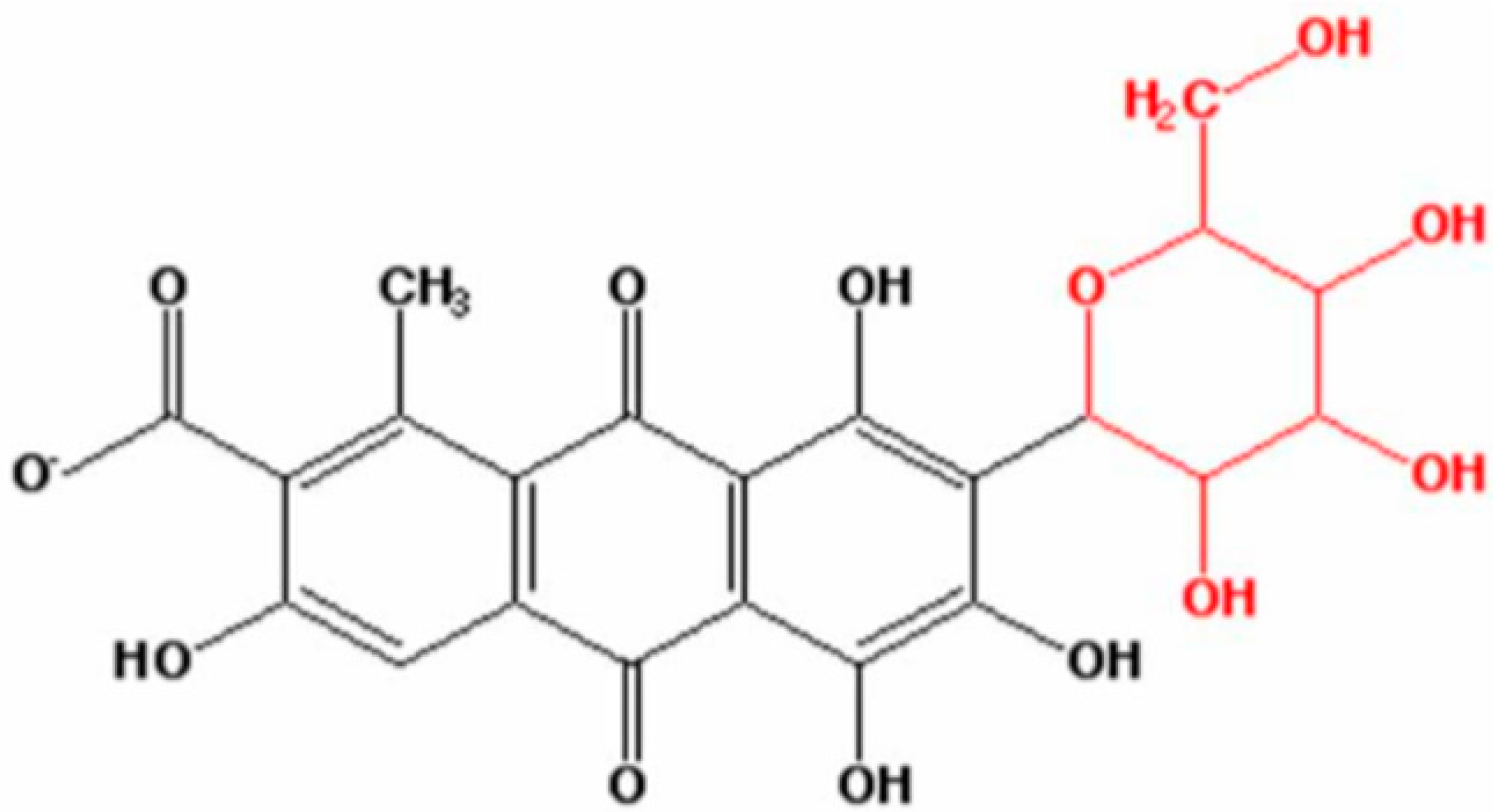
Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures
Abstract
:1. Introduction
2. Experimental Details
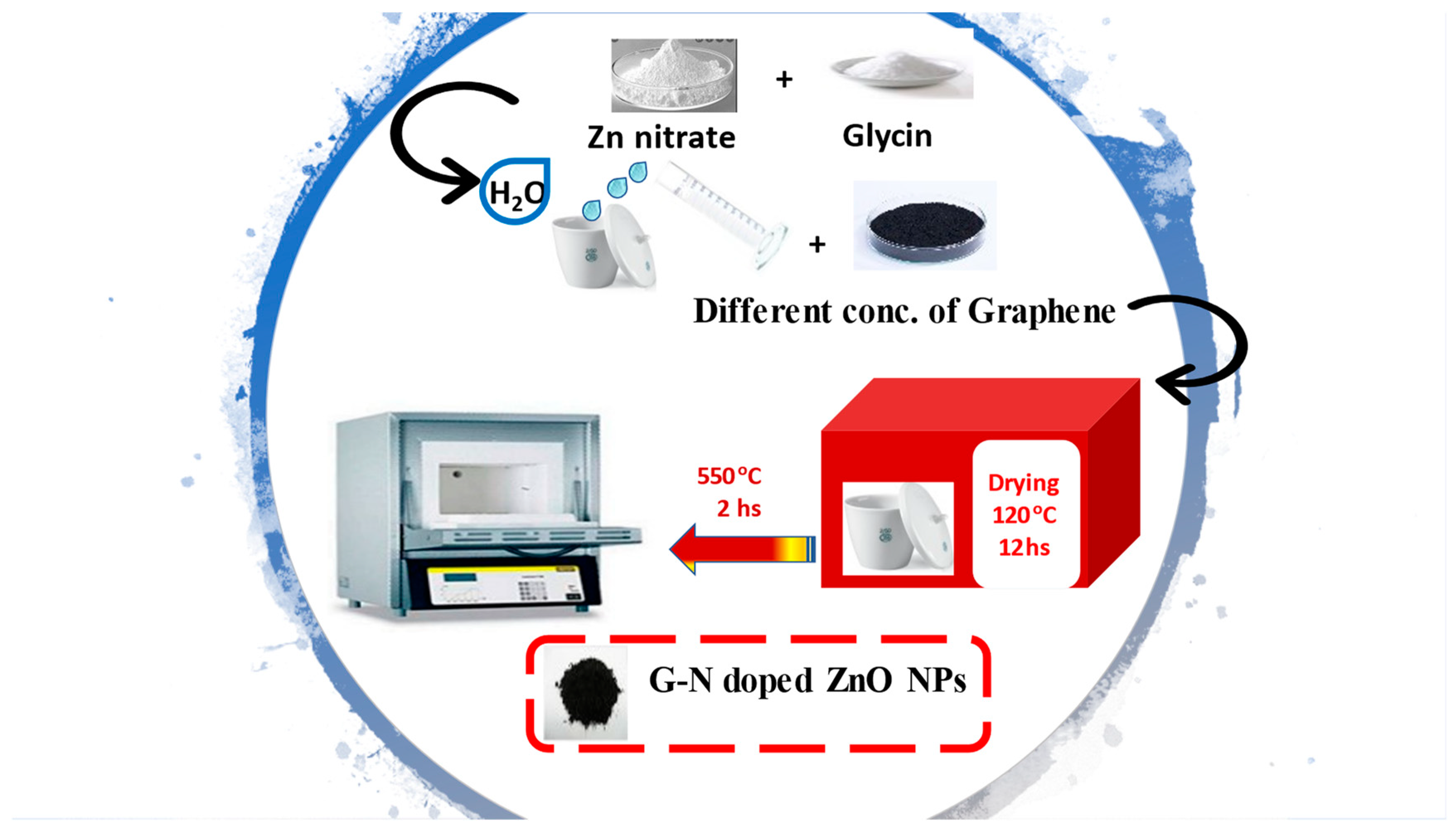
2.1. Synthesis of Nanostructured G/N-Doped ZnO
2.2. Characterization Techniques and Devices
2.3. Degradation Studies
2.3.1. In Situ Photocatalytic Degradation Studies
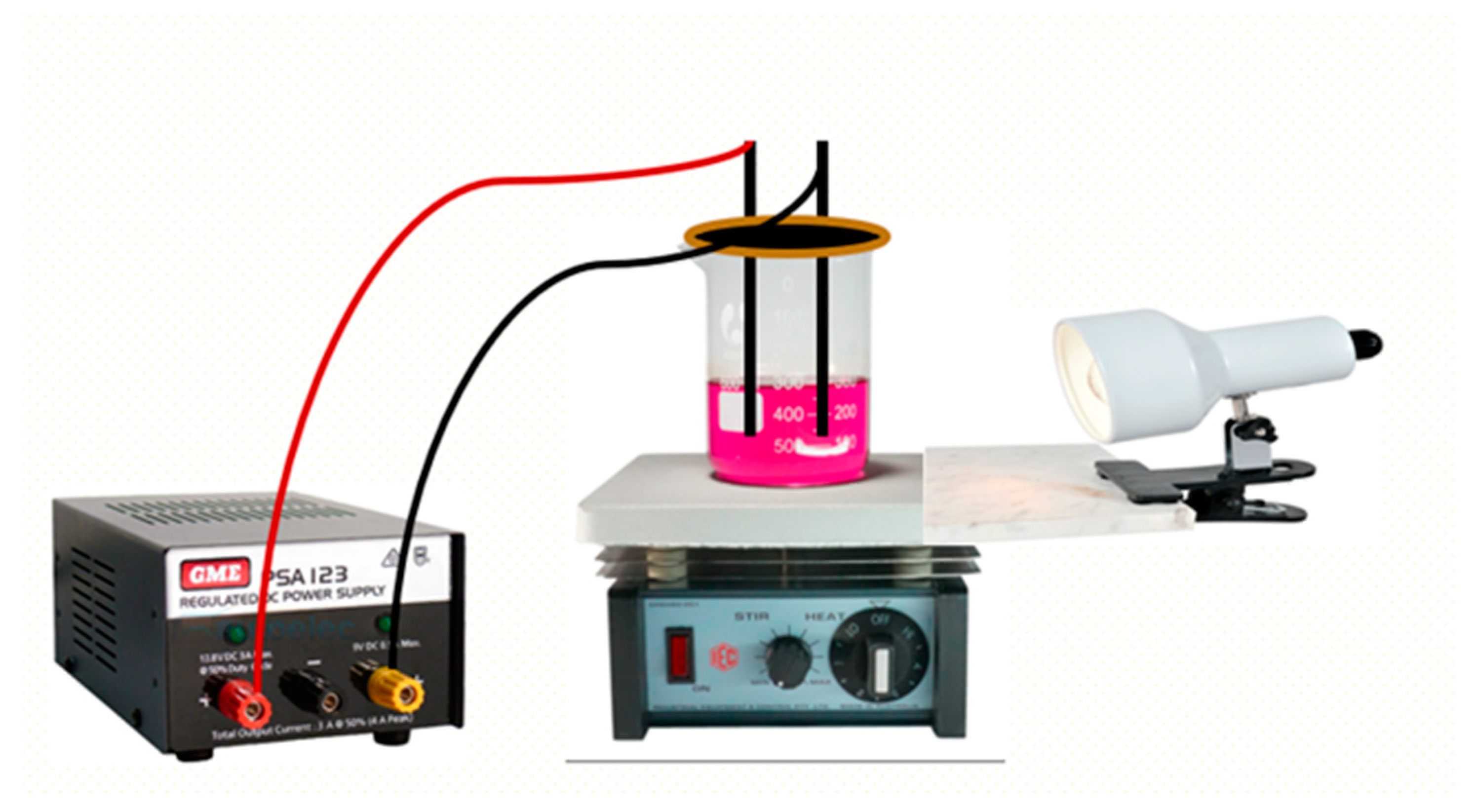
2.3.2. Electro- and Photoelectrochemical Degradation Experiment
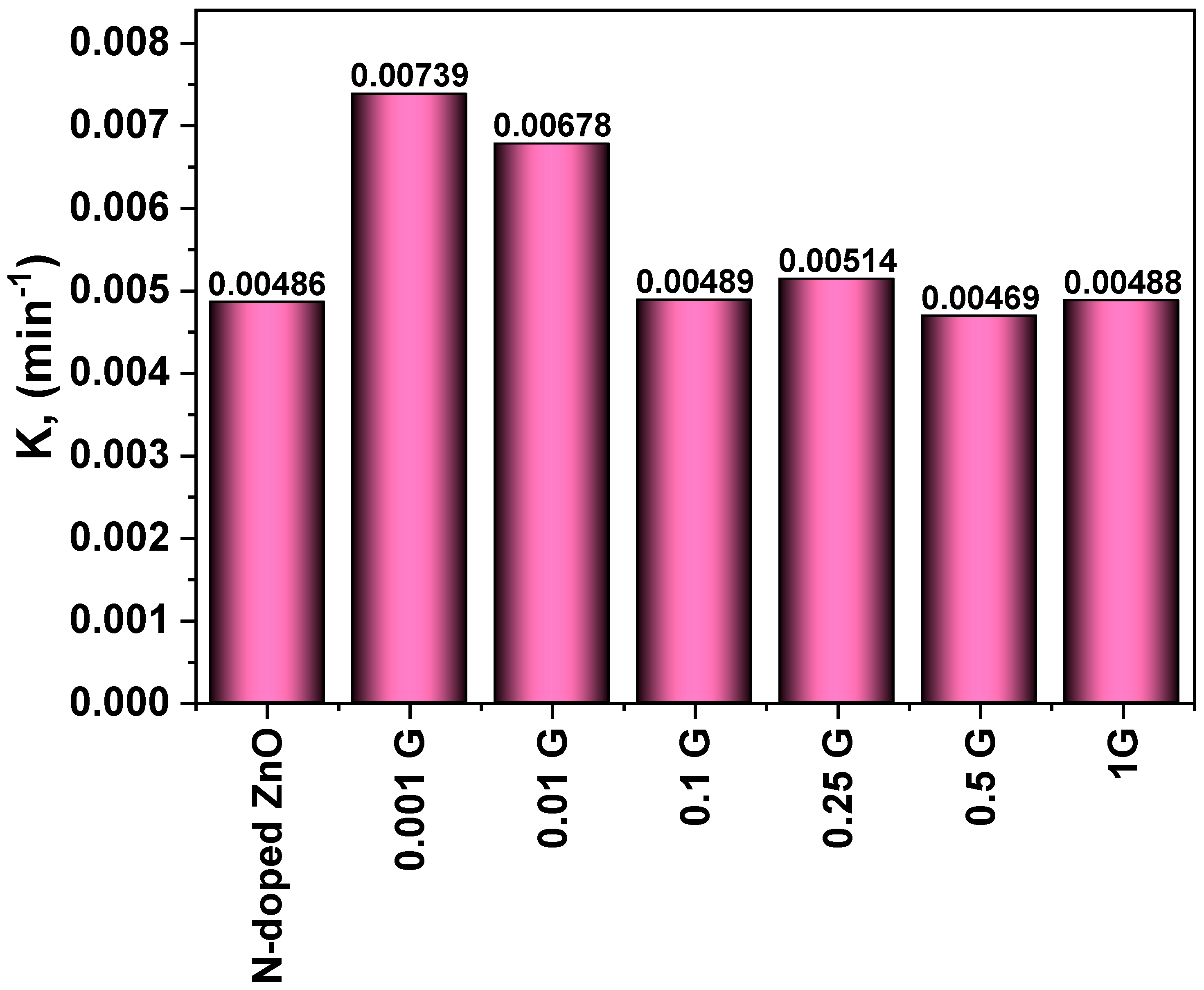
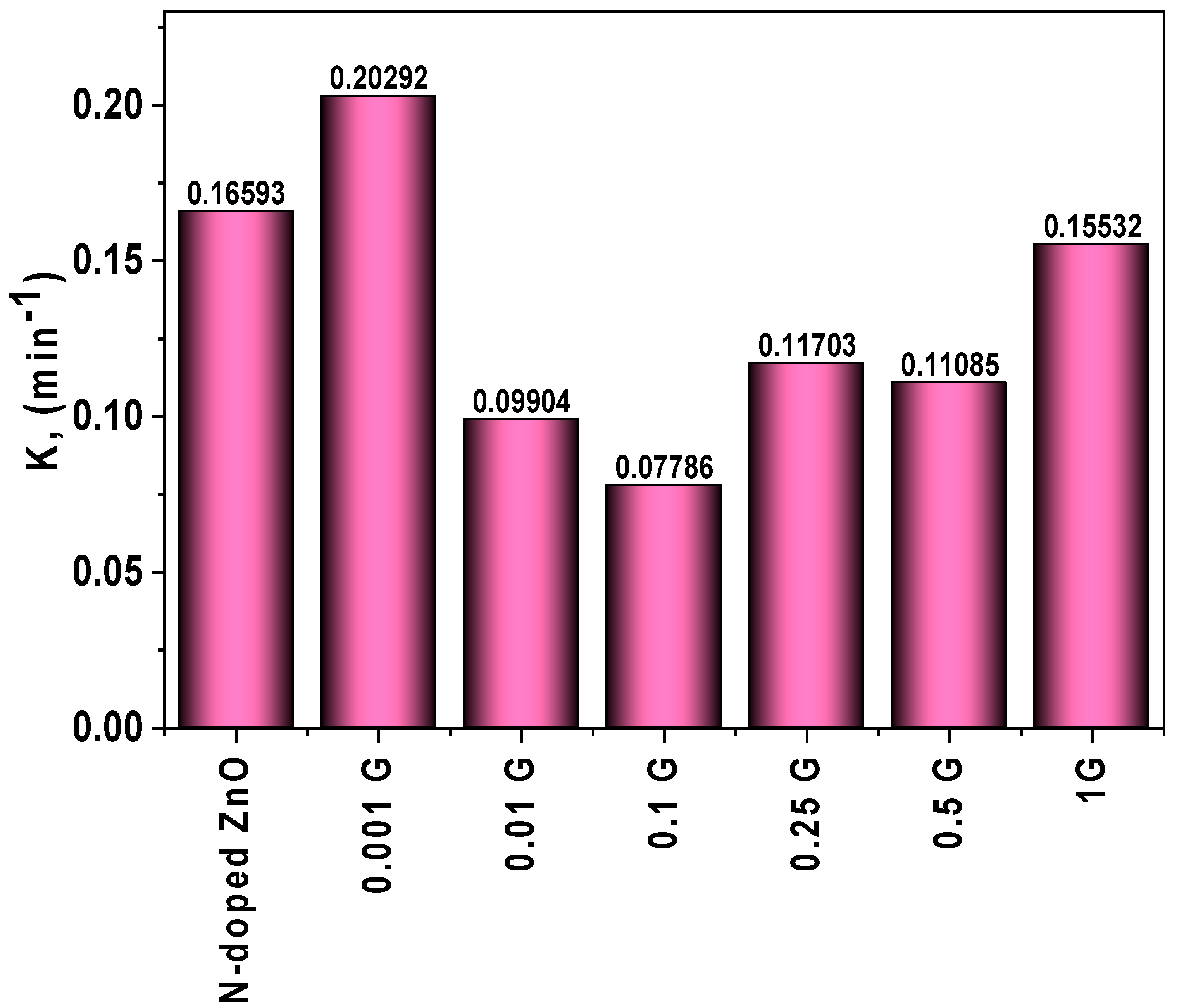
2.4. Kinetic Studies
3. Results and Discussion
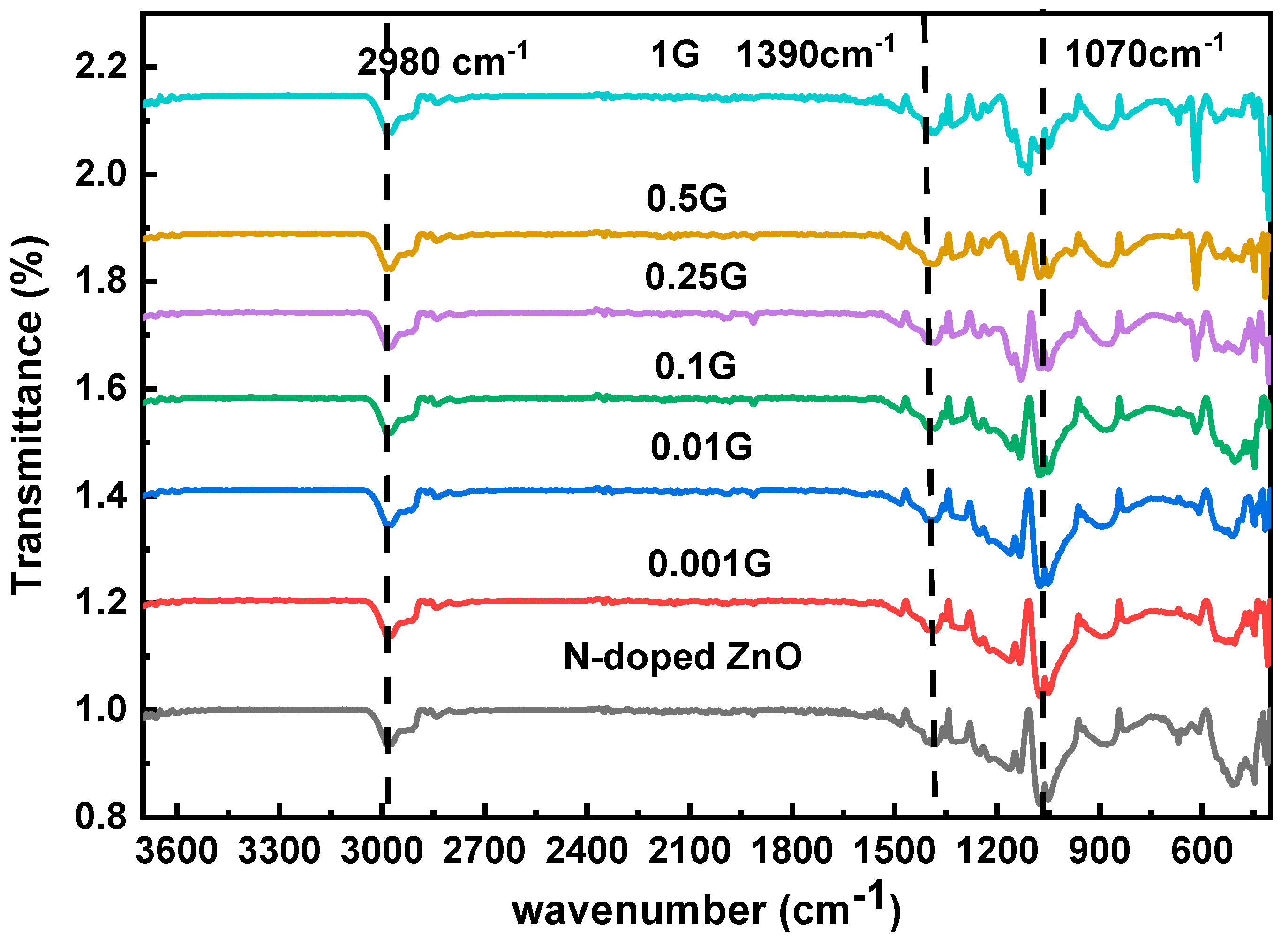
3.1. FT-IR Characterization
3.2. X-ray Diffraction Characterization
3.3. Optical Characterization
3.4. Morphology and Compositional Analysis
3.5. Degradation of Carmine Dye Methods
3.5.1. Photocatalytic Degradation of Carmine
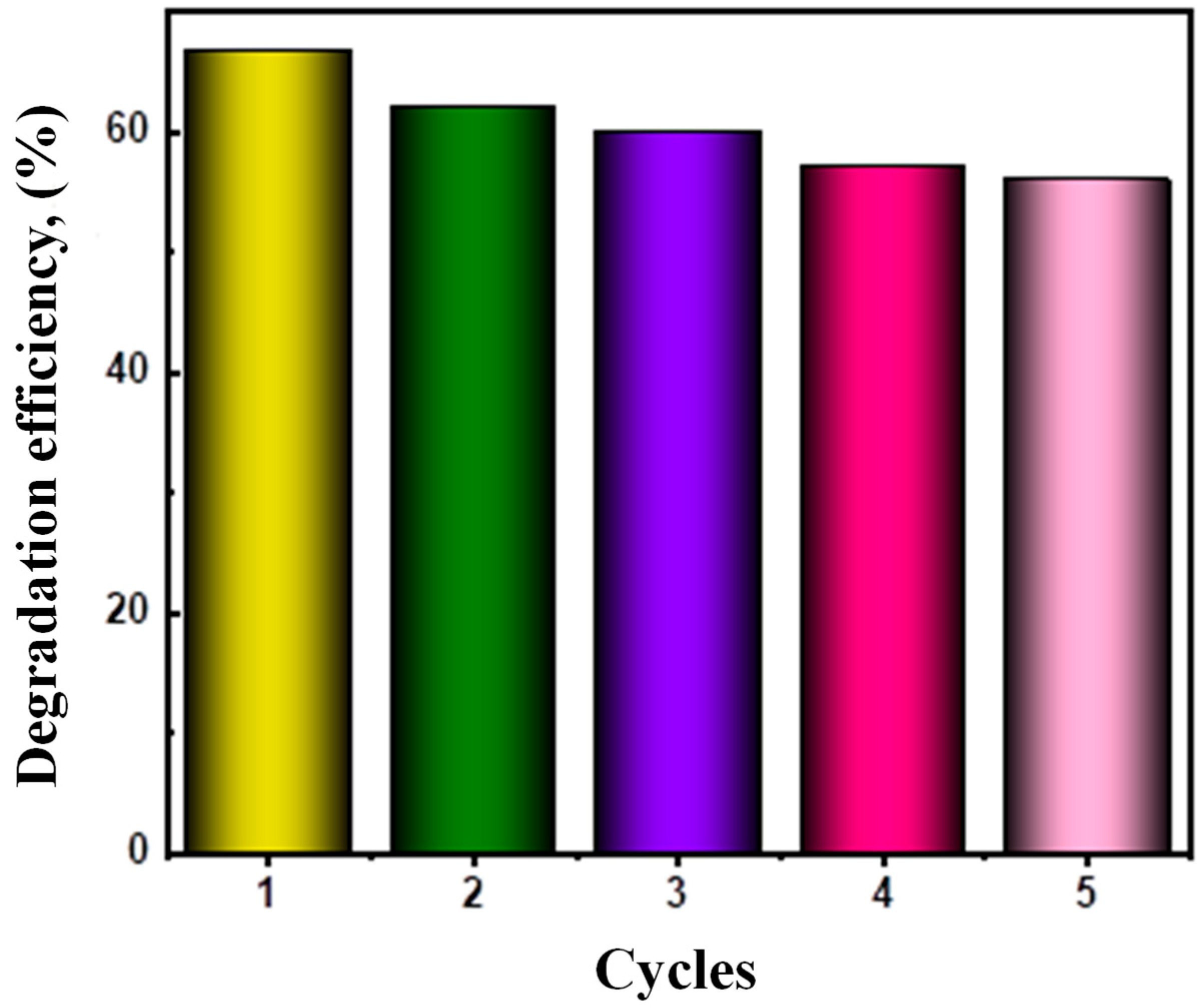
Reusability and Stability
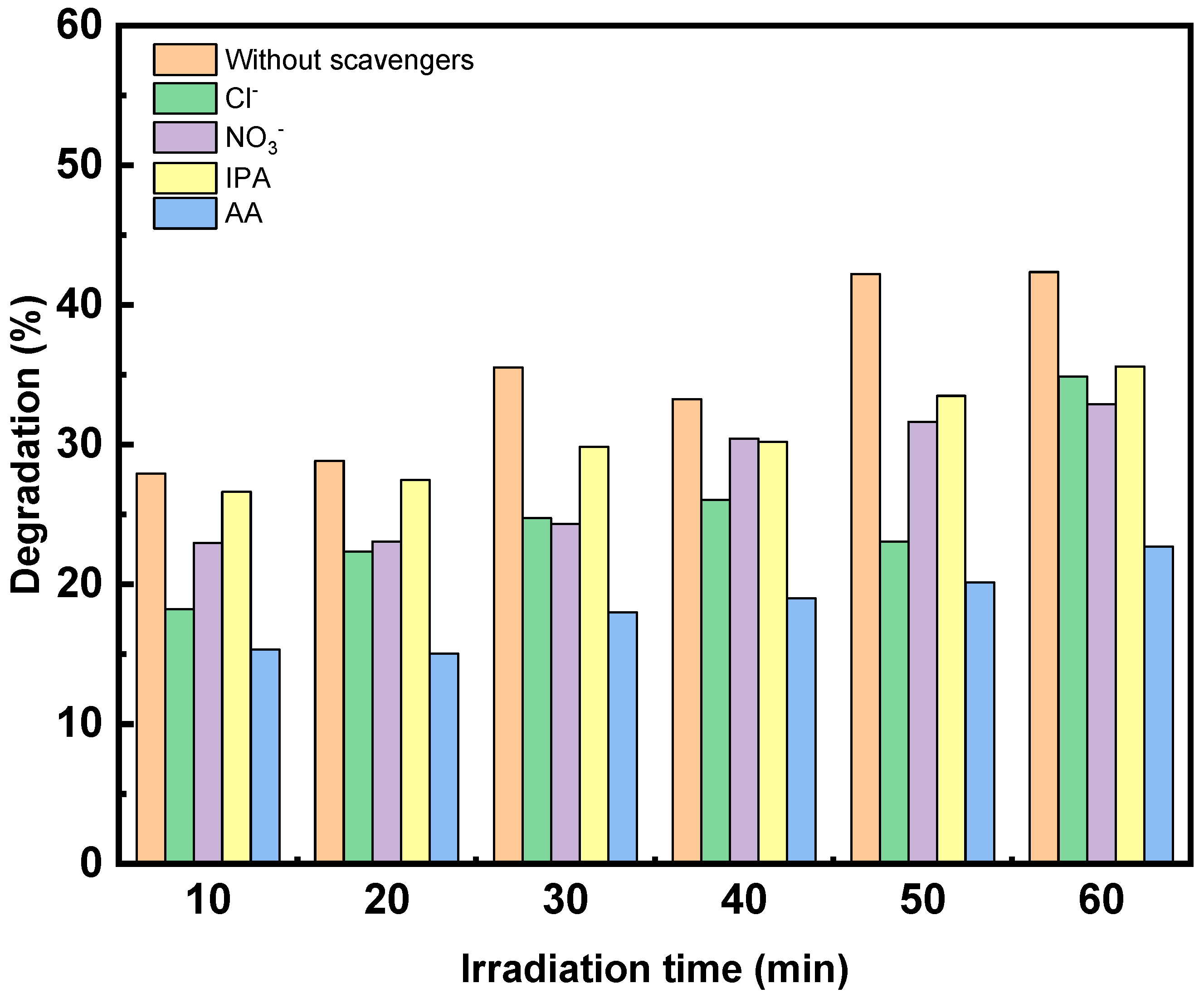
Influence of Radicals Scavengers on the Photocatalytic Activity
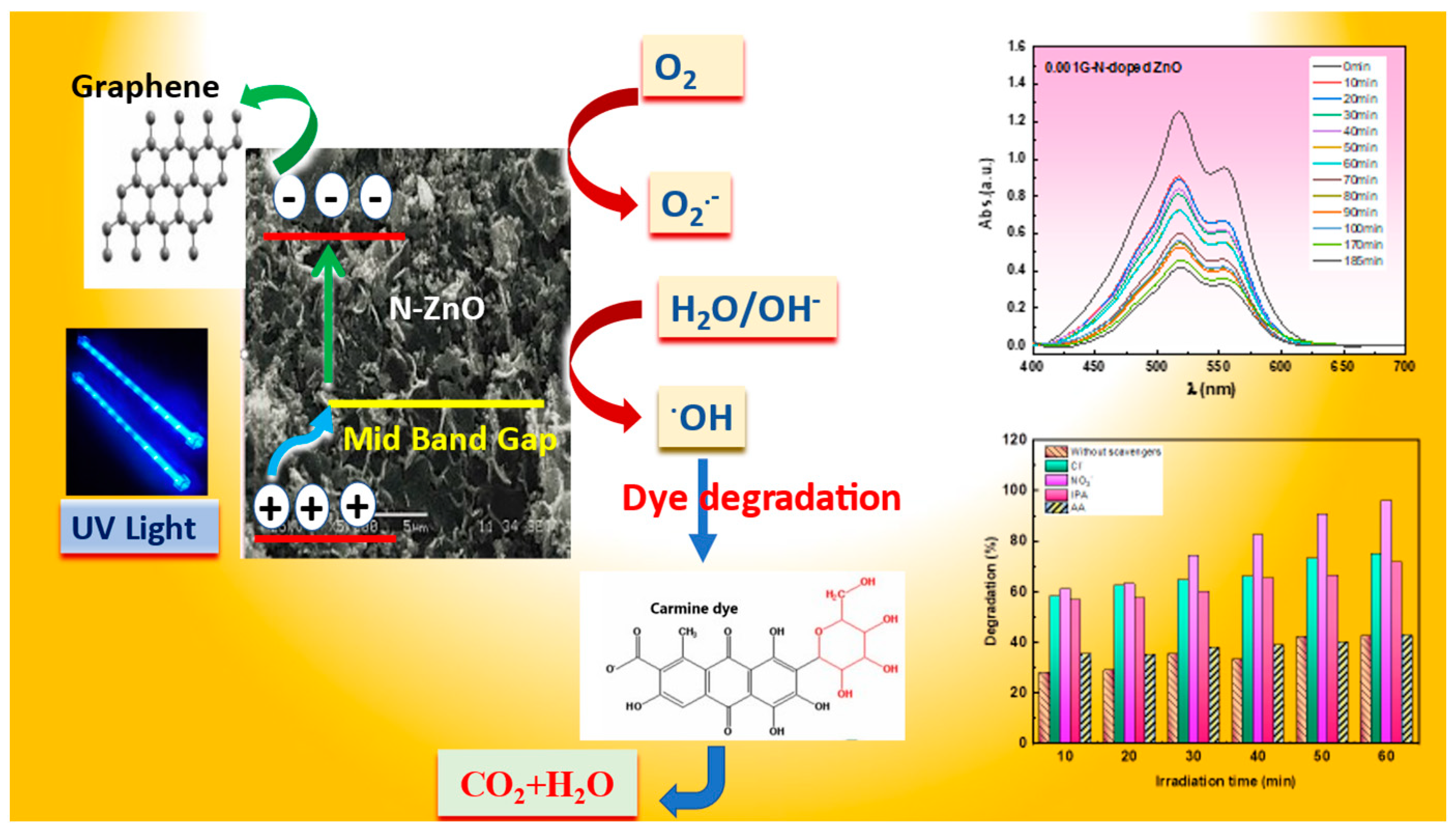
Mechanism of the Photocatalyst of Carmine Dye Degradation
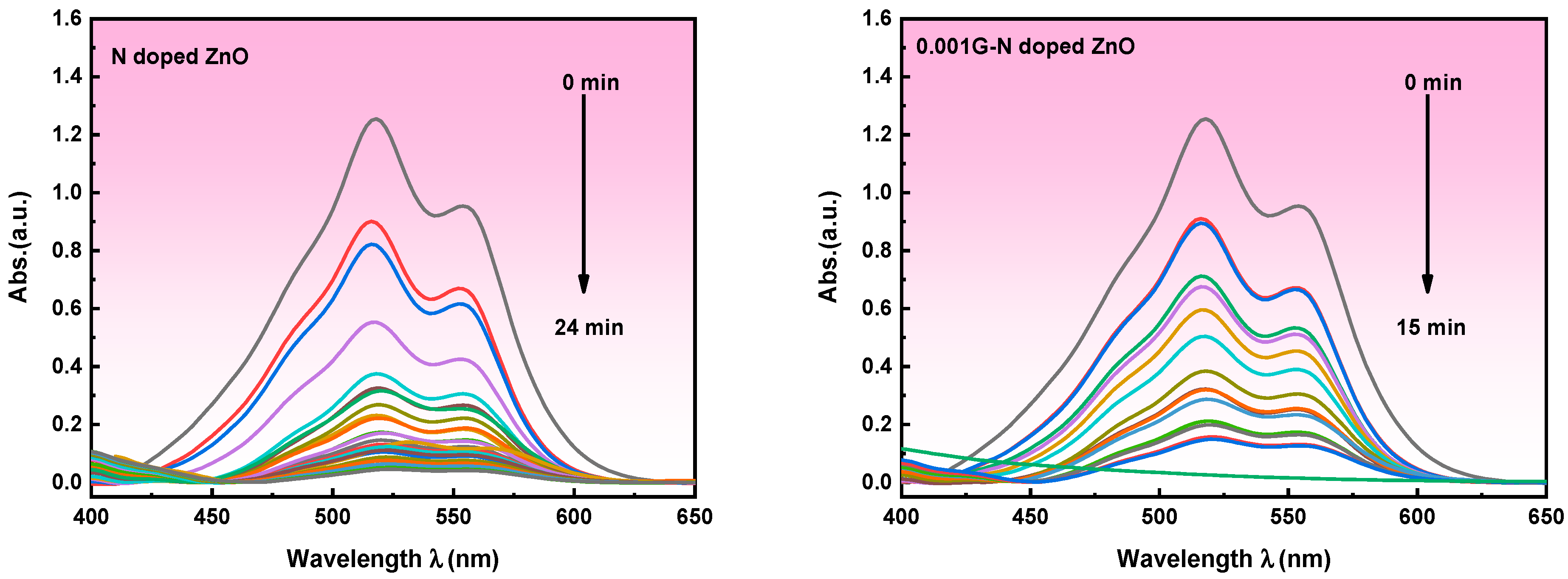
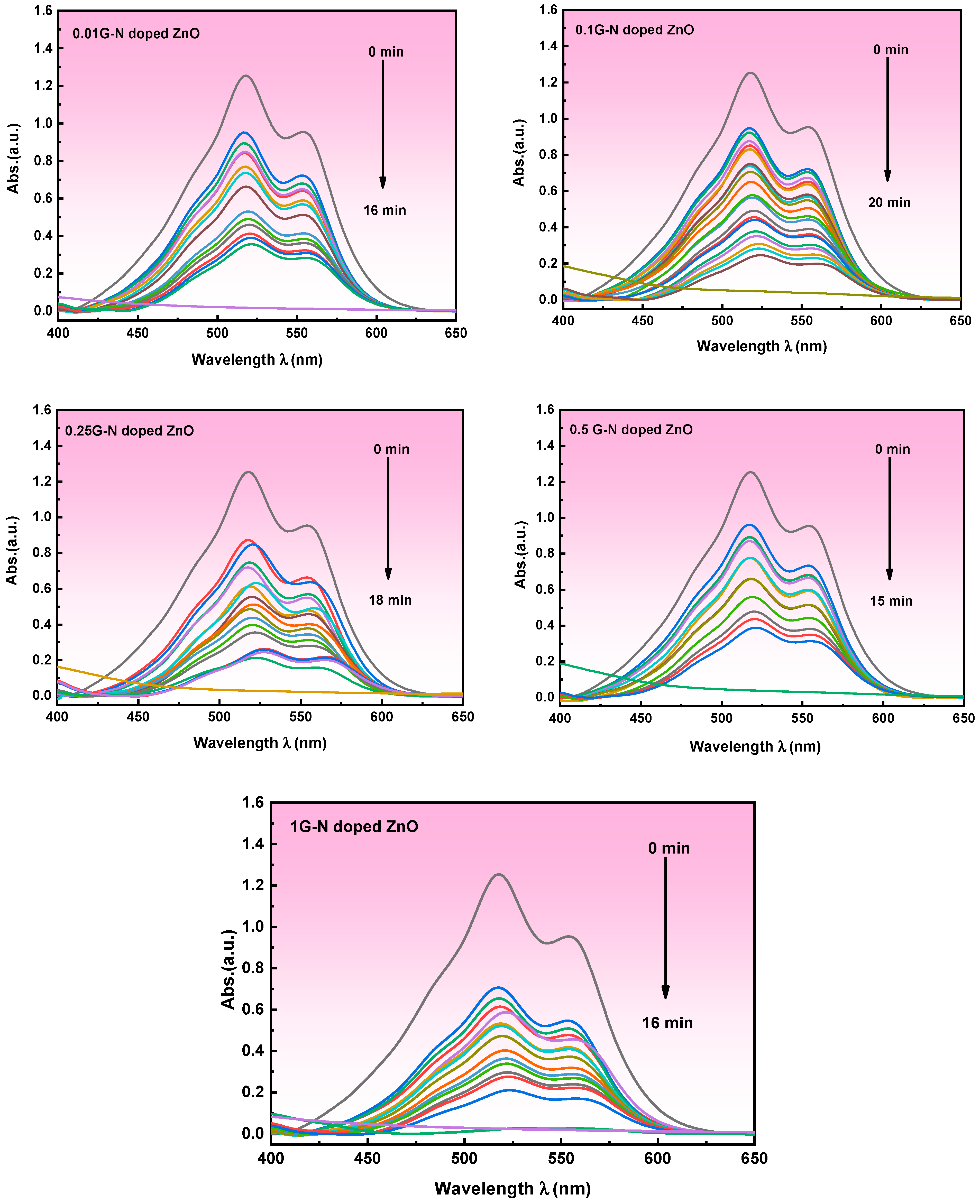
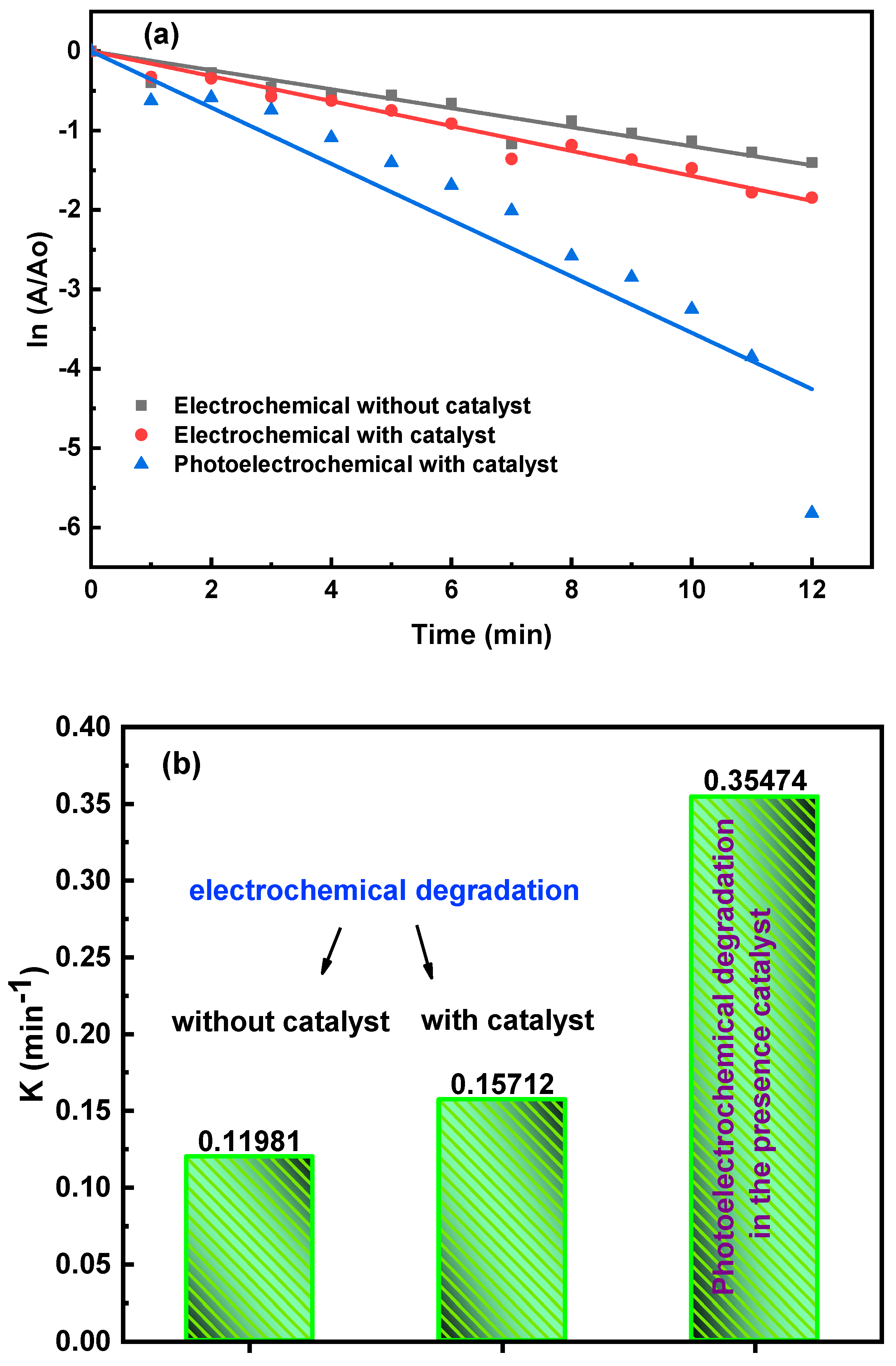
3.5.2. Indirect Electrochemical Degradation of Carmine Dye
Mechanism of Indirect Electrochemical Oxidation of Carmine Dye Degradation
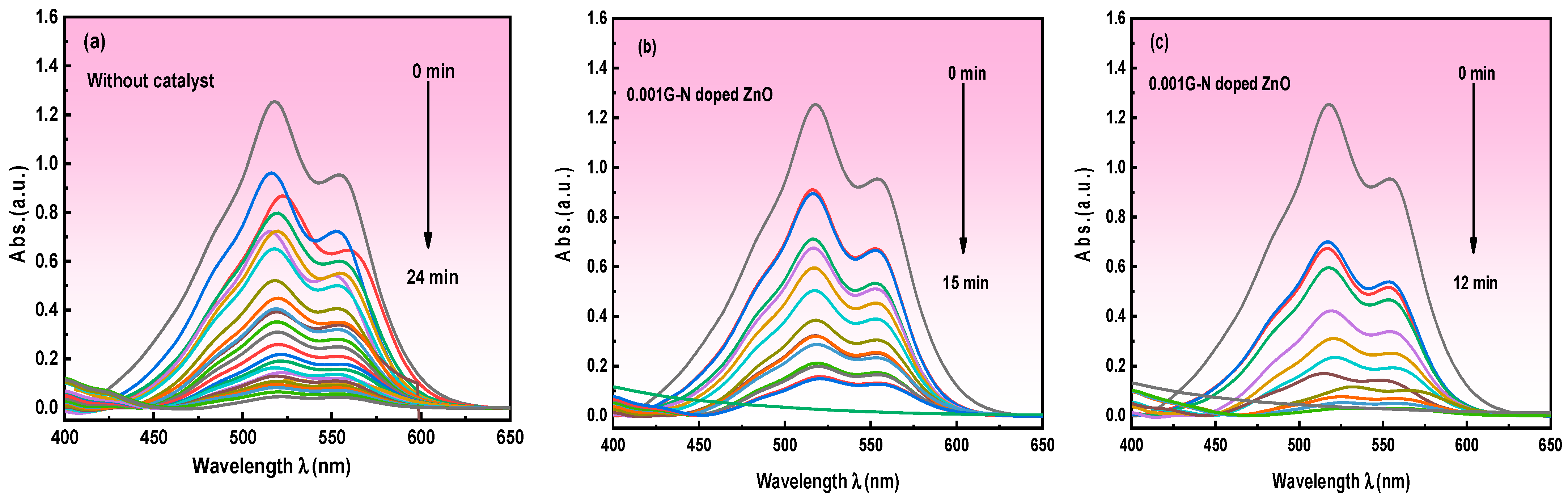
3.5.3. Photoelectrochemical Degradation (P.E.C.) of Carmine Dye
Mechanism of Photoelectrochemical Oxidation of Carmine Dye Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barka, N.; Assabbane, A.; Nounah, A.; Ichou, Y.A. Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. J. Hazard. Mater. 2008, 152, 1054–1059. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. J. Chem. Eng. