Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries
Abstract
:1. Introduction


1.1. Research Significance
1.2. Definition of Abbreviations Used in the Manuscript Script
1.3. Research Methodology
2. GPC Manufacturing Studies
2.1. Fly Ash Based GPC

2.2. GGBFS Based GPC
2.3. Effect of Molar Ratios of Alkaline Solution
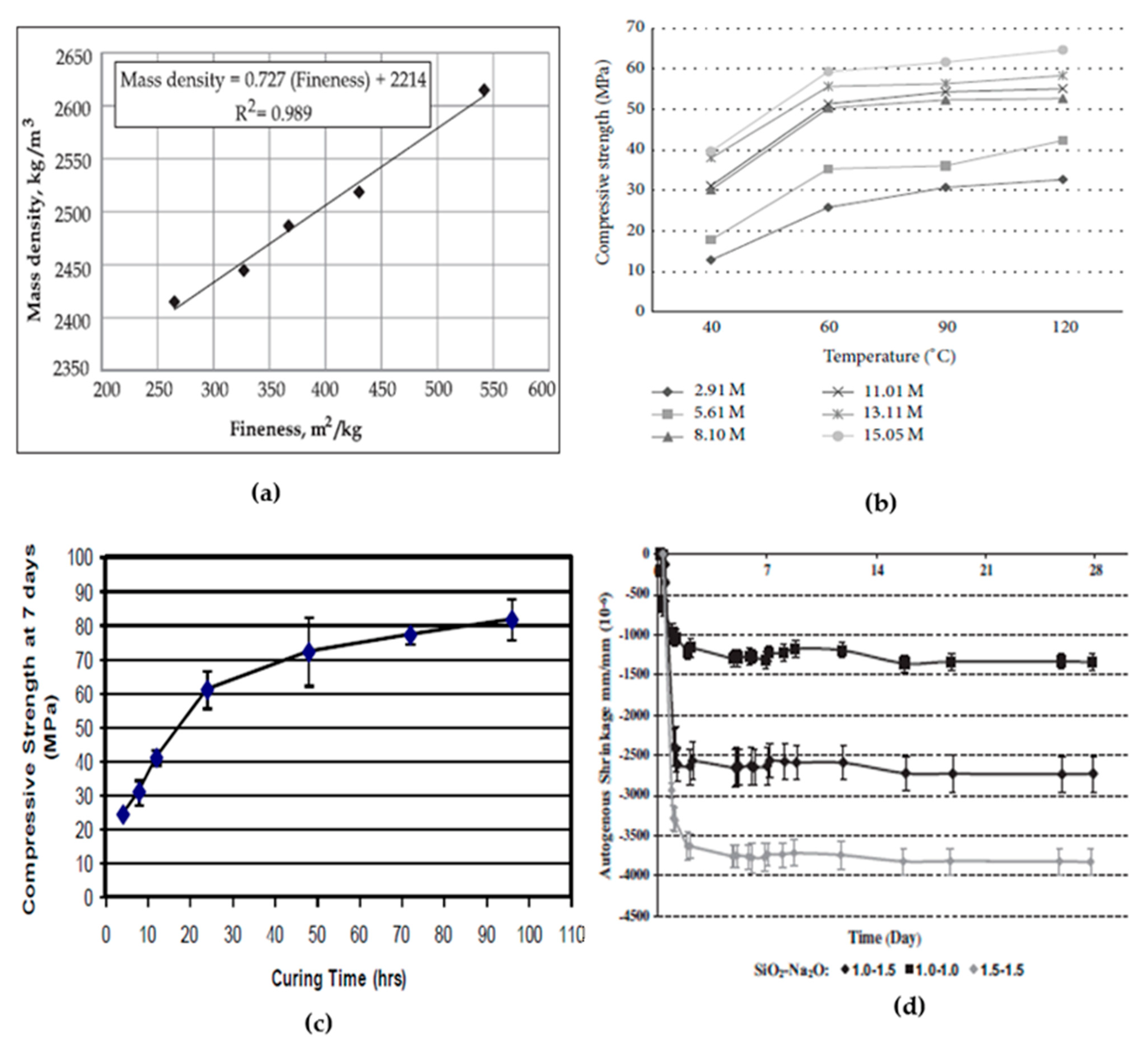
2.4. Effect of Calcination Temperature
2.5. Effect of Particle Size Fraction and Types of Aggregates
2.6. Effect of the Alkali Metal Activator
2.7. Effect of Ratio of Activator Liquid to Fly Ash/Slag
2.8. ITZ (Interfacial Transition Zone)
2.9. Effect of Curing Conditions
2.10. Effect of Calcium Content
2.11. Effect of Superplasticizer Addition
2.12. Effect of Handling Time
2.13. Effect of Silicate and Alumina
3. Durability and Other Related Aspect Studies
3.1. Effect of Sulphate Attack
3.2. Effect of Acid Attack
3.3. Effect of Sea Water
3.4. Effect on Carbonation
3.5. Effect of Alkali-Silica Reaction and Leaching
3.6. Effect of Elevated Temperature
3.7. Effect on the Bond Strength
4. Geopolymer Material Applications
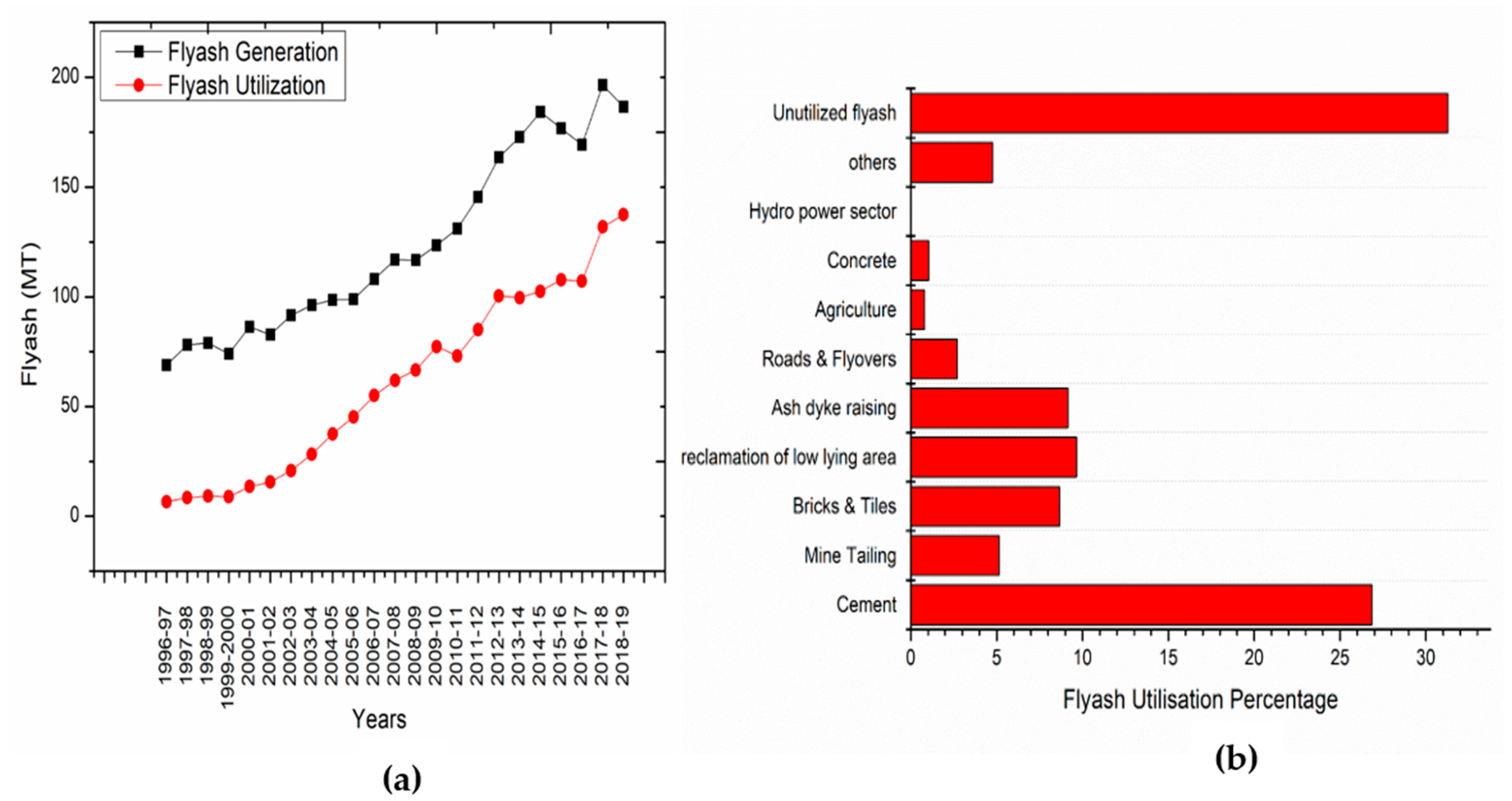
5. Sustainability
6. Conclusions
- Geopolymers are a perfect alternative to OPC concrete in concrete performance in terms of strength, durable properties, and sustainability.
- GPC reduces carbon footprints by using industrial solid waste like fly ash and slag and reducing cultivated land for dumping.
- GPC has reduced the cost of concrete by the use of industrial waste in the concrete production.
- The GPC shows better mechanical properties compared to OPC concrete.
- The GPC shows better durable properties, for all extreme environmental conditions i.e., acid attack, seawater conditions, sulphate attack, carbonation of concrete, chloride penetration, alkali-aggregate reactions and elevated temperature, than OPC concrete.
- The embodied energy of the GPC is less compared to the OPC concrete for the same compressive strength.
- The GPC materials have found a number of applications in infrastructure development and other various fields, and have become a proven material for the sustainable development in the construction industry.
- The application of GPC and economical production techniques in the construction industry create employment and increase energy efficiency.
7. Recommendations and Future Scope
- The requirement to implement GPC on major structural projects such as roads, bridges, buildings, hospitals, and other structures.
- More research on the other non-ferrous solid waste used in the GPC and analysis of such material’s capacity to work as a binder in the GPC.
- Further research on the hazards associated with some hazardous material components of GPC for its use on a large scale in the construction sector, and research on ways to reduce the hazard vulnerability in production/application of GPC.
- Further research work on long term effect on strength and durability characteristics.
- Further study on social/national standards attitude towards the application of GPC.
- Further research work to make geopolymer 3D printing process a viable construction approach.
