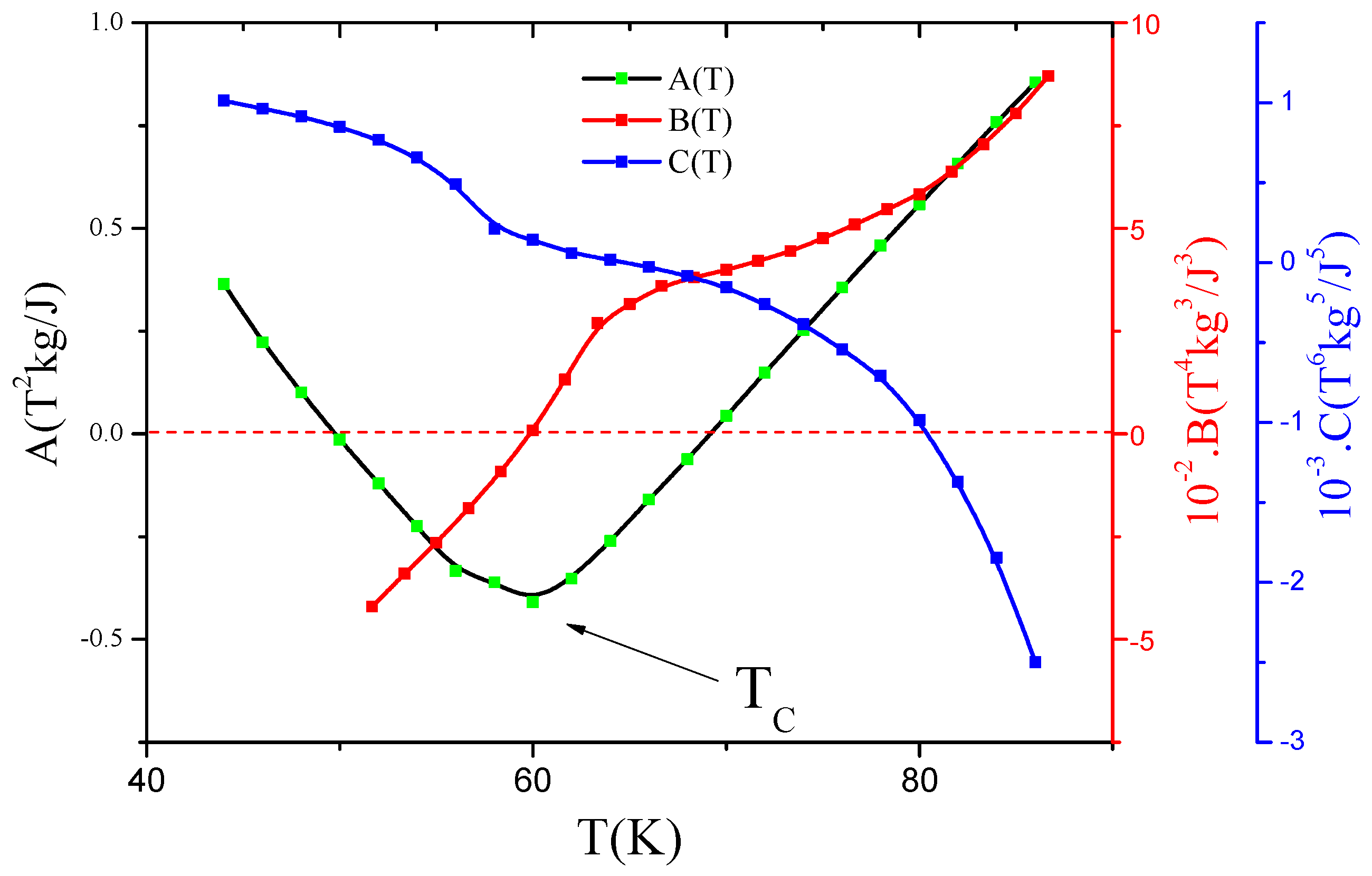
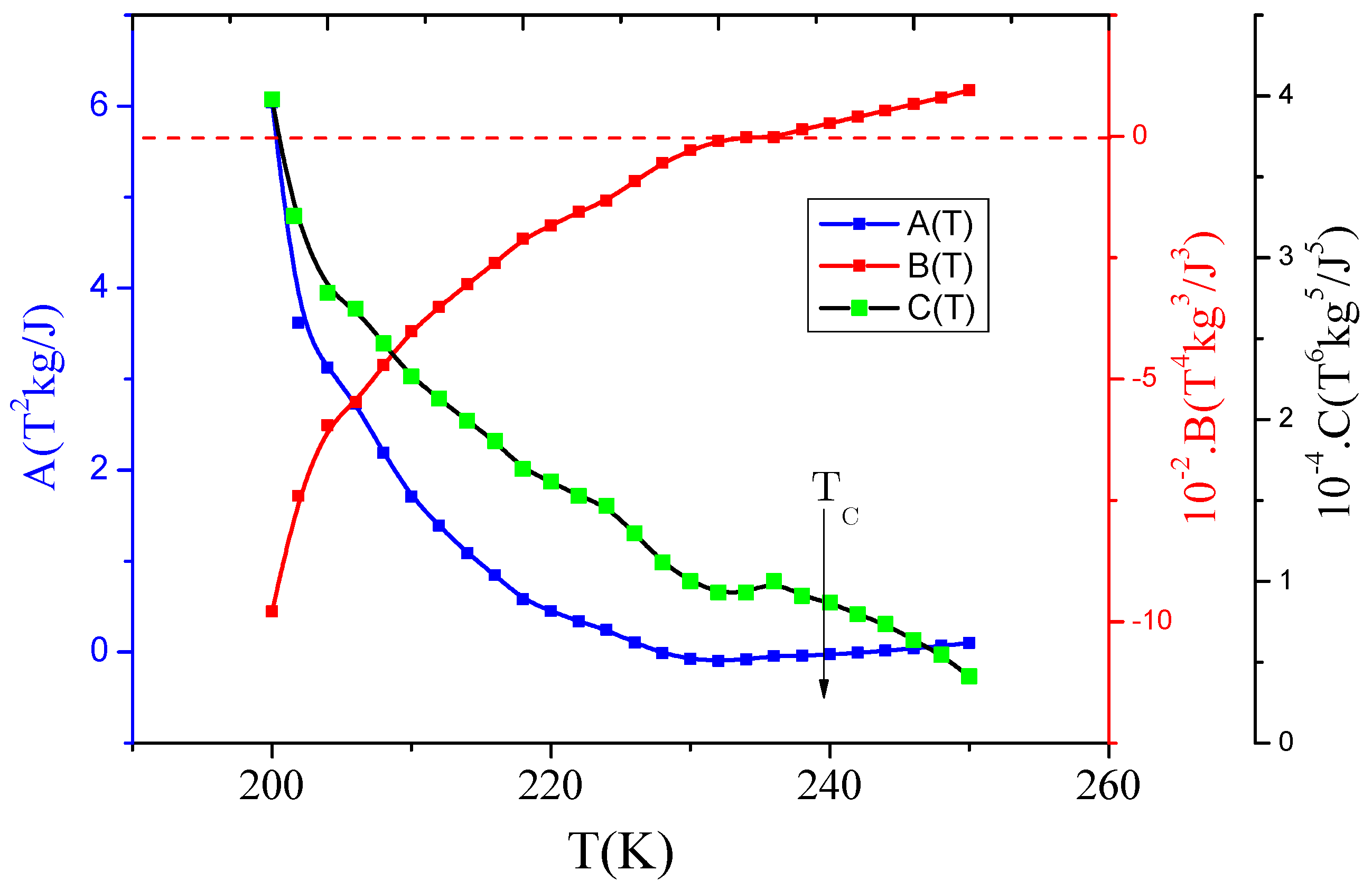
3.2. Phase Transition Nature according to Landau Model
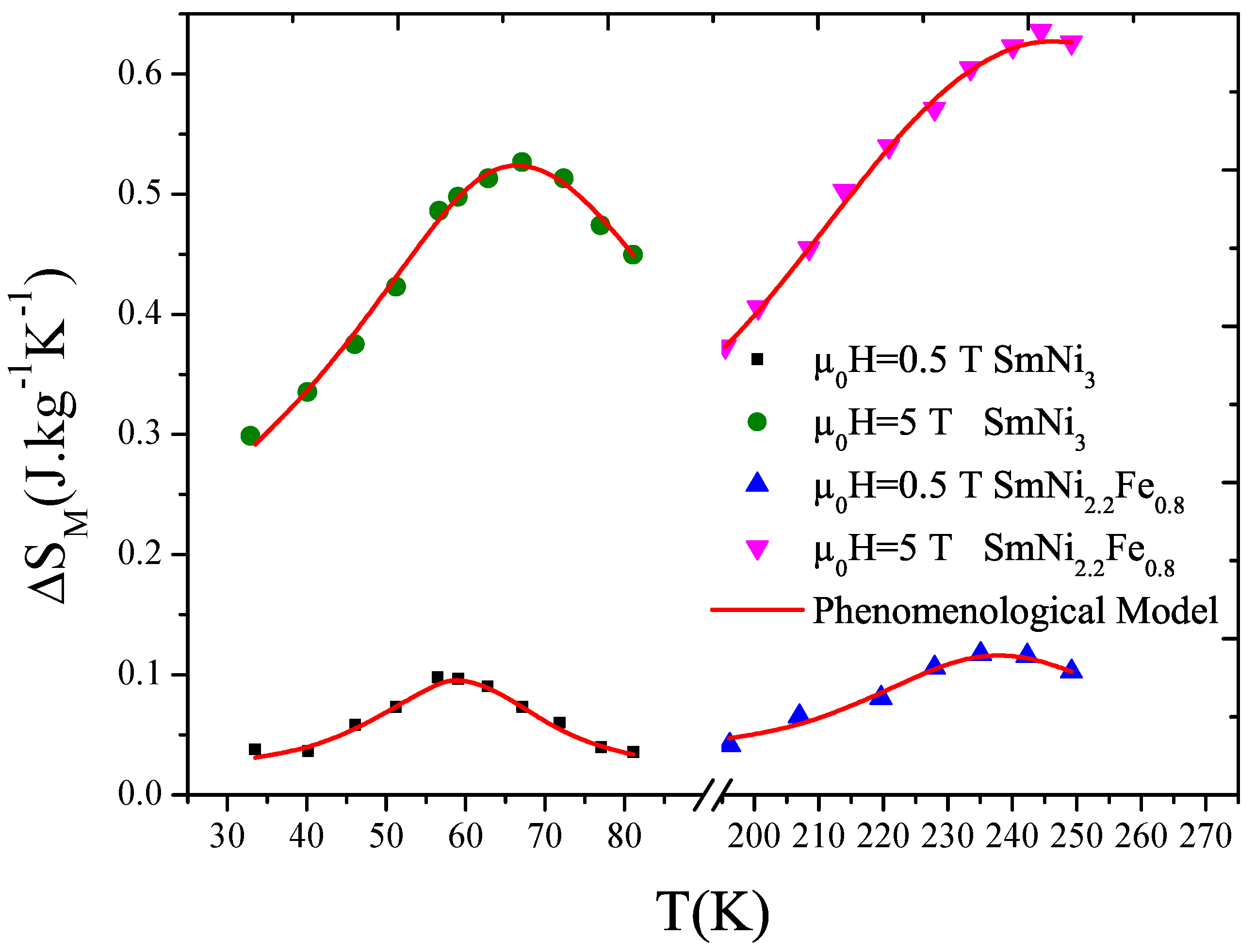
3.3. Magnetocaloric Effect
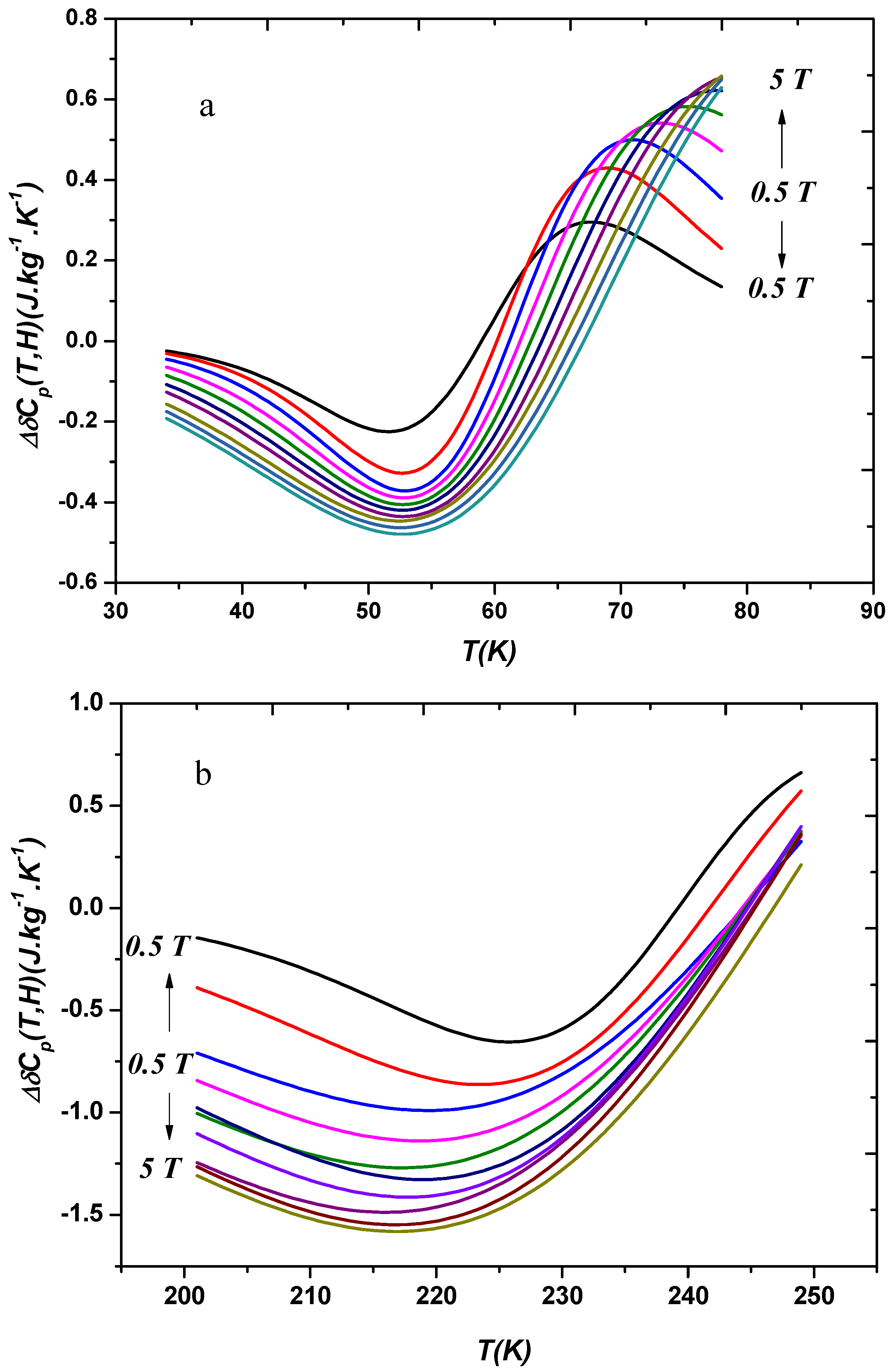
3.3.1. Phenomenological Model
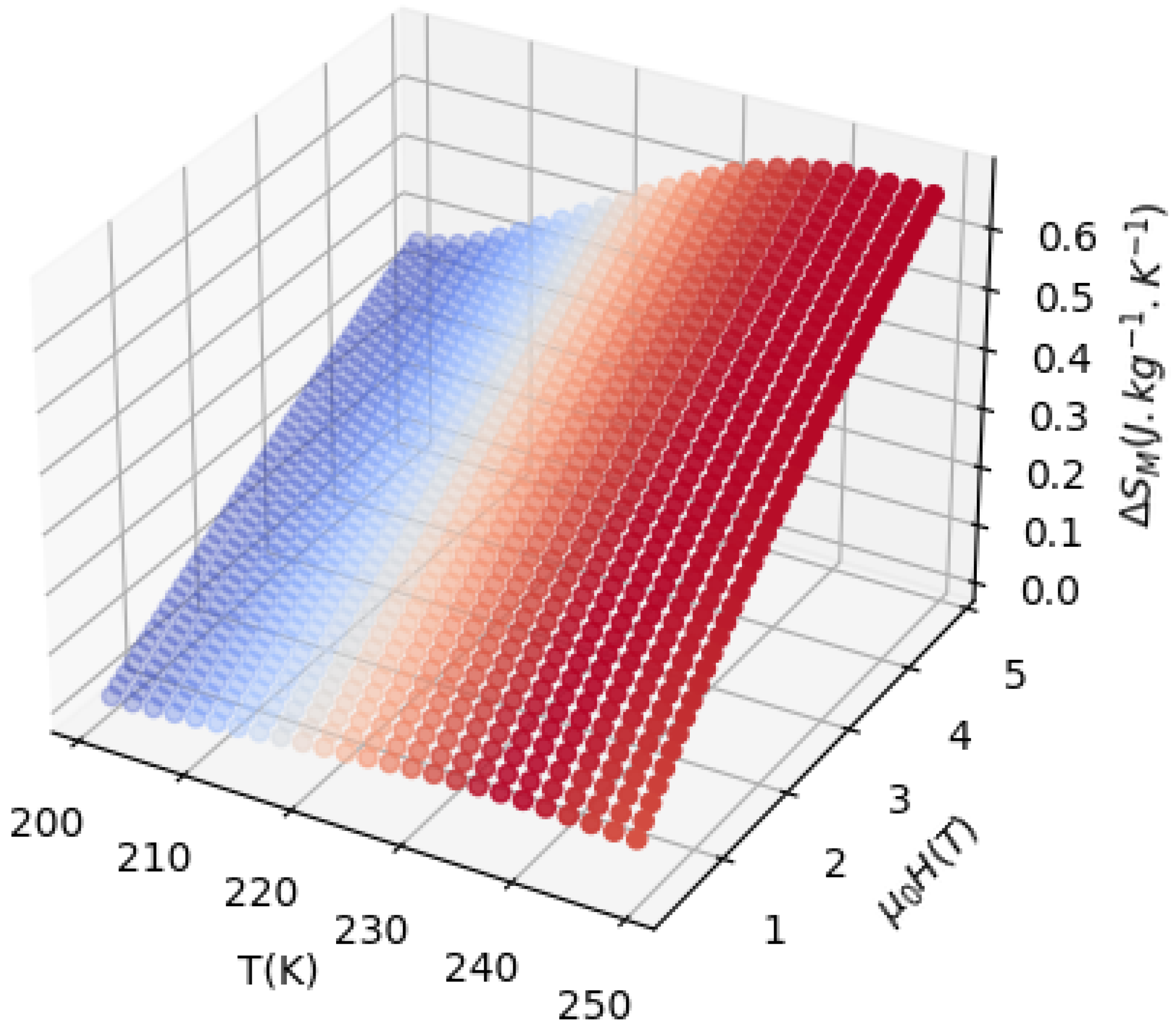
3.3.2. Results
3.3.3. Experimental Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bessais, L.; Younsi, K.; Khazzan, S.; Mliki, N. X-ray and intrinsic magnetic properties of nanocrystalline Sm2 (Fe, M)17 (M = Si, Ga, Co, Cr, Zr or Mo). Intermetallics 2011, 19, 997–1004. [Google Scholar] [CrossRef]
- Dematteis, E.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Hydrogen storage properties of Mn and Cu for Fe substitution in TiFe0.9 intermetallic compound. J. Alloys Compd. 2021, 851, 156075. [Google Scholar] [CrossRef]
- Iwase, K.; Mori, K. Crystal structure of intermetallic compound Y5Co19 and its hydride phases. Int. J. Hydrogen Energy 2021, 46, 9142–9150. [Google Scholar] [CrossRef]
- Ćwik, J.; Koshkid’ko, Y.; Nenkov, K.; Tereshina-Chitrova, E.; Kolchugina, N. Correlation between the structure and thermomagnetic properties of pseudo-binary (Tb, Er) Ni2 solid solutions. J. Alloys Compd. 2021, 859, 157870. [Google Scholar] [CrossRef]