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Dapson, R.W. The history, chemistry and modes of action of carmine and related dyes. Biotech. Histochem. 2007, 82, 173–187. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Basic principles of the electrochemical mineralization of organic pollutants for wastewater treatment. In Electrochemistry for the Environment; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photochemical oxidation of reactive azo dye with UV-H2O2 process. Dyes Pigment. 2004, 62, 269–275. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Zhou, M.; He, J. Degradation of cationic red X-GRL by electrochemical oxidation on modified PbO2 electrode. J. Hazard. Mater. 2008, 153, 357–363. [Google Scholar] [CrossRef]
- Fukunaga, M.T.; Guimarães, J.R.; Bertazzoli, R. Kinetics of the oxidation of formaldehyde in a flow electrochemical reactor with TiO2/RuO2 anode. J. Chem. Eng. 2008, 136, 236–241. [Google Scholar] [CrossRef]
- Harikishore, M.; Sandhyarani, M.; Venkateswarlu, K.; Nellaippan, T.A.; Rameshbabu, N. Effect of Ag Doping on Antibacterial and Photocatalytic Activity of Nanocrystalline TiO2. Procedia Mater. Sci. 2014, 6, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Pletcher, D.; Walsh, F.C. Electrodeposited lead dioxide coatings. Chem. Soc. Rev. 2011, 40, 3879–3894. [Google Scholar] [CrossRef] [Green Version]
- Hassanvand, A.; Sohrabi, M.; Royaee, S.J.; Jafarikajour, M. Preparation and characterization of nitrogen doped TiO2 nanoparticles as an effective catalyst in photodegradation of phenol under visible light. In Advanced Materials Research; Scientific.Net: Baech, Switzerland, 2014. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Janssen, L.J.J.; Koene, L. The role of electrochemistry and electrochemical technology in environmental protection. J. Chem. Eng. 2002, 85, 137–146. [Google Scholar] [CrossRef]
