Evaluation of the Structural Deviation of Cu/Cu2O Nanocomposite Using the X-ray Diffraction Analysis Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Synthesis of Cu/Cu2O PNCs
2.3. Characterizations
3. Results
3.1. The Formation of Cu/Cu2O Powder Nanocomposite
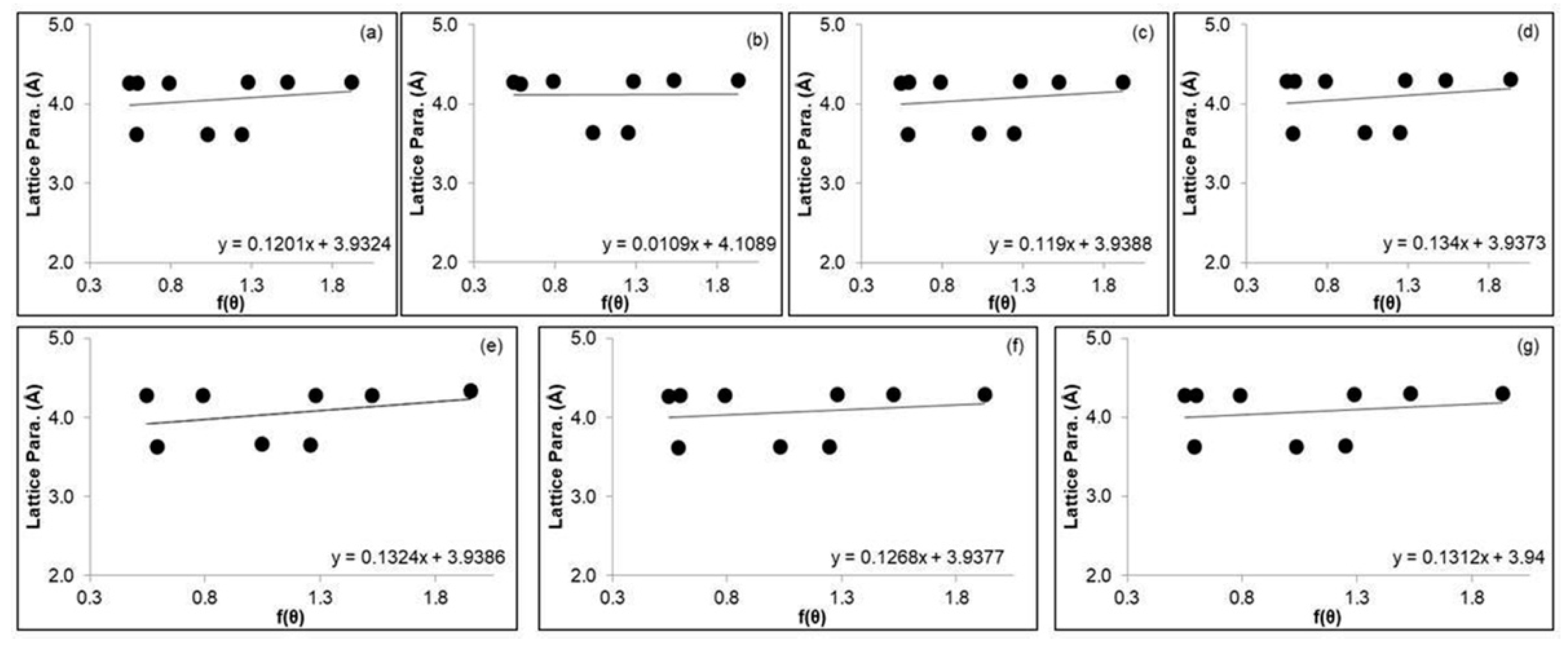
3.2. The XRD Analysis of Cu/Cu2O Powder Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Panah, S.M.; Ramakrishna, S.; Jiangyong, H. Visible light driven heterojunction photocatalyst of CuO–Cu2O thin films for photocatalytic degradation of organic pollutants. J. Nanomater. 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Meher, S.R.; Illyaskutty, N.; Alex, Z.C. Facile synthesis of Cu2O and CuO nanoparticles and study of their structural, optical and electronic properties. J. Alloys Compd. 2018, 743, 737–745. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO—Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Kou, T.; Wang, Y.; Zhang, C.; Sun, J.; Zhang, Z. Adsorption behavior of methyl orange onto nanoporous core–shell Cu@Cu2O nanocomposite. Chem. Eng. J. 2013, 223, 76–83. [Google Scholar] [CrossRef]
- Dai, J.; Fan, X.M.; Liu, H.; Wang, J.; Li, H.R.; Zhang, F.Z. Fabrication and high visible light photocatalytic properties of Cu/Cu2O nanocomposites by the one—pot solution—phase hydrothermal method. J. Nanosci. Nanotechnol. 2012, 12, 6412–6419. [Google Scholar] [CrossRef]
- Huan, K.; Tang, L.; Deng, D.; Wang, H.; Si, X.; Yan, X.; Luo, L. A Two-Step Electrodeposition of Pd—Cu/Cu2O Nanocomposite on FTO Substrate for Non—Enzymatic Hydrogen Peroxide Sensor. Curr. Anal. Chem. 2021, 17, 1373–1381. [Google Scholar] [CrossRef]
