Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review
Abstract
:1. Introduction
2. Hydrogen-Based Energy Technology
3. Synthesis of Noble-Metal Nanoparticles
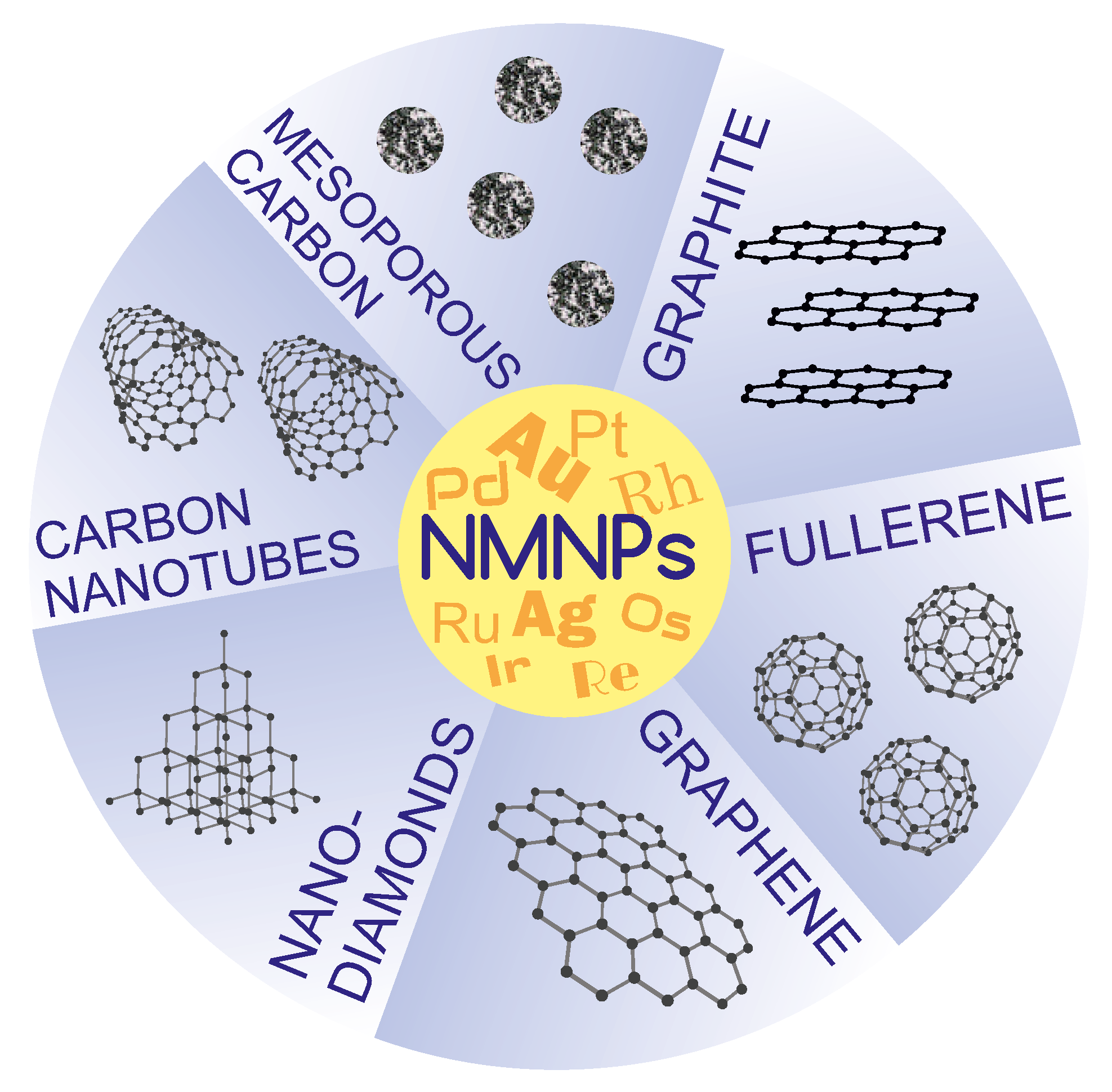
4. Carbon-Supported Noble-Metal Nanoparticles
4.1. Methods of Obtaining NMNPs/CMs Nanocomposites
4.1.1. Noble-Metal Nanoparticles on Mesoporous Carbon Materials
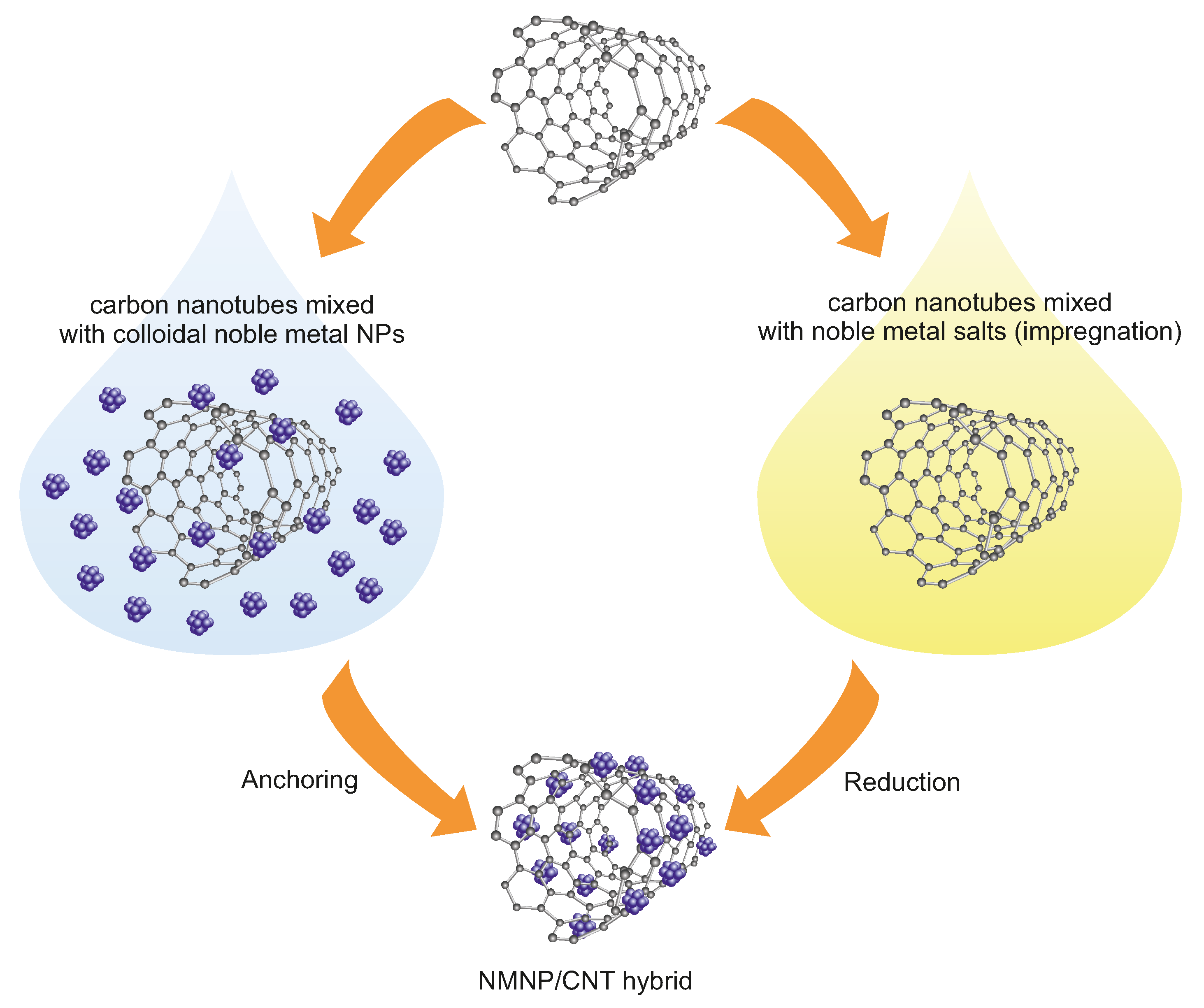
4.1.2. Noble-Metal Nanoparticles on Carbon Nanotubes
4.1.3. Noble-Metal Nanoparticles on Graphene
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic Acid |
| AFC | Alkaline Fuel Cells |
| ALD | Atomic Layer Deposition |
| BDD | Boron-Doped Diamond |
| C | Carbon |
| CA | Carbon Aerogel |
| CB | Carbon Black |
| CCA | Citric Acid |
| CM | Carbonaceous Material |
| CNF | Carbon Nanofiber |
| CNO | Carbon Nanoonion |
| CNT | Carbon Nanotube |
| CP | Carbon paper |
| CTAB | Cetyltrimethylammonium Bromide |
| CQD | Carbon Quantum Dot |
| CX | Carbon Xerogels |
| DEFC | Direct Ethanol Fuel Cell |
| DEG | Diethylene Glycol |
| DMFC | Direct Methanol Fuel Cell |
| EG | Ethylene Glycol |
| EOR | Ethanol Oxidation Reaction |
| G | Graphene |
| GCE | Glassy Carbon Electrode |
| GMC | Graphitic Mesoporous Carbon |
| GNS | Graphene Nanosheet |
| GO | Graphene Oxide |
| HA | Hydroxylamine |
| HEA | High-Entropy Alloy |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic Acid |