- Grigoras, M.; Lostun, M.; Borza, F.; Porcescu, M.; Lupu, N. The effect of the Mo addition on the magnetic properties and phase constituents of the Ce-(FeCo)-B ribbons. Intermetallics 2022, 141, 107425. [Google Scholar] [CrossRef]
- Biswas, A.; Del Rose, T.; Mudryk, Y.; Ribeiro, P.; Alho, B.; de Sousa, V.; Nóbrega, E.; von Ranke, P.; Pecharsky, V. Hidden first-order phase transitions and large magnetocaloric effects in GdNi1−xCox. J. Alloys Compd. 2022, 897, 163186. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Li, S.; Wang, J.; Ren, Z. Achievement of giant cryogenic refrigerant capacity in quinary rare-earths based high-entropy amorphous alloy. J Mater Sci Technol 2022, 102, 66–71. [Google Scholar] [CrossRef]
- Yao, K.; Xu, Y. Magnetic properties, magnetic transition and large magneto-caloric effect in the Ho0.2Er0.2Tm0.2Ni0.2Cu0.2 amorphous ribbon. Solid State Commun. 2022, 345, 114702. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Li, S.; Wang, J.; Ren, Z. Magnetic properties and promising magnetocaloric performances in the antiferromagnetic GdFe2Si2 compound. Sci. China Mater 2022, 65, 1345–1352. [Google Scholar] [CrossRef]