- Kamazani, M.M.; Zarghami, Z.; Rahmatolahzadeh, R.; Ramezani, M. Solvent-free synthesis of Cu-Cu2O nanocomposites via green thermal decomposition route using novel precursor and investigation of its photocatalytic activity. Adv. Powder Technol. 2017, 28, 2078–2086. [Google Scholar] [CrossRef]
- Nikam, A.; Bhagavatula, P.; Kulkarni, A. Wet Chemical Synthesis of Metal Oxide Nanoparticles: A Review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Dey, P.C.; Sarkar, S.; Das, R. X-ray diffraction study of the elastic properties of jagged spherical CdS nanocrystals. Mater. Sci.-Pol. 2020, 38, 271–278. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson–Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I.; Shapur, N.; Abdin, T.; Gofrit, O.N.; Pode, D.; Duvdevani, M. X-ray diffraction and SEM study of kidney stones in Israel: Quantitative analysis, crystallite size determination, and statistical characterization. Environ. Geochem. Health 2011, 33, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Nguyen, H.M. Role of capping agent in wet synthesis of nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar]
- Elechiguerra, J.L.; Lopez, L.L.; Liu, C.; Gutierrez, D.G.; Bragado, A.C.; Yacaman, M.J. Corrosion at the nanoscale: The case of silver nanowires and nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Jasbi, N.E.; Dorranian, D. Effect of aging on the properties of TiO2 nanoparticle. J. Theor. Appl. Phys. 2016, 10, 157–161. [Google Scholar] [CrossRef]
- Collins, D.; Luxton, T.; Kumar, N.; Shah, S.; Walker, V.K.; Shah, V. Assessing the Impact of Copper and Zinc Oxide Nanoparticles on Soil: A Field Study. PLoS ONE 2012, 7, e42663. [Google Scholar] [CrossRef]