- Further research work to draw relationships between composition, structure, and strength characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shobeiri, V.; Bennet, B.; Xie, T.; Visintin, P. A comprehensive assessment of the global warming potential of geopolymer concrete. J. Clean. Prod. 2021, 297, 126669. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.U.; Memon, A.S.; Khizar, K.M.; Faisal, J.M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Isleem, H.F.; de Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Sustainable utilization of red mud waste (bauxite reidue) and slag for the production of geopolymer composites: A review. Case Stud. Constr. Mater. 2022, 16, e00994. [Google Scholar] [CrossRef]
- Albitar, M.; Visintin, P.; Ali, M.S.M.; Drechsler, M. Assessing Behaviour of Fresh and Hardened Geopolymer Concrete Mixed with Class-F Fly Ash. KSCE J. Civ. Eng. 2015, 19, 1445–1455. [Google Scholar] [CrossRef]
- Bhavan, I. Indian Minerals Yearbook 2019; (Advance Release), Slag-Iron and Steel; Government of India, Ministry of Mines, Indian Bureau of Mines, ENVIS-Centre on Environmental Problems of Mining: Dhanbad, India, 2019.
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Mater. Educ. 1994, 16, 91–138. [Google Scholar] [CrossRef]
- Wongkvanklom, A.; Posi, P.; Kampala, A.; Kaewngao, T.; Chindaprasirt, P. Beneficial utilization of recycled asphaltic concrete aggregate in high calcium fly ash geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00615. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Cement and Concrete Composites Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Chi, M. Effects of the alkaline solution/binder ratio and curing condition on the mechanical properties of alkali-activated fly ash mortars. Sci. Eng. Compos. Mater. 2017, 24, 773–782. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.H.; Sarbini, N.N. Effect of binder to fine aggregate content on performance of sustainable alkali activated mortars incorporating solid waste materials. Chem. Eng. Trans. 2018, 63, 667–672. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2017, 103, 21–34. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.; Qiu, J.; Sun, X. Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags. Materials 2019, 12, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K. Effects of Ultrafine Fly Ash on Setting, Strength, and Porosity of Geopolymers Cured at Room Temperature. J. Civ. Eng. 2016, 29, 1–5. [Google Scholar] [CrossRef]
- Kawade, U.R.; Hussain, M.; Shirule, P.P.A. Enhancement of compressive strength of geopolymer concrete by varying ratio of Na2SiO3/NaoH and by varying molarity of NaoH. Int. J. Latest Res. Eng. Technol. 2016, 2, 47–50. [Google Scholar]
- Luhar, S.; Khandelwal, U. A Study on Water Absorption and Sorptivity of Geopolymer Concrete. Int. J. Civ. Eng. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Mix design of fly ash based geopolymer concrete. Adv. Struct. Eng. Mater. 2015, 3, 1619–1633. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Effect of ground granulated blast furnace slag and fly ash ratio and the curing conditions on the mechanical properties of geopolymer concrete. Struct. Concr. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Davidovits, J.; Quentin, S. Geopolymers Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. J. Mater. 2014, 62, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, L.; Karthikeyan, S.; Nathiya, S.; Suganya, K. Geopolymer Concrete an Eco-Friendly Construction Material. Int. J. Res. Eng. Technol. 2014, 3, 164–167. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Dev, N. Review on the effect of different parameters on behavior of Geopolymer Concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 11276–11281. [Google Scholar] [CrossRef]
- Nagalia, G.; Park, Y.; Abolmaali, A.; Aswath, P. Compressive Strength and Microstructural Properties of Fly Ash–Based Geopolymer Concrete. J. Mater. Civ. Eng. 2016, 18, 040161441-11. [Google Scholar] [CrossRef]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Effect of Concentration of Sodium Hydroxide and Degree of Heat Curing on Fly Ash-Based Geopolymer Mortar. Indian J. Mater. Sci. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Bidwe, S.S.; Hamane, A.A. Effect of different molarities of Sodium Hydroxide solution on the Strength of Geopolymer con-crete. Am. J. Eng. Res. 2015, 4, 139–145. Available online: https://www.ajer.org/papers/v4(03)/S04301390145.pdf (accessed on 9 March 2022).