- Li, L.; Yan, M. Recent progresses in exploring the rare earth based intermetallic compounds for cryogenic magnetic refrigeration. J. Alloys Compd 2020, 823, 153810. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.; Wu, B.; Geng, S.; Zhang, Y. Magnetocaloric effect and refrigeration performance in RE60Co20Ni20 (RE = Ho and Er) amorphous ribbons. J. Magn. Magn. Mater. 2020, 498, 166179. [Google Scholar] [CrossRef]
- Jaballah, H.; Bouzidi, W.; Fersi, R.; Mliki, N.; Bessais, L. Structural, magnetic and magnetocaloric properties of (Pr, Sm)2Fe17 compound at room temperature. J. Phys. Chem. Solids 2021, 161, 110438. [Google Scholar] [CrossRef]
- Xu, P.; Ma, Z.; Wang, P.; Wang, H.; Li, L. Excellent cryogenic magnetocaloric performances in ferromagnetic Sr2GdNbO6 double perovskite compound. Mater. Today Phys. 2021, 20, 100470. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Ye, S.; Li, Y.; Liu, G.; Huo, D.; Yan, M. Magnetic properties and excellent cryogenic magnetocaloric performances in B-site ordered RE2ZnMnO6 (RE = Gd, Dy and Ho) perovskites. Acta Mater. 