- Kant, R.; Dwivedi, C.; Pathak, S.; Dutta, V. Fabrication of ZnO nanostructures using Al doped ZnO (AZO) templates for application in photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 447, 200–212. [Google Scholar] [CrossRef]
- Hussain, S.; Steter, J.R.; Gul, S.; Motheo, A.J. Photo-assisted electrochemical degradation of sulfamethoxazole using a Ti/Ru0.3Ti0.7O2 anode: Mechanistic and kinetic features of the process. J. Environ. Manag. 2017, 201, 153–162. [Google Scholar] [CrossRef]
- Sharma, V.; Prasad, M.; Ilaiyaraja, P.; Sudakar, C.; Jadkar, S. Electrodeposition of highly porous ZnO nanostructures with hydrothermal amination for efficient photoelectrochemical activity. Int. J. Hydrogen Energy 2019, 44, 11459–11471. [Google Scholar] [CrossRef]
- Salem, M.; Massoudi, I.; Akir, S.; Litaiem, Y.; Gaidi, M.; Khirouni, K. Photoelectrochemical and opto-electronic properties tuning of ZnO films: Effect of Cu doping content. J. Alloys Compd. 2017, 722, 313–320. [Google Scholar] [CrossRef]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and enhanced photocatalytic property of TiO2 -ZnO composite photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Zou, Y.; Shen, X.; Zhu, L.; Liao, G. Solvothermal synthesis and photocatalytic properties of ZnO micro/nanostructures. Ceram. Int. 2019, 45, 1724–1729. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Reis, R.Y.N.; Lima, A.E.B.; Costa, M.J.S.; Cruz-Filho, J.F.; Moura, J.P.C.; Santos, R.S.; Luz, G.E. Enhanced photoelectrocatalytic performance of ZnO films doped with N2 by a facile electrochemical method. Surf. Interfaces 2020, 21, 100675. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Peng, T. New insight into the enhanced photocatalytic activity of N-, C- and S-doped ZnO photocatalysts. Appl. Catal. B Environ. 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Gionco, C.; Fabbri, D.; Calza, P.; Paganini, M.C. Synthesis, Characterization, and Photocatalytic Tests of N-Doped Zinc Oxide: A New Interesting Photocatalyst. J. Nanomat. 2016, 2016, 4129864. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of Graphene and graphene-based nanocomposites progress in materials science mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Chen, C.S.; Cao, S.Y.; Zeng, B.; Ning, X.T.; Liu, T.G.; Chen, X.H.; Xiao, Y.; Yu, W.W. Synthesis and photocatalytic property of graphene/multi-walled carbon nanotube/ZnO nanocrystalline aggregates hybrids by spray drying method. Funct. Mater. Lett. 2014, 7, 2014. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Synthesis of N-doped ZnO nanoparticles with cabbage morphology as a catalyst for the efficient photocatalytic degradation of methylene blue under U.V. and visible light. RSC Adv. 2019, 9, 7509–7535. [Google Scholar] [CrossRef] [Green Version]
- Segala, K.; Dutra, R.L.; Franco, C.v.; Pereira, A.S.; Trindade, T. In situ and Ex situ preparations of ZnO/poly-{trans- [RuCl 2(vpy)4]/styrene} nanocomposites. J. Braz. Chem. Soc. 2010, 21, 2010. [Google Scholar] [CrossRef] [Green Version]
- Suresh, M.; Sivasamy, A. Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq. 2020, 317, 114112. [Google Scholar] [CrossRef]
- Sun, S.; Chang, X.; Li, X.; Li, Z. Synthesis of N-doped ZnO nanoparticles with improved photocatalytical activity. Ceram. Int. 2013, 39, 5197–5203. [Google Scholar] [CrossRef]