- Kabir, S.M.A.A.; Alengaram, U.J.; Jumaat, M.Z.; Sharmin, A.; Islam, A. Influence of Molarity and Chemical Composition on the Development of Compressive Strength in POFA Based Geopolymer Mortar. Adv. Mater. Sci. Eng. 2015, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Aldawsari, S.; Kampmann, R.; Harnisch, J.; Rohde, C. Setting Time, Microstructure, and Durability Properties of Low Calcium Fly Ash/Slag Geopolymer: A Review. Materials 2022, 15, 876. [Google Scholar] [CrossRef]
- Topark-Ngarm, P.; Chindaprasirt, P.; Sata, V. Setting Time, Strength, and Bond of High-Calcium Fly Ash Geopolymer Concrete. J. Mater. Civ. Eng. 2015, 27, 04014198. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Sodium hydroxide effect on the mechanical properties of flyash-slag based geopolymer concrete. Struct. Concr. 2020, 22, E368–E379. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Bella, N.; Gudiel, E.; Soriano, L.; Font, A.; Borrachero, M.V.; Paya, J.; Monzó, J.M. Formulation of Alkali-Activated Slag Binder Destined for Use in Developing Countries. Appl. Sci. 2020, 10, 9088. [Google Scholar] [CrossRef]
- Ban, C.C.; Ken, P.W.; Ramli, M. Effect of Sodium Silicate and Curing Regime on Properties of Load Bearing Geopolymer Mortar Block. J. Mater. Civ. Eng. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Amran, Y.H.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Yu, J.; Ji, F.; Gu, L.; Chen, Y.; Shan, X. Mechanical Property and Microstructure of Fly Ash-Based Geopolymer Activated by Sodium Silicate. KSCE J. Civ. Eng. 2021, 25, 1765. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Molin, D.C.C.D.; Vilela, A.C.F.; da Silva, F.J.; Pavão, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci 2018, 53, 4709–4733. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Freire, C.B.; Dos Santos, B.L.D.; De Miranda, I.L.F.; Rodrigues, M.A.; Lameiras, F.S. Influence of the Kaolinite Calcination Conditions on the Compressive Strength of Geopolymer. KnE Eng. 2020, 5, 1–10. [Google Scholar]
- Boakye, K.; Khorami, M.; Ganjian, E.; Saidani, M. A review of the Effect of Calcination Temperature on the Properties of Calcined Clay Concrete. Int J. Eng Technol. Inf. 2021, 2, 72–74. [Google Scholar] [CrossRef]
- Ghani, U.; Hussain, S.; Amin, N.; Imtiaz, M.; Khan, S.A. Role of calcination on geopolymerization of lateritic clay by alkali treatment. J. Saudi Chem. Soc. 2021, 25, 101198. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G.; Kong, D.L.Y. Effect of aggregate size on spalling of geopolymer and Portland cement concretes subjected to elevated temperatures. Constr. Build. Mater. 2012, 36, 365–372. [Google Scholar] [CrossRef]
- Gluth, G.J.G.; Rickard, W.D.A.; Werner, S.; Pirskawetz, S. Acoustic emission and microstructural changes in fly ash geopolymer concretes exposed to simulated fire. Mater. Struct. 2016, 49, 5243–5254. [Google Scholar] [CrossRef]
- Yliniemi; Paiva; Ferreira; Tiainen; Illikainen. Development and incorporation of lightweight waste-based geopolymer aggregates in mortar and concrete. Constr. Build. Mater. 2017, 131, 784–792. [Google Scholar] [CrossRef]
- Sreenivasulu, C.; Ramakrishnaiah, A.; Guru Jawahar, J. Mechanical Properties of Geopolymer Concrete Using Granite Slurry as Sand Replacement. Int. J. Adv. Eng. Technol. 2015, 8, 83–91. [Google Scholar]
- Mahaboob, S.; Reddy, C.H.B.; Vasugi, K. Strength behaviour of geopolymer concrete replacing fine aggregates by M- sand and E-waste. Int. J. Eng. Trends Technol. 2016, 40, 401–407. [Google Scholar] [CrossRef]
- Saravanan, S.; Elavenil, S. Strength properties of geopolymer concrete using mssand by assessing their mechanical characteristics. J. Eng. Appl. Sci. 2018, 13, 4028–4041. [Google Scholar]
- Shaikh, F.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Taher, S.M.S.; Saadullah, S.T.; Haido, J.H.; Tayeh, B.A. Behavior of geopolymer concrete deep beams containing waste aggregate of glass and limestone as a partial replacement of natural sand. Case Stud. Constr. Mater. 2021, 15, e00744. [Google Scholar] [CrossRef]
- Matsuda, A.; Maruyama, I.; Meawad, A.; Pareek, S.; Araki, Y. Reaction, Phases, and Microstructure of Fly Ash-Based Alkali-Activated Materials. J. Adv. Concr. Technol. 2019, 17, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Ghani, N.T.; Elsayed, H.A.; AbdelMoied, S. Geopolymer synthesis by the alkali-activation of blastfurnace steel slag and its fire-resistance. HBRC Journal 2018, 14, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Hosan, A.; Haque, S.; Shaikh, F. Comparative Study of Sodium and Potassium Based Fly Ash Geopolymer at Elevated Temperatures. In Proceedings of the International Conference on Performance-based and Life-Cycle Structural Engineering, Brisbane, Australia, 9–11 December 2015. [Google Scholar] [CrossRef] [Green Version]
- Khater, H.M. Studying the effect of thermal and acid exposure on alkali-activated slag geopolymer. Adv. Cem. Res. 2014, 29, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Cui, N.; Xian, Y.; Liu, J.; Xing, C.; Xie, N.; Wang, D. Microstructure Evolution Mechanism of Geopolymers with Exposure to High-Temperature Environment. Crystals 2021, 11, 1062. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G. The shrinkage of alkali activated fly ash. Cem. Concr. Res. 2015, 68, 75–82. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Shayan, A. Effect of activator and water to binder ratios on setting and strength of geopolymer concrete. In Proceedings of the ARRB 27th Conference, Melbourne, VIC, Australia, 16–18 November 2016. [Google Scholar]