2020, 194, 354–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Guo, D.; Wang, J.; Ren, Z. Magnetic properties and promising cryogenic magneto-caloric performances of Gd20Ho20Tm20Cu20Ni20 amorphous ribbons. Chin. Phys. B 2021, 30, 017501. [Google Scholar] [CrossRef]
- Zarkevich, N.; Zverev, V. Viable materials with a giant magnetocaloric effect. Crystals 2020, 10, 815. [Google Scholar] [CrossRef]
- Tishin, A.; Spichkin, Y. The Magnetocaloric Effect and Its Applications; CRC Press: Boca Raton, FL, USA, 2016; Volume 12. [Google Scholar]
- Jaballah, H.; Nouri, K.; Mliki, N.; Bessais, L.; Jemmali, M. Universality class change from Mean-Field to 3D-Heisenberg in magnetocaloric compounds SmNi3−xFex. Chem. Phys. Lett. 2021, 787, 139260. [Google Scholar] [CrossRef]
- Fan, J.; Huang, C.; Liu, H.; Yang, Y.E.; Llamazares, J.; Valdés, C.; Gorria, P.; Ma, C.; Zhu, Y.; Yang, H. Critical behavior in hexagonal Y2 Fe17: Magnetic interaction crossover from 3D to 2D Ising model. CrystEngComm 2021, 23, 3411–3418. [Google Scholar] [CrossRef]
- Nouri, K.; Jemmali, M.; Walha, S.; Zehani, K.; Bessais, L.; Salah, A.B. The isothermal section phase diagram of the Sm-Fe-Ni ternary system at 800 °C. J. Alloys Compd. 2016, 661, 508–515. [Google Scholar] [CrossRef]