| HER | Hydrogen Evolution Reaction |
| HOR | Hydrogen Oxidation Reaction |
| MCFC | Molten Carbonate Fuel Cells |
| MEA | Membrane-Electrode Assembly |
| MeCpPtMe3 | Methylcyclopentadienyl)-trimethyl platinum |
| MNC | Marimo Nanocarbon |
| MOR | Methanol Oxidation Reaction |
| MWCNT | Multiwalled Carbon Nanotube |
| N-GN | Nitrogen-doped Graphite Nanosheet |
| NMNP | Noble Metal Nanoparticle |
| NP | Nanoparticle |
| NW | Nanowire |
| NYPA | Naphthalen-1-ylmethylphosphonic Acid |
| ORR | Oxygen Reduction Reaction |
| PC | Porous Carbon |
| PCN | Porous Carbon Nanosphere |
| PDDA | poly(diallyldimethyl ammonium) chloride |
| PEG | Polyethylene Glycol |
| PEI | Polyethyleneimine |
| PEMFC | Proton-Exchange Membrane Fuel Cell |
| POLE | Polyoxyethylene Lauryl Ether |
| prGO | Partially Reduced Graphene Oxide |
| PSD | Potentiostatic Deposition |
| PSWD | Potential Square Wave Deposition |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinyl Pyrrolidone |
| PyrC60 | Fullerene-pyrolidine |
| RFC | Regenerative Fuel Cells |
| rGO | Reduced Graphene Oxide |
| SA | Sodium Ascorbate |
| SAC | Single-Atom Catalyst |
| SC | Sodium Citrate |
| SDBS | Sodium Dodecyl Benzene Sulfonate |
| SDS | Sodium Dodecyl Sulfate |
| SEED | Substrate Enhanced Electroless Deposition |
| SOFC | Solid-Oxide Fuel Cells |
| SWCNT | Single-Walled Carbon Nanotube |
| TA | Tannic Acid |
| TEG | Tetraethylene Glycol |