- Lam, N.H.; Le, N.; Kim, E.S.; Tamboli, M.S.; Tamboli, A.M.; Truong, N.T.N.; Jung, J.H. Powder X-ray diffraction analysis of Cu/Cu2O nanocomposites synthesized by colloidal solution method. Korean J. Chem. Eng. 2022. [CrossRef]
- Mishra, S.K.; Roy, H.; Lohar, A.K.; Samanta, S.K.; Tiwari, S.; Dutta, K. A comparative assessment of crystallite size and lattice strain in differently cast A356 aluminium alloy. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012001. [Google Scholar] [CrossRef]
- Hameed, T.A.; Wassel, A.R.; IRadaf, M.E. Investigating the effect of thickness on the structural, morphological, optical and electrical properties of AgBiSe2 thin films. J. Alloys Compd. 2019, 805, 1–11. [Google Scholar] [CrossRef]
- Ansari, A.R.; Hammad, A.H.; Abdel-wahab, M.S.; Shariq, M.; Imran, M. Structural, optical and photoluminescence investigations of nanocrystalline CuO thin films at different microwave powers. Opt. Quantum Electron. 2020, 52, 426–441. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal based synthesis of silver nanoparticles—an effect of temperature on the size of particles. Colloids Surf. B 2009, 74, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Shen, Z.G.; Zhong, J.; Hu, T.T.; Chen, J.F.; Ma, Z.Q.; Yun, J. Preparation of amorphous cefuroxime axetil nanoparticles by controlled nanoprecipitation method without surfactants. Int. J. Pharm. 2006, 323, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2017, 13, 1011–1019. [Google Scholar] [CrossRef]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A.B. Role of Temperature in the Growth of Silver Nanoparticles Through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2010, 6, 32. [Google Scholar] [CrossRef]