- Kabir, R.; Saifullah, M.A.K.; Ahmed, A.Z.; Masum, S.M.; Molla, M.A.I. Synthesis of n-doped zno nanocomposites for sunlight photocatalytic degradation of textile dye pollutants. J. Compos. Sci. 2020, 4, 49. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Chandekar, K.v.; Shkir, M.; AlFaify, S.; Ibrahim, E.H.; Ahmad, Z.; Kilany, M.; Al-Shehri, B.M.; Al-Namshah, K.S. Novel Mg@ZnO nanoparticles synthesized by facile one-step combustion route for anti-microbial, cytotoxicity and photocatalysis applications. J. Nanostruct. Chem. 2021, 11, 147–163. [Google Scholar] [CrossRef]
- Elias, M.; Uddin, M.N.; Saha, J.K.; Hossain, M.A.; Sarker, D.R.; Akter, S.; Siddiquey, I.A.; Uddin, J. A highly efficient and stable photocatalyst; N-doped ZnO/C.N.T. composite thin film synthesized via simple sol-gel drop coating method. Molecules 2021, 26, 1470. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic activity of graphene oxide/zinc oxide nanocomposites with embedded metal nanoparticles for the degradation of organic dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Iwase, A.; Bell, N.J.; Kudo, A.; Amal, R. Semiconductor/reduced graphene oxide nanocomposites derived from photocatalytic reactions. Catal. Today 2011, 164, 353–357. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.H.; Lin, S.; Liang, C.; Wang, H.; Ye, C.X.; Wang, Y.J.; Zhang, R.; Fan, D.Y.; Yang, H.J.; et al. A facile route to reduced graphene oxide-zinc oxide nanorod composites with enhanced photocatalytic activity. Powder Technol. 2014, 257, 113–119. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S. Photocatalytic Degradation of Methylene Blue Using Visible Light Active N-Doped ZnO. Adv. Mater. Res. 2015, 1101, 299–302. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. Asian Ceram. Soci. 2015, 3, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Peter, C.N.; Anku, W.W.; Sharma, R.; Joshi, G.M.; Shukla, S.K.; Govender, P.P. N-doped ZnO/graphene oxide: A photostable photocatalyst for improved mineralization and photodegradation of organic dye under visible light. Ionics 2019, 25, 327–339. [Google Scholar] [CrossRef]