| THF | tetrahydrofuran |
| TREG | Triethylene Glycol |
| URFC | Unitized Regenerative Fuel Cells |
References
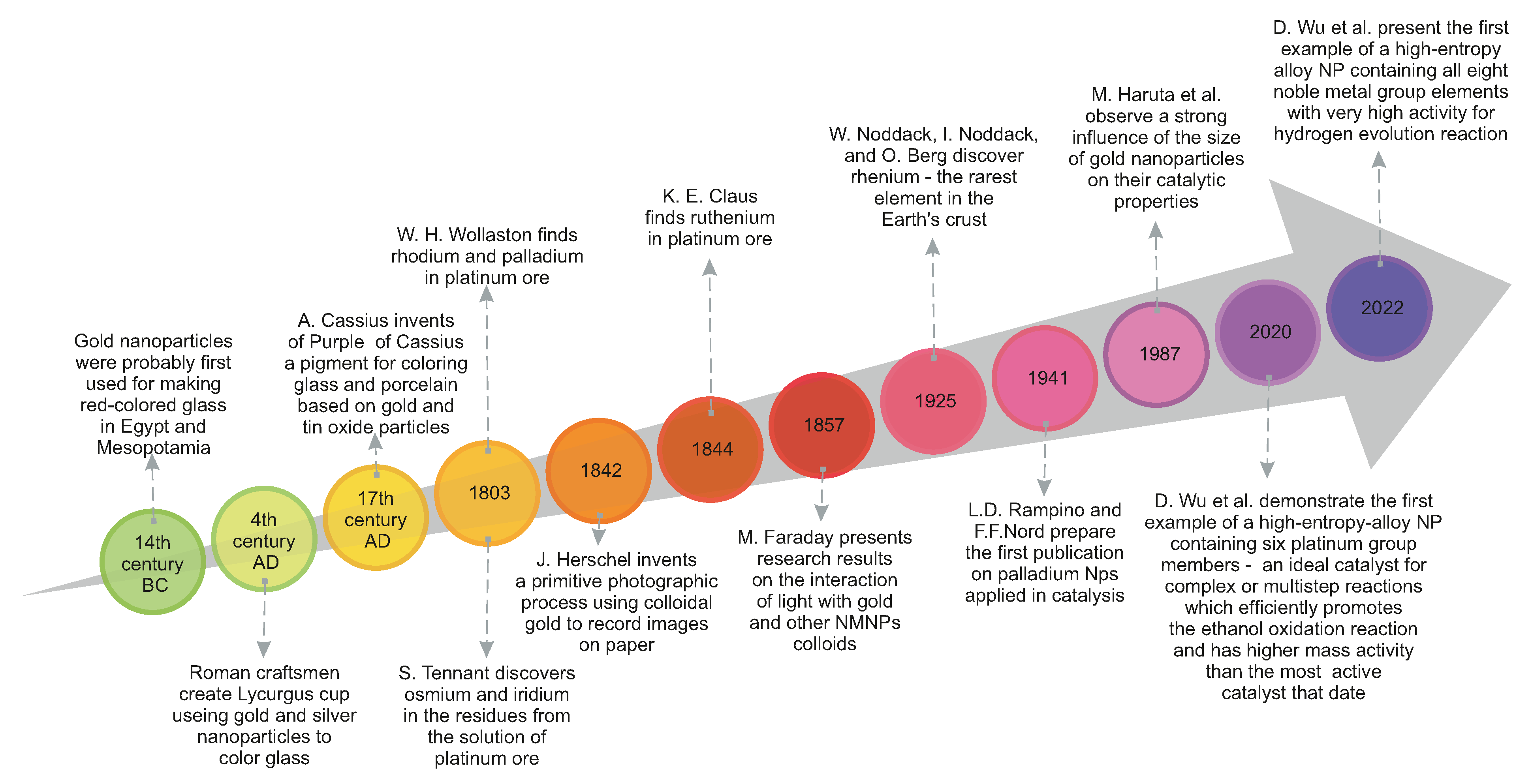
- Habashi, F. Gold–An historical introduction. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Vajtai, R. Springer Handbook of Nanomaterials; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry and Biology; World Scientific: Singapore, 2017. [Google Scholar]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Sotoodeh Jahromi, A.; Ghasemi, Y. Ancient and novel forms of silver in medicine and biomedicine. J. Adv. Med Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Mingos, D.M.P.; Broda, J. Gold Clusters, Colloids and Nanoparticles I; Springer: Berlin/Heidelberg, Germany, 2014; Volume 161. [Google Scholar]
- Tweney, R.D. Discovering discovery: How Faraday found the first metallic colloid. Perspect. Sci. 2006, 14, 97–121. [Google Scholar] [CrossRef]
- Faraday, M.X. The Bakerian Lecture.—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Wu, D.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 2020, 142, 13833–13838. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Brenan, J.M. The platinum-group elements: “Admirably adapted” for science and industry. Elements 2008, 4, 227–232. [Google Scholar] [CrossRef]