- Kasture, M.B.; Patel, P.; Prabhune, A.A.; Ramana, C.V.; Kulkarni, A.A.; Prasad, B.L.V. Synthesis of silver nanoparticles by sophorolipids: Effect of temperature and sophorolipid structure on the size of particles. J. Chem. Sci. 2008, 120, 515–520. [Google Scholar] [CrossRef]
- Albetran, H.M. Thermal expansion coefficient determination of pure, doped, and co-doped anatase nanoparticles heated in sealed quartz capillaries using in—situ high—temperature synchrotron radiation diffraction. Heliyon 2020, 6, e04501. [Google Scholar] [CrossRef]
- Aravand, M.A.; Semsarzadeh, M.A. Particle Formation by Emulsion Inversion Method: Effect of the Stirring Speed on Inversion and Formation of Spherical Particles. Macromol. Symp. 2008, 274, 141–147. [Google Scholar] [CrossRef]
- Lemos, Y.P.; Marfil, P.H.M.; Nicoletti, V.R. Particle size characteristics of buriti oil microcapsules produced by gelatin—sodium alginate complex coacervation: Effect of stirring speed. Int. J. Food Prop. 2017, 20, 1438–1447. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction a Practical Approach, 1st ed.; Plenum Press: New York, NY, USA, 1998; p. 513. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: New York, NY, USA, 2001; pp. 363–383. [Google Scholar]
- Das, R.; Sarkar, S. Determination of structural elements of synthesized silver nano—hexagon from X-ray diffraction analysis. Indian J. Pure Appl. Phys. 2018, 56, 765–772. [Google Scholar]
- Chen, Y.J.; Li, M.H.; Huang, J.C.A.; Chen, P. Cu/Cu2O nanocomposite films as a p-type modified layer for efficient perovskite solar cells. Sci. Rep. 2018, 8, 7464. [Google Scholar] [CrossRef]
- Tang, L.; Huan, K.; Deng, D.; Han, L.; Zeng, Z.; Luo, L. Glucose sensor based on Pd nanosheets deposited on Cu/Cu2O nanocomposites by galvanic replacement. Colloids Surf. 2020, 188, 110797. [Google Scholar] [CrossRef] [PubMed]