- Modwi, A.; ben Aissa, M.A.; Taha, K.K.; Khezami, L.; El Ghoul, J.; Al-Ayed, A.S.; Bououdina, M. Fabrication of (Y2O3)n–ZnO nanocomposites by high-energy milling as potential photocatalysts. J. Mater. Sci. Mater Elect. 2021, 32, 3415–3430. [Google Scholar] [CrossRef]
- Morsi, M.S.; Al-Sarawy, A.A.; El-Dein, W.A.S. Electrochemical degradation of some organic dyes by electrochemical oxidation on a pb/pbo2 electrode. Desalin. Water Treat. 2011, 26, 301–308. [Google Scholar] [CrossRef] [Green Version]
- King, F. Environmental Electrochemistry: Fundamentals and Applications in Pollution Abatement. J. Environ. Qual. 1998, 27, 1550. [Google Scholar] [CrossRef]
- Luo, J.; Yartym, J.; Hepel, M. Photoelectrochemical Degradation of Orange II Textile Dye on Nanostructured WO3 Film Electrodes. J. New Mater. Electrochem. Syst. 2002, 5, 315–322. [Google Scholar]
- Allami, S.; Abid Ali, Z.D.; Li, Y.; Hamody, H.; Jawad, B.H.; Liu, L.; Li, T. Photoelectrochemical performance of N-doped ZnO branched nanowire photoanodes. Heliyon 2017, 3, e00423. [Google Scholar] [CrossRef] [PubMed]
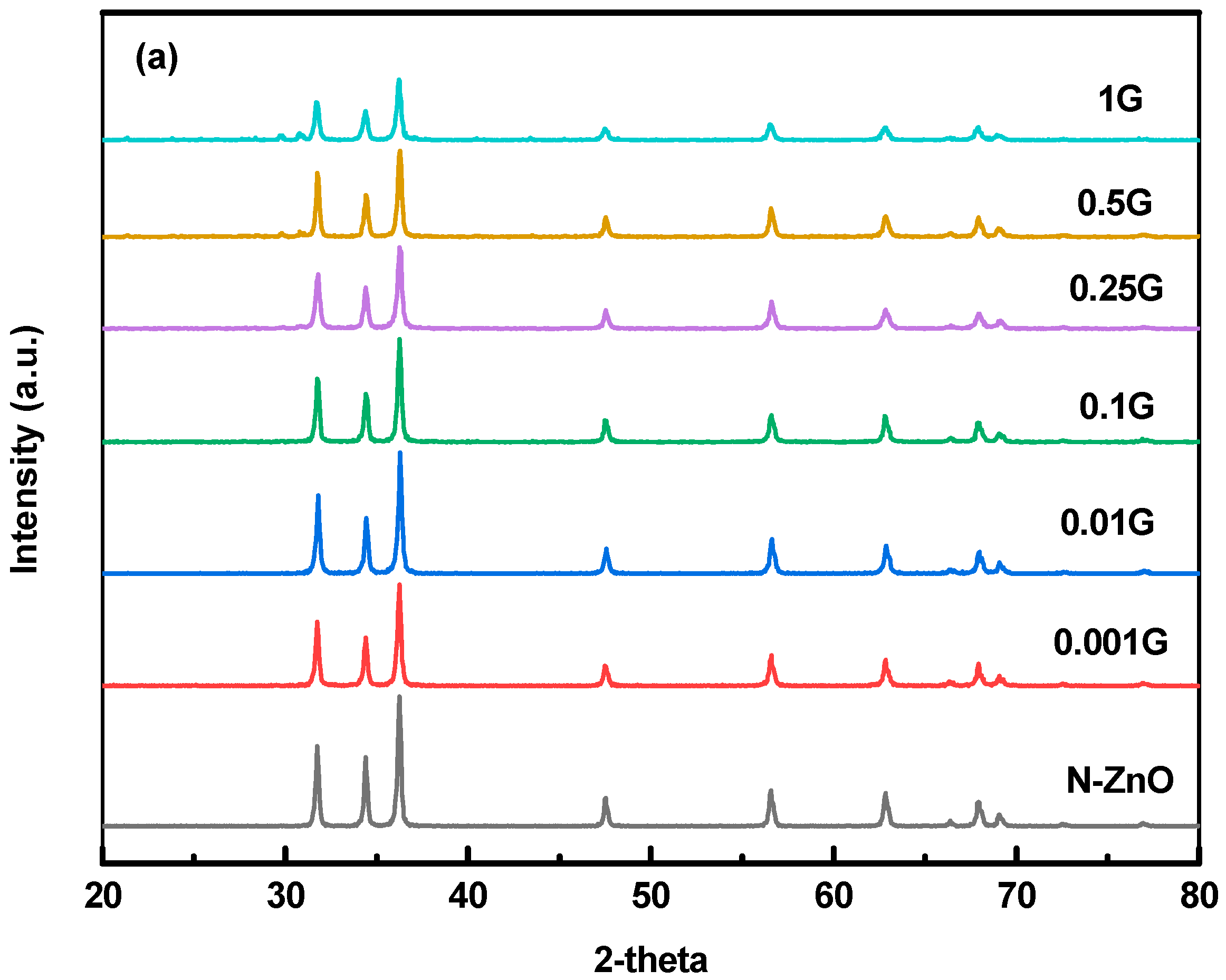
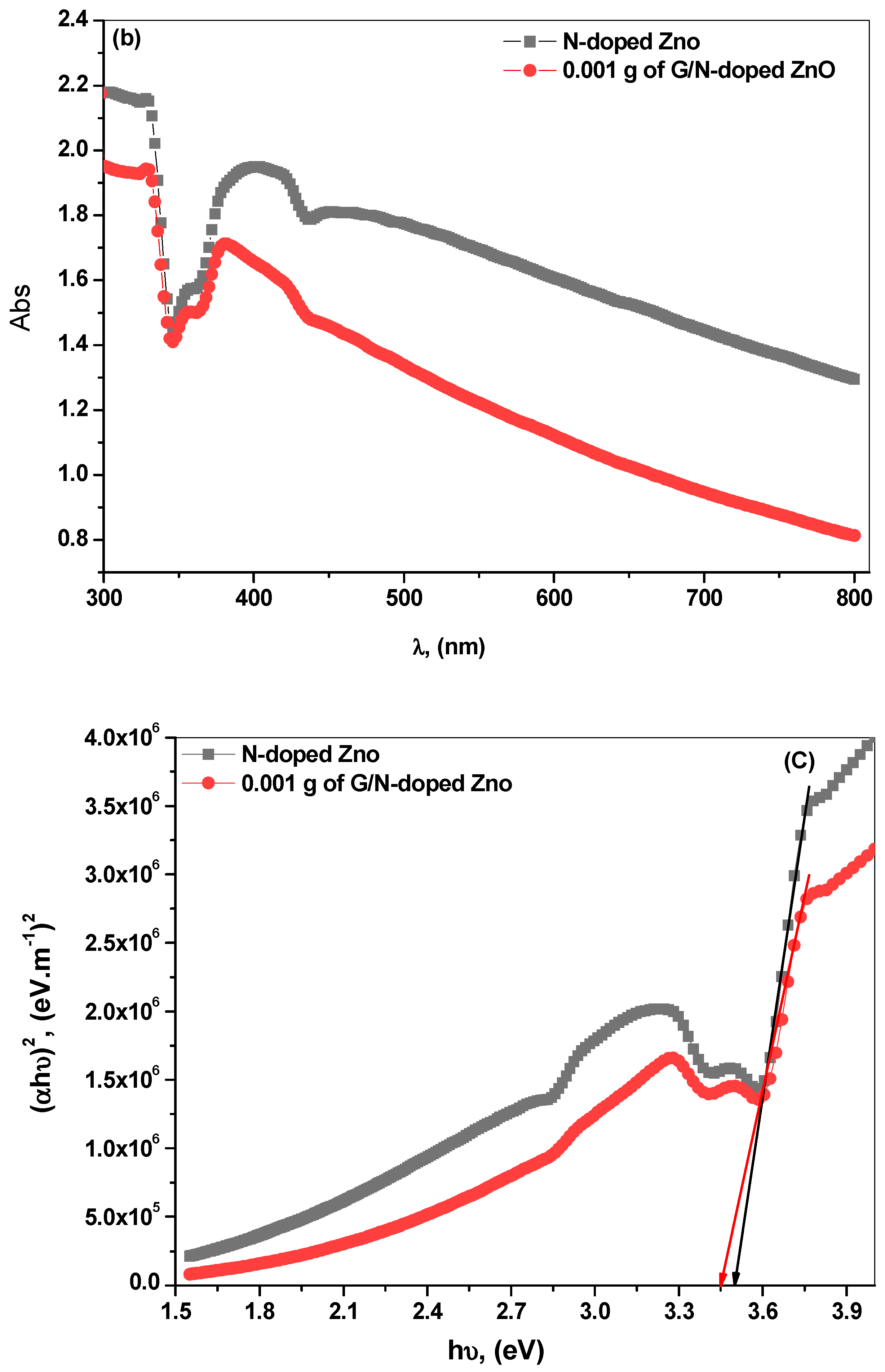
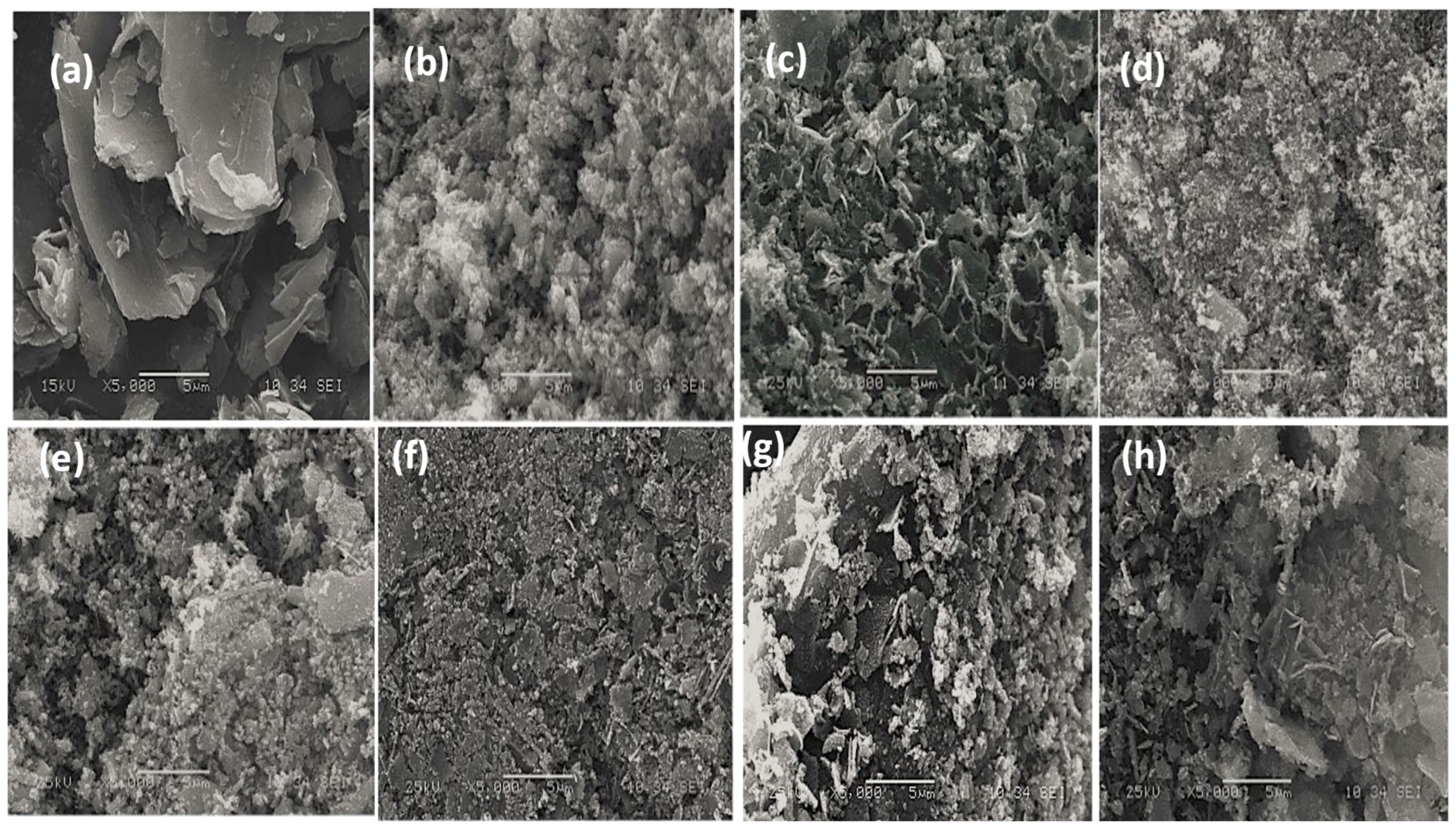
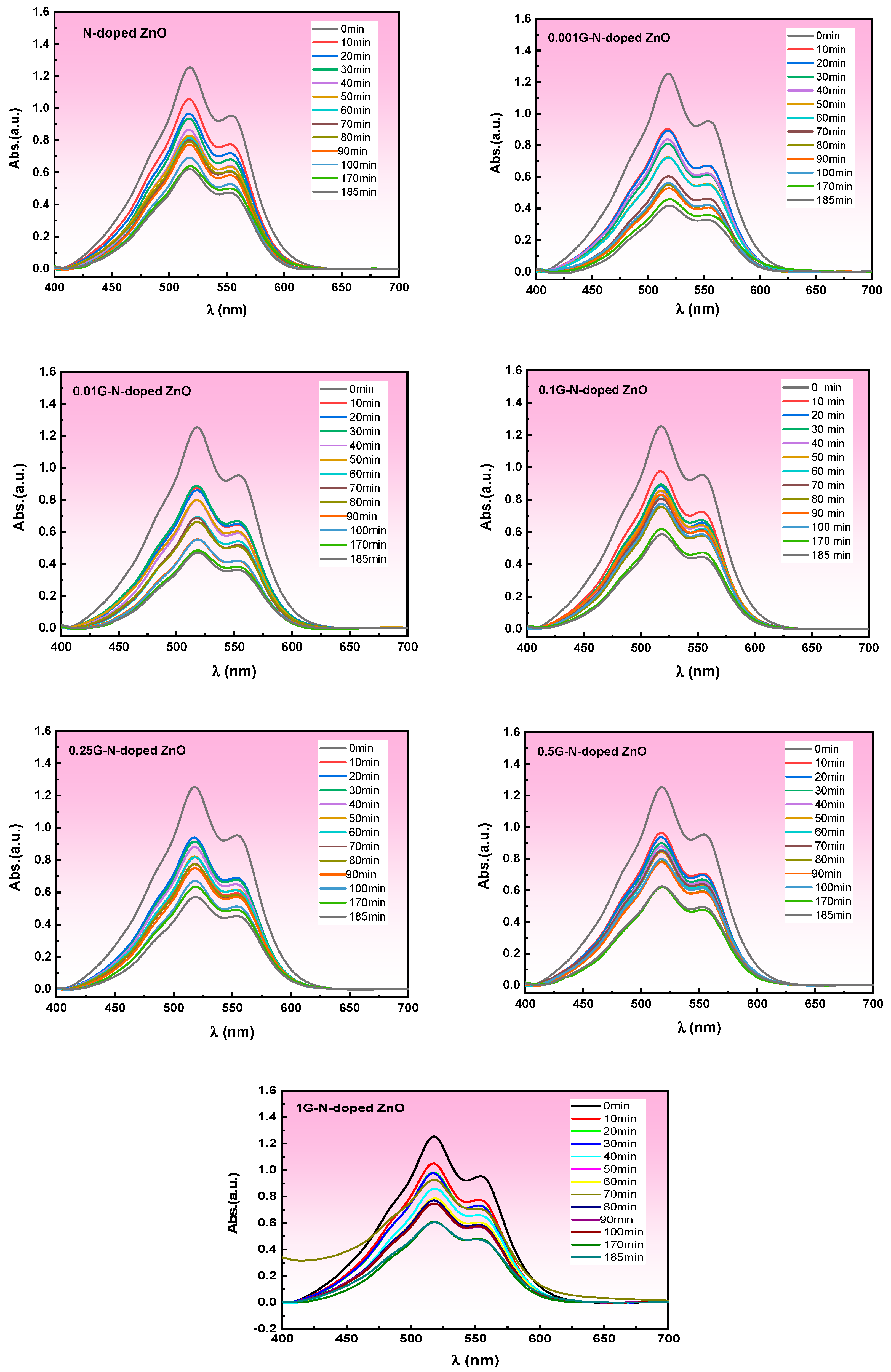
| Samples | Mean Values of the Grain Size (nm) | Mean Values of Dislocation Density, (nm)2 | Mean Values of Lattice Strain |
|---|---|---|---|
| G/N-doped ZnO | 66.53 | 2.259 × 10−4 | 5.210 × 10−4 |
| 0.001 g of G/N-doped ZnO | 31.51 | 1.007 × 10−3 | 1.100 × 10−3 |
| 0.01 g of G/N-doped ZnO | 58.33 | 3.181 × 10−4 | 6.105 × 10−4 |
| 0.1 g of G/N-doped ZnO | 45.54 | 5.450 × 10−4 | 7.938 × 10−4 |