- Földeàki, M.; Chahine, R.; Bose, T.K. Magnetic measurements: A powerful tool in magnetic refrigerator design. J. Appl. Phys. 1995, 77, 3528–3537. [Google Scholar] [CrossRef]
- Bessais, L.; Dorolti, E.; Djega-Mariadassou, C. High coercivity in nanocrystalline carbides Sm (Fe, Ga)9C. Appl. Phys. Lett. 2005, 87, 192503. [Google Scholar] [CrossRef]
- Khazzan, S.; Mliki, N.; Bessais, L.; Djega-Mariadassou, C. Rare-earth iron-based intermetallic compounds and their carbides: Structure and magnetic behaviors. J. Magn. Magn. Mater. 2010, 322, 224–229. [Google Scholar] [CrossRef]
- Bensalem, R.; Tebib, W.; Alleg, S.; Sunol, J.J.; Bessais, L.; Greneche, J.M. Magnetic properties of nanostructured Fe92P8 powder mixture. J. Alloys Compd. 2009, 471, 24–27. [Google Scholar] [CrossRef]
- Hamrita, A.; Slimani, Y.; Salem, M.K.B.; Hannachi, E.; Bessais, L.; Azzouz, F.B.; Salem, M.B. Superconducting properties of polycrystalline YBa2Cu3O7−d prepared by sintering of ball-milled precursor powde. Ceram. Int. 2014, 40, 1461–1470. [Google Scholar] [CrossRef]