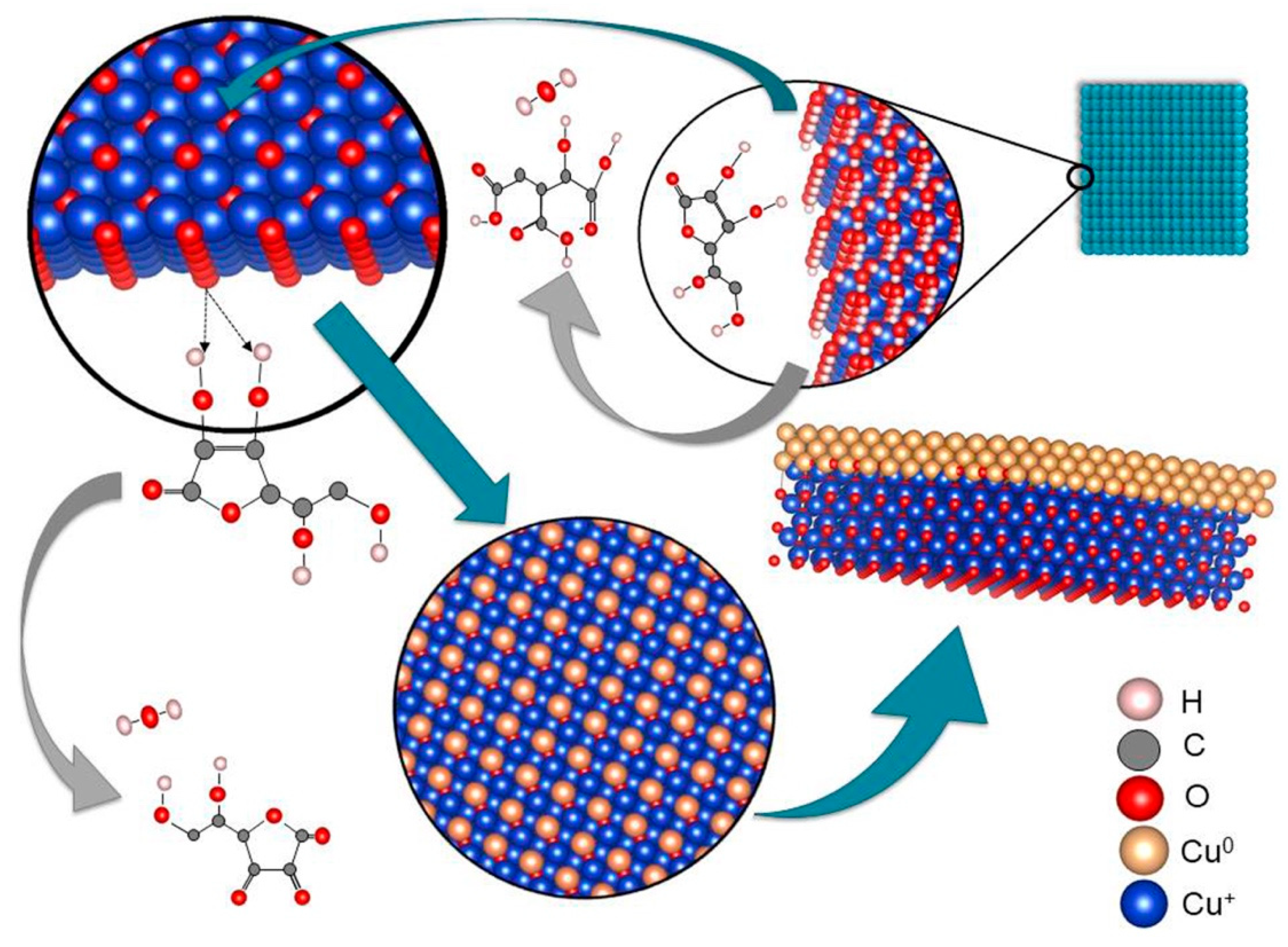

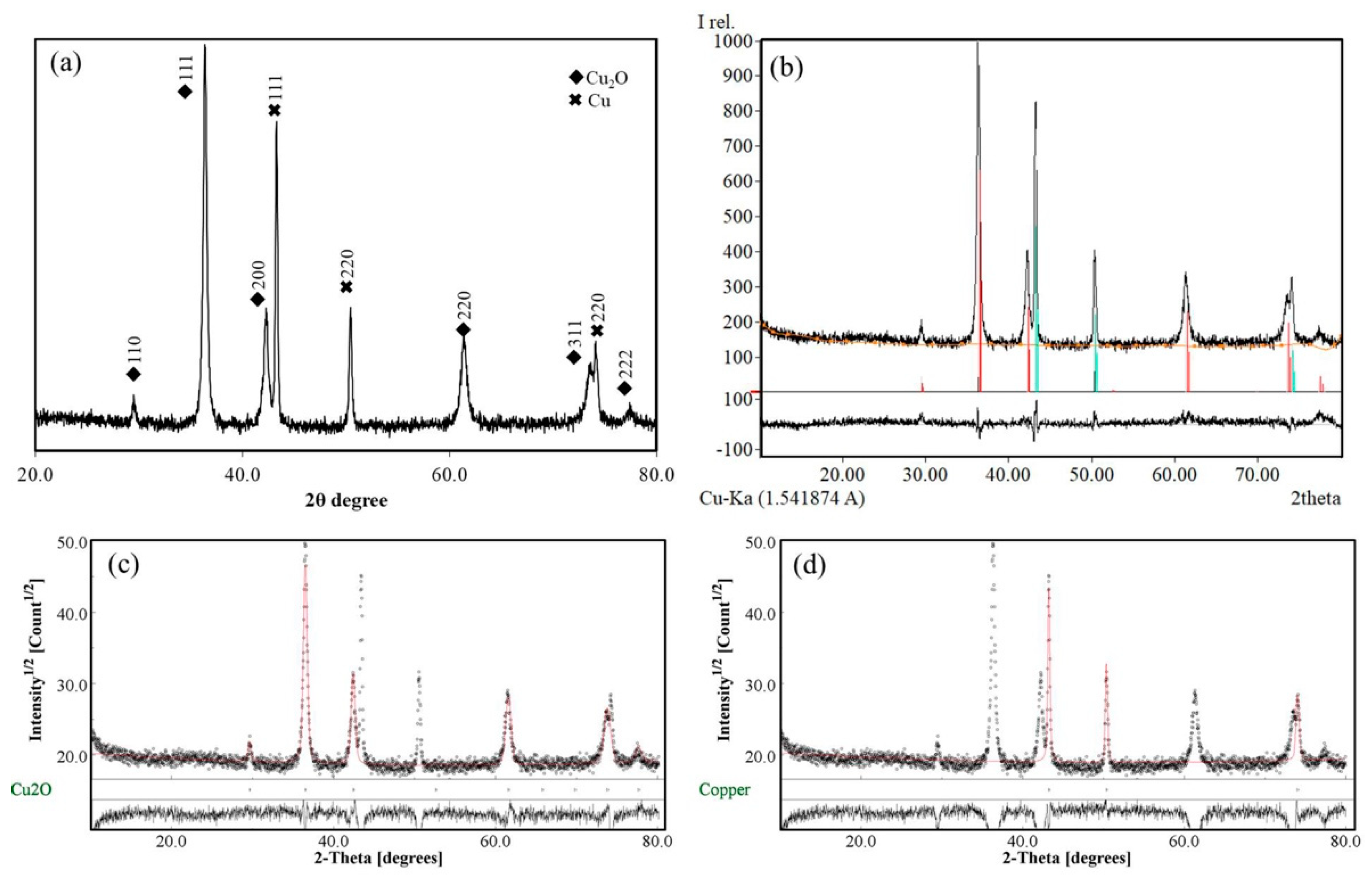


| Code | CuSO4·5H2O (M) | PEG (g/L) | NaOH (M) | C6H8O6 (mmol) | NaBH4 (M) | Tv (°C) | Sv (rpm) |
|---|---|---|---|---|---|---|---|
| ST | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 40 | 250 |
| T1 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 60 | 250 |
| T2 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 80 | 250 |
| T3 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 100 | 250 |
| S1 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 40 | 350 |
| S2 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 40 | 450 |
| S3 | 1.0 | 1.0 | 1.0 | 0.25 | 1.0 | 40 | 550 |
| Title 1 | ST | S1 | S2 | S3 | T1 | T2 | T3 | |
|---|---|---|---|---|---|---|---|---|
| Crystallographic Parameters (from ICDD) | Crystal system | Cubic | Cubic | Cubic | Cubic | Cubic | Cubic | Cubic |
| Space group | Pn—3 m | Pn—3 m | Pn—3 m | Pn—3 m | Pn—3 m | Pn—3 m | Pn—3 m | |
| a = b = c | 4.260 | 4.258 | 4.258 | 4.258 | 4.267 | 4.267 | 4.258 | |
| α = β = γ | 90 | 90 | 90 | 90 | 90 | 90 | 90 | |
| Volume of cell (nm3) | 0.0773 | 0.0772 | 0.0772 | 0.0772 | 0.0777 | 0.0777 | 0.0772 | |
| Inter—planar spacing (Å) | 1.8703 | 1.9517 | 1.8727 | 1.8803 | 1.9537 | 1.8765 | 1.8800 | |
| Lattice parameter [Å] | 4.0531 | 4.1206 | 4.0586 | 4.0730 | 4.0466 | 4.0657 | 4.0728 | |
| Full width at half maximum | 0.3218 | 0.3273 | 0.3367 | 0.3554 | 0.3341 | 0.3354 | 0.3530 | |
| Dislocation—δ (nm) | 0.0877 | 0.0894 | 0.0960 | 0.1069 | 0.0932 | 0.0952 | 0.1054 | |
| Lattice strain (%) | 0.0708 | 0.0729 | 0.0741 | 0.0783 | 0.0744 | 0.0739 | 0.0778 | |