| 0.25 g of G/N-doped ZnO | 43.13 | 6.613 × 10−4 | 8.580 × 10−4 |
| 0.5 g of G/N-doped ZnO | 44.03 | 5.518 × 10−4 | 8.058 × 10−4 |
| 1 g of G/N-doped ZnO | 38.90 | 8.986 × 10−4 | 9.876 × 10−4 |
| Samples | Carmine Dye (185 min) Photocatalysis In Situ | Carmine Dye (15 min) Electrocatalysis | ||
|---|---|---|---|---|
| K min−1 | Degradation (%) | K min−1 | Degradation (%) | |
| G/N-doped ZnO | 0.00486 | 50.60 | 0.16593 | 74.96 |
| 0.001 g of G/N-doped ZnO | 0.00739 | 66.76 | 0.20292 | 100 |
| 0.01 g of G/N-doped ZnO | 0.00678 | 62.52 | 0.09904 | 71.99 |
| 0.1 g of G/N-doped ZnO | 0.00489 | 53.23 | 0.07786 | 70.20 |
| 0.25 g of G/N-doped ZnO | 0.00514 | 54.41 | 0.11703 | 83.40 |
| 0.5 g of G/N-doped ZnO | 0.00469 | 50.11 | 0.11085 | 96.92 |
| 1 g of G/N-doped ZnO | 0.00488 | 51.74 | 0.15532 | 98.27 |
| Photocatalyst | Method of Preparation | Organic Solution | Irradiation Time, (min) | Source | Type of Treatment | % Degradation | K, (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| G/N-doped ZnO | Combustion method | Carmine dye | 185 | UV light | Photocatalysis | 66.76% | 0.0022 | Present work |
| G/N-doped ZnO | Combustion method | Carmine dye | 15 | - | EO | 100% | ------- | Present work |
| RGO-N-ZnO nanocomposite | Hydrothermal method | MB | 120 | Visible light | Photocatalysis | 98.5% | ------- | [29] |
| N-ZnO NPs | Mechanochemical method | M.B. | - | Visible light | Photocatalysis | 98% | ------- | [38] |
| N-ZnO N.C.B.s | Hydrothermal method | M.B. | - | UV light –visible light | Photocatalysis | 99.6% | 0.0579– 0.0585 | [27] |
| N-ZnO NPs | Microemulsion method | MG | 90 | visible light | Photocatalysis | 100% | ------- | [39] |
| 7 wt% of N-ZnO NPs | Mechanochemical method | M.B. | - | sunlight | Photocatalysis | 98.11% | ------- | [31] |