- Rietveld, H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J.; Fernandez-Diaz, M.T.; Martinez, J.L. Neutron diffraction study on structural and magnetic properties of La2NiO4. J. Phys. Condens. Matter 1991, 3, 3215. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Banerjee, A. Phase separation and the effect of quenched disorder in Pr0.5Sr0.5MnO3. J. Phys Condens. Matter 2008, 20, 275207. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.W.; Morrish, X.A.H. Negative exchange interactions and Curie temperatures for Sm2 Fe17 sand Sm2 Fe 17 Ny. Phys. Rev. B 1997, 55, 3670–3676. [Google Scholar] [CrossRef]
- Kuchin, A.G.; Platonov, S.P.; Lukoyanov, A.V.; Volegov, A.S.; Gaviko, V.S.; Mukhachev, R.D.; Yakovleva, M.Y. Remarkable increase of Curie temperature in doped GdFeSi compound. Intermetallics 2021, 133, 107183. [Google Scholar] [CrossRef]
- Hamad, M.A. Theoretical work on magnetocaloric effect in La0.75Ca0.25MnO3. J. Adv. Ceram. 2012, 1, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Fujieda, S.; Fujita, A.; Fukamichi, K. Reduction of hysteresis loss in itinerant-electron metamagnetic transition by partial substitution of Pr for La in La (FexSi1−x)13. J. Magn. Magn. Mater. 2007, 310, e1004–e1005. [Google Scholar] [CrossRef]