- Zulkifly, K.; Cheng-Yong, H.; Yun-Ming, L.; Bayuaji, R.; Abdullah, M.M.A.B.; Ahmad, S.B.; Stachowiak, T.; Szmidla, J.; Gondro, J.; Jez, B.; et al. Elevated-Temperature Performance, Combustibility and Fire Propagation Index of Fly Ash-Metakaolin Blend Geopolymers with Addition of Monoaluminium Phosphate (MAP) and Aluminum Dihydrogen Triphosphate (ATP). Materials 2021, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Korniejenko, K.; Figiela, B.; Ziejewska, C.; Marczyk, J.; Bazan, P.; Hebda, M.; Choińska, M.; Lin, W.-T. Fracture Behavior of Long Fiber Reinforced Geopolymer Composites at Different Operating Temperatures. Materials 2022, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Nurruddin, M.F.; Haruna, S.; Mohammed, B.S.; Sha΄aban, I.G. Methods of curing geopolymer concrete: A review. International. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N. Sulfate Resistance of Alkali Activated Pozzolans. Int. J. Concr. Struct. Mater. 2015, 9, 145–158. [Google Scholar] [CrossRef] [Green Version]
- San Nicolas, R.; Provis, J.L. The Interfacial Transition Zone in Alkali-Activated Slag Mortars. Front. Mater. 2015, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, H. Study of the Interfacial Transition Zone Characteristics of Geopolymer and Conventional Concretes. Gels 2022, 8, 105. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, C.S.; Peng, H.; Fan, F. Experimental Study of the Geopolymeric Recycled Aggregate Concrete. J. Mater. Civ. Eng. 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Demie, S.; Nuruddin, M.F.; Shafiq, N.; Fadhil, M.; Shafiq, N. Effects of micro-structure characteristics of interfacial transition zone on the compressive strength of self-compacting geopolymer concrete. Constr. Build. Mater. 2020, 41, 91–98. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Al Bakri, A.M.M.; Kamarudin, H.; Nizar, I.K.; Saif, A.A. Effects of elevated temperatures on the thermal behavior and mechanical performance of fly ash geopolymer paste, mortar and lightweight concrete. Constr. Build. Mater. 2014, 50, 377–387. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Effect of Liquid to Binder Ratio and Curing Temperature on the Engineering Properties of the Geopolymer Concrete. Silicon. 2022, 14, 1743–1757. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.M.; Binhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Effect of Curing Profile on Kaolin-based Geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.A. Optimum curing temperature and duration of alkali-activated glass inorganic binders. J. Chin. Inst. Eng. 2020, 43, 592. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, A.M.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I.; Kabir, S.M.A.A. Engineering properties and carbon footprint of ground granulated blast- furnace slag-palm oil fuel ash-based structural geopolymer concrete. Constr. Build. Mater. 2015, 101, 503–521. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Engineering Properties of Alkali Activated Natural Pozzolan Concrete. ACI Mater. J. 2011, 108, 64–72. [Google Scholar]
- Saif, M.S.; El-Hariri, M.O.R.; Sarie-Eldin, A.I.; Tayeh, B.A.; Farag, M.F. Impact of Ca+ content and curing condition on durability performance of Metakaolin-based Geopolymer Mortars. Case Stud. Constr. Mater. 2022, 16, e00922. [Google Scholar] [CrossRef]
- Chotetanorm, C.; Chindaprasirt, P.; Sata, V.; Rukzon, S.; Sathonsaowaphak, A. High-Calcium Bottom Ash Geopolymer: Sorptivity, Pore Size, and Resistance to Sodium Sulfate Attack. J. Mater. Civ. Eng. 2013, 25, 105–111. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Verma, M.; Nigam, M. Mechanical Behaviour of Self Compacting and Self Curing Concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 14361–14366. [Google Scholar] [CrossRef]
- Maanser, A.; Benouis, A.; Ferhoune, N. Effect of high temperature on strength and mass loss of admixtured concretes. Constr. Build. Mater. 2018, 166, 916–921. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Effect of SNF-Based Superplasticizer on Physical, Mechanical and Thermal Properties of the Geopolymer Concrete. Silicon 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash-based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Kusbiantoro, A.; Ibrahim, M.S.; Muthusamy, K.; Alias, A. Development of sucrose and citric acid as the natural-based admixture for fly ash-based geopolymer. Procedia Environ. Sci. 2013, 17, 596–602. [Google Scholar] [CrossRef] [Green Version]
- European Federation for Specialist Construction Chemicals and Concrete Systems. European Guidelines for Self-Compacting Concrete: Specification, Production and Use; Association House: Farnham, UK, 2005. [Google Scholar]
- Kaur, M.; Singh, J.; Kaur, M. Synthesis of fly ash based geopolymer mortar considering different concentrations and combinations of alkaline activator solution. Ceram. Int. 2018, 44, 1534–1537. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Flyash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Priyanka, R.; Selvaraj, R.; Rajesh, B. Characterization Study on Clay Based Geopolymer Concrete. Int. J. Res. Engg. Appl. Sci. 2015, 5, 49–55. [Google Scholar]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; Van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non. Cryst. Solids. 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P. Effect of SiO2 and Al2 O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Law, D. A state-of-the-art review on the durability of geopolymer concrete for sustainable structures and infrastructure. Constr. Build. Mater. 2021, 291, 123381. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Law, D. Durability performance of reinforced waste-based geopolymer foam concrete under exposure to various corrosive environments. Case Stud. Constr. Mater. 2021, 16, e00703. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; Van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. Constr. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Saavedra, W.G.V.; Angulo, D.E.; De Gutiérrez, R.M. Fly Ash Slag Geopolymer Concrete: Resistance to Sodium and Magnesium Sulfate Attack. J. Mater. Civ. Eng. 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Sukmak, P.; De Silva, P.; Horpibulsuk, S.; Chindaprasirt, P. Sulfate Resistance of Clay-Portland Cement and Clay High-Calcium Fly Ash Geopolymer. J. Mater. Civ. Eng. 2015, 27, 04014158. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Hussin, W.M.; Azreen, M.; Tahir, M.M. Sulphate Resistance of Geopolymer Concrete Prepared from Blended Waste Fuel Ash. J. Mater. Civ. Eng. 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Manjeeth, K.V.; Rama, J.S.K. An Experimental Investigation on the behaviour of Portland Cement Concrete and Geopolymer Concrete in acidic environment. Int. J. Civ. Eng. 2015, 2, 40–44. [Google Scholar] [CrossRef]