| N-ZnO-GO | Wet chemical method | B.G. | 90 | Visible light | Photocatalysis | ------- | ------- | [40] |
| N-ZnO | Solvent-free mechanochemical method | M.B. | - | Visible light | Photocatalysis | 100% | ------- | [41] |
| N-ZnO | Simple vacuum atmosphere method | MO | 100 | Visible light | Photocatalysis | 100% | ------- | [30] |
| GO-ZnO | Simple one-pot method | MB | 90 | Sunlight | photocatalysis | 84% | ------- | [34] |
| GO-ZnO-Ag | Simple one-pot method | MB | 40 | Sun light | Photocatalysis | 100% | 0.1112 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, F.; Hussien, M.S.A.; AlAbdulaal, T.H.; Ismail, A.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Khairy, Y.; Ali, T.E.; Ibrahim, M.A. Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures. Crystals 2022, 12, 535. https://doi.org/10.3390/cryst12040535
El-Sayed F, Hussien MSA, AlAbdulaal TH, Ismail A, Zahran HY, Yahia IS, Abdel-wahab MS, Khairy Y, Ali TE, Ibrahim MA. Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures. Crystals. 2022; 12(4):535. https://doi.org/10.3390/cryst12040535
Chicago/Turabian StyleEl-Sayed, Fatma, Mai S. A. Hussien, Thekrayat H. AlAbdulaal, Ahmed Ismail, Heba Y. Zahran, Ibrahim S. Yahia, Mohamed Sh. Abdel-wahab, Yasmin Khairy, Tarik E. Ali, and Medhat A. Ibrahim. 2022. "Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures" Crystals 12, no. 4: 535. https://doi.org/10.3390/cryst12040535
APA StyleEl-Sayed, F., Hussien, M. S. A., AlAbdulaal, T. H., Ismail, A., Zahran, H. Y., Yahia, I. S., Abdel-wahab, M. S., Khairy, Y., Ali, T. E., & Ibrahim, M. A. (2022). Comparative Degradation Studies of Carmine Dye by Photocatalysis and Photoelectrochemical Oxidation Processes in the Presence of Graphene/N-Doped ZnO Nanostructures. Crystals, 12(4), 535. https://doi.org/10.3390/cryst12040535