| Magnetocaloric Sample | M | M | |||
|---|---|---|---|---|---|
| SmNi | 5.14 | 1.69 | 60 | −0.06 | −0.2 |
| SmNiFe | 8.59 | 1.90 | 239 | −0.08 | −0.24 |
| Magnetocaloric Sample | J·(kg·K) | K | J·kg | J·(kg·K) |
|---|---|---|---|---|
| SmNi | 0.1 | 31 | 3.1 | −0.23–0.3 |
| SmNiFe | 0.12 | 50 | 6 | −0.66–0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaballah, H.; Nouri, K.; Mliki, N.; Bessais, L.; Jemmali, M. Investigation of Magnetic Entropy Change in Intermetallic Compounds SmNi3−xFex Based on Maxwell Relation and Phenomenological Model. Crystals 2022, 12, 481. https://doi.org/10.3390/cryst12040481
Jaballah H, Nouri K, Mliki N, Bessais L, Jemmali M. Investigation of Magnetic Entropy Change in Intermetallic Compounds SmNi3−xFex Based on Maxwell Relation and Phenomenological Model. Crystals. 2022; 12(4):481. https://doi.org/10.3390/cryst12040481
Chicago/Turabian StyleJaballah, Hamdi, Kamel Nouri, Najeh Mliki, Lotfi Bessais, and Mosbah Jemmali. 2022. "Investigation of Magnetic Entropy Change in Intermetallic Compounds SmNi3−xFex Based on Maxwell Relation and Phenomenological Model" Crystals 12, no. 4: 481. https://doi.org/10.3390/cryst12040481