- Hosan, A.; Shaikh, F.U.A. Compressive strength development and durability properties of high volume slag and slag-fly ash blended concretes containing nano-CaCO3. J. Mater. Res. Technol. 2021, 10, 1310–1322. [Google Scholar] [CrossRef]
- Wiyono, D.; Hardjito, D.; Antoni, P.; Hardjito, D. Improving the durability of pozzolan concrete using alkaline solution and geopolymer coating. Procedia Eng. 2015, 125, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.V.; Edouard, J.-B.; Sobhan, K. Durability of Fly Ash-Based Geopolymer Structural Concrete in the Marine Environment. J. Mater. Civ. Eng. 2013, 6, 781–787. [Google Scholar] [CrossRef]
- Olivia, M.; Nikraz, H. Properties of fly ash geopolymer concrete designed by Taguchi method. Mater. Des. 2012, 36, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a blended slag-fly ash geopolymer concrete in field conditions after 8 years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Sanusi, O.; Tempest, B.; Ogunro, V.O.; Gergely, J. Leaching Characteristics of Geopolymer Cement Concrete Containing Recycled Concrete Aggregates. J. Hazard. Toxic Radioact. Waste 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Impact of Alkali Silica Reaction on Fly Ash-Based Geopolymer Concrete. J. Mater. Civ. Eng. 2013, 25, 131–139. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Examination of Chloride-Induced Corrosion in Reinforced Geopolymer Concretes. J. Mater. Civ. Eng. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P.; Balchandra, A.; Peyvandi, A. Characterization of Alkali-Activated Non-wood Biomass Ash–Based Geopolymer Concrete. J. Mater. Civ. Eng. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Kaya, M.; Uysal, M.; Yilmaz, K.; Atis, C.D. Behaviour of Geopolymer Mortars after Exposure to Elevated Temperatures. Mater. Sci. 2018, 24, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- Ren, W.; Xu, J.; Bai, E. Strength and Ultrasonic Characteristics of Alkali-Activated Fly Ash-Slag Geopolymer Concrete after Exposure to Elevated Temperatures. J. Mater. Civ. Eng. 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G.; Collins, F. Cement and Concrete Research Effect of transient creep on compressive strength of geopolymer concrete for elevated temperature exposure. Cem. Concr. Res. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Hakamy, A.; Sherif, M.A.; Zeyad, A.M.; Agwa, I.S. Effect of air agent on mechanical properties and microstructure of lightweight geopolymer concrete under high temperature. Case Stud. Constr. Mater. 2022, 16, e00951. [Google Scholar] [CrossRef]
- Sherif, M.A.; Zeyad, A.M.; Tayeh, B.A.; Agwa, I.S. Effect of high temperatures on mechanical, radiation attenuation and microstructure properties of heavyweight geopolymer concrete. Struct. Eng. Mech. 2021, 80, 181–199. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.L. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Snell, C.; Tempest, B.; Gentry, T. Comparison of the Thermal Characteristics of Portland Cement and Geopolymer Cement Concrete Mixes. J. Archit. Eng. 2017, 23, 1–10. [Google Scholar] [CrossRef]
- Rivera, O.G.; Long, W.R.; Weiss, C.A., Jr.; Moser, R.D.; Williams, B.A.; Torres-Cancel, K.; Gore, E.R.; Allison, P.G. Effect of elevated temperature on alkali-activated geopolymeric binders compared to portland cement-based binders. Cem. Concr. Res. 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Sarker, P.K. Bond strength of reinforcing steel embedded in fly ash-based geopolymer concrete. Mater. Struct. 2011, 44, 1021–1030. [Google Scholar] [CrossRef]
- Luan, C.; Wang, Q.; Yang, F.; Zhang, K.; Utashev, N.; Dai, J.; Shi, X. Practical Prediction Models of Tensile Strength and Reinforcement-Concrete Bond Strength of Low-Calcium Fly Ash Geopolymer Concrete. Polymers 2021, 13, 875. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Yu, T.; Hadi, M.N.S. Factors Affecting the Bond Strength Between the Fly Ash-based Geopolymer Concrete and Steel Reinforcement. Structures 2018, 14, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Tekle, B.H.; Khennane, A.; Kayali, O. Bond Properties of Sand-Coated GFRP Bars with Fly Ash–Based Geopolymer Concrete. J. Compos. Constr. 2016, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sing Ng, T.; Amin, A.; Foster, S.J. The behaviour of steel-fibre-reinforced geopolymer concrete beams in shear. Mag. Concr. Res. 2013, 65, 308–318. [Google Scholar] [CrossRef]
- Maranan, G.; Manalo, A.; Karunasena, K.; Benmokrane, B. Bond Stress-Slip Behavior: Case of GFRP Bars in Geopolymer Concrete. J. Mater. Civ. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Yost, J.R.; Radlińska, A.; Ernst, S.; Salera, M. Structural behavior of alkali activated fly ash concrete. Part 1: Mixture design, material properties and sample fabrication. Mater. Struct. 2012, 46, 435–447. [Google Scholar] [CrossRef]
- Prakash, A.S.; Kumar, G.S. Experimental Study on Geopolymer Concrete using Steel Fibres. Int. J. Eng. Trends Technol. 2015, 21, 396–399. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.S.M.; Visintin, P. Experimental study on fly ash and lead smelter slag-based geopolymer concrete columns. Constr. Build. Mater. 2017, 141, 104–112. [Google Scholar] [CrossRef]
- AS 3600–2009: Australian Standard for Concrete Structures; Standards Australia Limited: Sydney, NSW, Australia, 2001. Available online: www.standards.org.au (accessed on 9 March 2022).