| Crystallite size (nm) | 3.3881 | 3.3547 | 3.2389 | 3.0694 | 3.2862 | 3.2516 | 3.0906 | |
| Nelson Riley | Precise lattice parameter | 3.9324 | 4.1089 | 3.9388 | 3.9373 | 3.9386 | 3.9377 | 3.9400 |
| Williamson-Hall | Induced—lattice strain (%) | 0.0623 | 0.1358 | 0.2280 | 0.1949 | 0.1832 | 0.1915 | 0.1461 |
| Induced—crystallite size (nm) | 0.2512 | 0.1454 | 0.0591 | −0.0189 | 0.0152 | 0.0106 | −0.0098 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, N.H.; Smith, R.P.; Le, N.; Thuy, C.T.T.; Tamboli, M.S.; Tamboli, A.M.; Alshehri, S.; Ghoneim, M.M.; Truong, N.T.N.; Jung, J.H. Evaluation of the Structural Deviation of Cu/Cu2O Nanocomposite Using the X-ray Diffraction Analysis Methods. Crystals 2022, 12, 566. https://doi.org/10.3390/cryst12040566
Lam NH, Smith RP, Le N, Thuy CTT, Tamboli MS, Tamboli AM, Alshehri S, Ghoneim MM, Truong NTN, Jung JH. Evaluation of the Structural Deviation of Cu/Cu2O Nanocomposite Using the X-ray Diffraction Analysis Methods. Crystals. 2022; 12(4):566. https://doi.org/10.3390/cryst12040566
Chicago/Turabian StyleLam, Nguyen Hoang, Ryan P. Smith, Nam Le, Chau Thi Thanh Thuy, Mohaseen S. Tamboli, Asiya M. Tamboli, Sultan Alshehri, Mohammed M. Ghoneim, Nguyen Tam Nguyen Truong, and Jae Hak Jung. 2022. "Evaluation of the Structural Deviation of Cu/Cu2O Nanocomposite Using the X-ray Diffraction Analysis Methods" Crystals 12, no. 4: 566. https://doi.org/10.3390/cryst12040566
APA StyleLam, N. H., Smith, R. P., Le, N., Thuy, C. T. T., Tamboli, M. S., Tamboli, A. M., Alshehri, S., Ghoneim, M. M., Truong, N. T. N., & Jung, J. H. (2022). Evaluation of the Structural Deviation of Cu/Cu2O Nanocomposite Using the X-ray Diffraction Analysis Methods. Crystals, 12(4), 566. https://doi.org/10.3390/cryst12040566