- Srividya, T.; Kannan, R.P.R.; Sivasakthi, M.; Sujitha, A.; Jeyalakshmi, R. A state-of-the-art on development of geopolymer concrete and its field applications. Case Stud. Constr. Mater. 2022, 16, e00812. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Figiela, B.; Šimonová, H.; Korniejenko, K. State of the art, challenges, and emerging trends: Geopolymer composite reinforced by dispersed steel fibers. Rev. Adv. Mater. Sci. 2022, 61, 1–15. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Arpajirakul, S.; Pungrasmi, W.; Likitlersuang, S. Efficiency of microbially-induced calcite precipitation in natural clays for ground improvement. Constr. Build. Mater. 2021, 282, 122722. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, J.; Wang, Y. Durability properties of sustainable alkali-activated cementitious materials as marine engineering material: A review. Mater. Today Sustain. 2022, 17, 100099. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; Liu, Y.; Wang, E.; He, Z.; Xu, H.; Ren, X. Preparation and characterization of lightweight aggregate foamed geopolymer concretes aerated using hydrogen peroxide. Constr. Build. Mater. 2020, 256, 119442. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, C.; Hao, H.; Li, J.; Wu, P.; Yang, Y.; Wang, Z. Steel fibre reinforced alkali-activated geopolymer concrete slabs subjected to natural gas explosion in buried utility tunnel. Constr. Build. Mater. 2020, 246, 118447. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Miraldo, S.; Pacheco-Torgal, F.; Aguiar, J.B. Cost-efficient one-part alkali-activated mortars with low global warming potential for floor heating systems applications. Eur. J. Environ. Civ. Eng. 2015, 21, 412–429. [Google Scholar] [CrossRef] [Green Version]
- Sierraab, V.; Chejne, F. Energy saving evaluation of microencapsulated phase change materials embedded in building systems. J. Energy Storage 2022, 49, 104102. [Google Scholar] [CrossRef]
- Rahjoo, M.; Goracci, G.; Martauz, P.; Rojas, E.; Dolado, J.S. Geopolymer Concrete Performance Study for High-Temperature Thermal Energy Storage (TES) Applications. Sustainability 2022, 14, 1937. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice Husk Ash-Based Geopolymer Binder: Compressive Strength, Optimize Composition, FTIR Spectroscopy, Microstructural, and Potential as Fire-Retardant Material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Rovnaník, P.; Kusák, I.; Bayer, P.; Schmid, P.; Fiala, L. Comparison of electrical and self-sensing properties of Portland cement and alkali-activated slag mortars. Cem. Concr. Res. 2019, 118, 84–91. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Miraldo, S.; Baklouti, S.; Ding, Y. An overview on the potential of geopolymers for concrete infrastructure rehabilitation. Constr. Build. Mater. 2012, 36, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Castel, A.; Khan, M. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials 2022, 15, 1725. [Google Scholar] [CrossRef]
- Abulencia, A.B.; Villoria, M.B.D.; Libre, R.G.D.; Quiatchon, P.R.J.; Dollente, I.J.R.; Guades, E.J.; Promentilla, M.A.B.; Garciano, L.E.O.; Ongpeng, J.M.C. Geopolymers as sustainable material for strengthening and restoring unreinforced masonry structures: A review. Buildings 2021, 11, 532. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Kantarc, F.; Maras, M.M. Formulation of a novel nano TiO2-modified geopolymer grout for application in damaged beam-column joints. Constr. Build. Mater. 2022, 317, 125929. [Google Scholar] [CrossRef]
- Rao, S.M.; Acharya, I.P. Synthesis and Characterization of Fly Ash Geopolymer Sand. J. Mater. Civ. Eng. 2014, 1, 912–917. [Google Scholar] [CrossRef]
- Kashani, A.; Ngo, T. Optimisation of mixture properties for 3D printing of geopolymer concrete. In Proceedings of the International Symposium on Automation and Robotics in Construction, Berlin, Germany, 20–25 July 2018; pp. 1–8. [Google Scholar]
- Bagheri, A.; Cremona, C. Formulation of mix design for 3D printing of geopolymers: A machine learning approach. Mater. Adv. 2020, 1, 720–727. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Sglavo, V.M. 3D printing of geopolymer-based concrete for building applications. Rapid Prototyp. J. 2020, 26, 1783–1788. [Google Scholar] [CrossRef]
- Voney, V.; Odaglia, P.; Brumaud, C.; Dillenburger, B.; Habert, G. From casting to 3D printing geopolymers: A proof of concept. Cem. Concr. Res. 2021, 143, 106374. [Google Scholar] [CrossRef]
- Gasca-Tirado, R.; Manzano-Ramírez, A.; RiveraMuñoz, E.M.; Velázquez-Castillo, R.; Apátiga-Castro, M.; Nava, R.; Rodríguez-López, A. Ion Exchange in Geopolymers. In New Trends in Ion Exchange Studies; Karakuş, S., Ed.; IntechOpen: London, UK, 2018; Chapter 5. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Zribi, M.; Adeniyi, F.; Saha, S.; Baklouti, S.; Shaikh, F.U.A.; Gökçe, H.S. A review on developments of environmentally friendly geopolymer technology. Materialia 2021, 20, 101212. [Google Scholar] [CrossRef]
- Jihui, Z. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar]
- Karthik, S.; Mohan, K.S.R. A Taguchi Approach for Optimizing Design Mixture of Geopolymer Concrete Incorporating Fly Ash, Ground Granulated Blast Furnace Slag and Silica Fume. Crystals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Reddy, B.V.V.; Jagadish, K.S. Embodied energy of common and alternative building materials and technologies. Energy Build. 2003, 35, 129–137. [Google Scholar] [CrossRef]
- Anvekar, S.R.; Manjunatha, L.R.; Anvekar, S.R.; Sagari, S.; Archana, K. An Economic and Embodied Energy Comparison of Geo-polymer, Blended Cement and Traditional Concretes. J. Civ. Eng. Technol. Res. 2014, 1, 33–40. [Google Scholar]
- Al-Hamrani, A.; Kucukvar, M.; Alnahhal, W.; Mahdi, E.; Onat, N.C. Green Concrete for a Circular Economy: A Review on Sustainability, Durability, and Structural Properties. Materials 2021, 14, 351. [Google Scholar] [CrossRef]
- de Azevedo, R.; Marvila, M.T.; de Oliveira, L.B.; Ferreira, W.M.; Colorado, H.; Rainho Teixeira, S.; Vieira, C.M.F. Circular economy and durability in geopolymers ceramics pieces obtained from glass polishing waste. Int. J. Appl. Ceram. Technol. 2021, 18, 1891–1900. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Torkashvand, M.; Mohammad, M.; Alghoul, M.A.; Gasaymeh, S.S.; Sopian, K. Wastes from the petroleum industries as sustainable resource materials in construction sectors: Opportunities, limitations, and directions. J. Clean. Prod. 2021, 284, 125459. [Google Scholar] [CrossRef]
- Mathew, B.J.; Sudhakar, M.; Natarajan, C. Strength, Economic and Sustainability Characteristics of Coal Ash –GGBS Based Geopolymer Concrete. Int.J. Comput. Eng. Res. 2013, 3, 207–212. [Google Scholar]
- Weil, M.; Dombrowski-Daube, K.; Buchwald, A. Geopolymer binders-Part 3: Ecological and economic analyses of geopolymer concrete mixes for external structural elements. ZKG Int. 2011, 64, 76–87. [Google Scholar]
- Youssef, N.; Lafhaj, Z.; Chapiseau, C. Economic Analysis of Geopolymer Brick Manufacturing: A French Case Study. Sustainability 2020, 12, 7403. [Google Scholar] [CrossRef]
- van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Shamsaei, E.; Bolt, O.; Basquiroto de Souza, F.; Benhelal, E.; Sagoe-Crentsil, K.; Sanjayan, J. Pathways to Commercialisation for Brown Coal Fly Ash-Based Geopolymer Concrete in Australia. Sustainability 2021, 13, 4350. [Google Scholar] [CrossRef]
- Munir, Q.; Kärki, T. Cost Analysis of Various Factors for Geopolymer 3D Printing of Construction Products in Factories and on Construction Sites. Recycling 2021, 6, 60. [Google Scholar] [CrossRef]
| Si/Al Ratio | Applications |
|---|---|
| 1 |
|
| 2 |
|
| 3 |
|
| >3 |
|
| 20–35 |
|
| Embodied Energy (MJ/kg) | OPC Concrete | Geopolymer Concrete | |||
|---|---|---|---|---|---|
| Mix Content (kg/m3) | Embodied Energy Content (MJ/kg) | Mix Content (kg/m3) | Embodied Energy Content (MJ/kg) | ||
| OPC | 4.2 | 370 | 1554 | 0.0 | 0.0 |
| Fly ash | 0.0 | 0.0 | 0.0 | 303.75 | 0.0 |
| GGBFS | 0.31 | 0.0 | 0.0 | 101.25 | 31.38 |
| NaOH | 20.5 | 0.0 | 0.0 | 40.5 | 830.25 |
| Na2SiO3 | 5.37 | 0.0 | 0.0 | 101.25 | 543.71 |
| Fine Aggregate | 0.02 | 683 | 13.66 | 683 | 13.66 |
| Coarse Aggregate | 0.22 | 1289 | 283.58 | 1269 | 279.18 |
| Water | 0.0 | 148 | 0.0 | 40.5 | 0.0 |
| Superplasticizer | 12.6 | 3.7 | 46.62 | 4.05 | 51.03 |
| Total | 2493.7 | 1897.86 | 2543.7 | 1749.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, M.; Dev, N.; Rahman, I.; Nigam, M.; Ahmed, M.; Mallick, J. Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries. Crystals 2022, 12, 514. https://doi.org/10.3390/cryst12040514
Verma M, Dev N, Rahman I, Nigam M, Ahmed M, Mallick J. Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries. Crystals. 2022; 12(4):514. https://doi.org/10.3390/cryst12040514
Chicago/Turabian StyleVerma, Manvendra, Nirendra Dev, Ibadur Rahman, Mayank Nigam, Mohd. Ahmed, and Javed Mallick. 2022. "Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries" Crystals 12, no. 4: 514. https://doi.org/10.3390/cryst12040514
APA StyleVerma, M., Dev, N., Rahman, I., Nigam, M., Ahmed, M., & Mallick, J. (2022). Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries. Crystals, 12(4), 514. https://doi.org/10.3390/cryst12040514