- Wu, D.; Kusada, K.; Nanba, Y.; Koyama, M.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Seo, O.; Gueye, I.; Kim, J.; et al. Noble-Metal High-Entropy-Alloy Nanoparticles: Atomic-Level Insight into the Electronic Structure. J. Am. Chem. Soc. 2022, 144, 3365–3369. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Kee, R.J.; Zhu, H.; Goodwin, D.G. Solid-oxide fuel cells with hydrocarbon fuels. Proc. Combust. Inst. 2005, 30, 2379–2404. [Google Scholar] [CrossRef]
- Cropper, M.A.; Geiger, S.; Jollie, D.M. Fuel cells: A survey of current developments. J. Power Sources 2004, 131, 57–61. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.; Santos, L.; Garcia, G.; Ticianelli, E.; Pastor, E.; Gonzalez, E. Carbon supported Pt–Cr alloys as oxygen-reduction catalysts for direct methanol fuel cells. J. Appl. Electrochem. 2006, 36, 355–362. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Ye, Q.; Deng, W.; Xu, B. RESEARCH PAPER. Acta-Phys.-Chim. Sin. 2006, 22, 419–423. [Google Scholar] [CrossRef]
- Ralph, T.; Hards, G.; Keating, J.; Campbell, S.; Wilkinson, D.; Davis, M.; St-Pierre, J.; Johnson, M. ChemInform Abstract: Low Cost Electrodes for Proton Exchange Membrane Fuel Cells. Performance in Single Cells and Ballard Stacks. J. Electrochem. Soc. 1997, 144, 3845–3857. [Google Scholar] [CrossRef]
- Panayiotou, G.P.; Kalogirou, S.A.; Tassou, S.A. PEM fuel cells for energy production in solar hydrogen systems. Recent Patents Mech. Eng. 2010, 3, 226–235. [Google Scholar] [CrossRef]
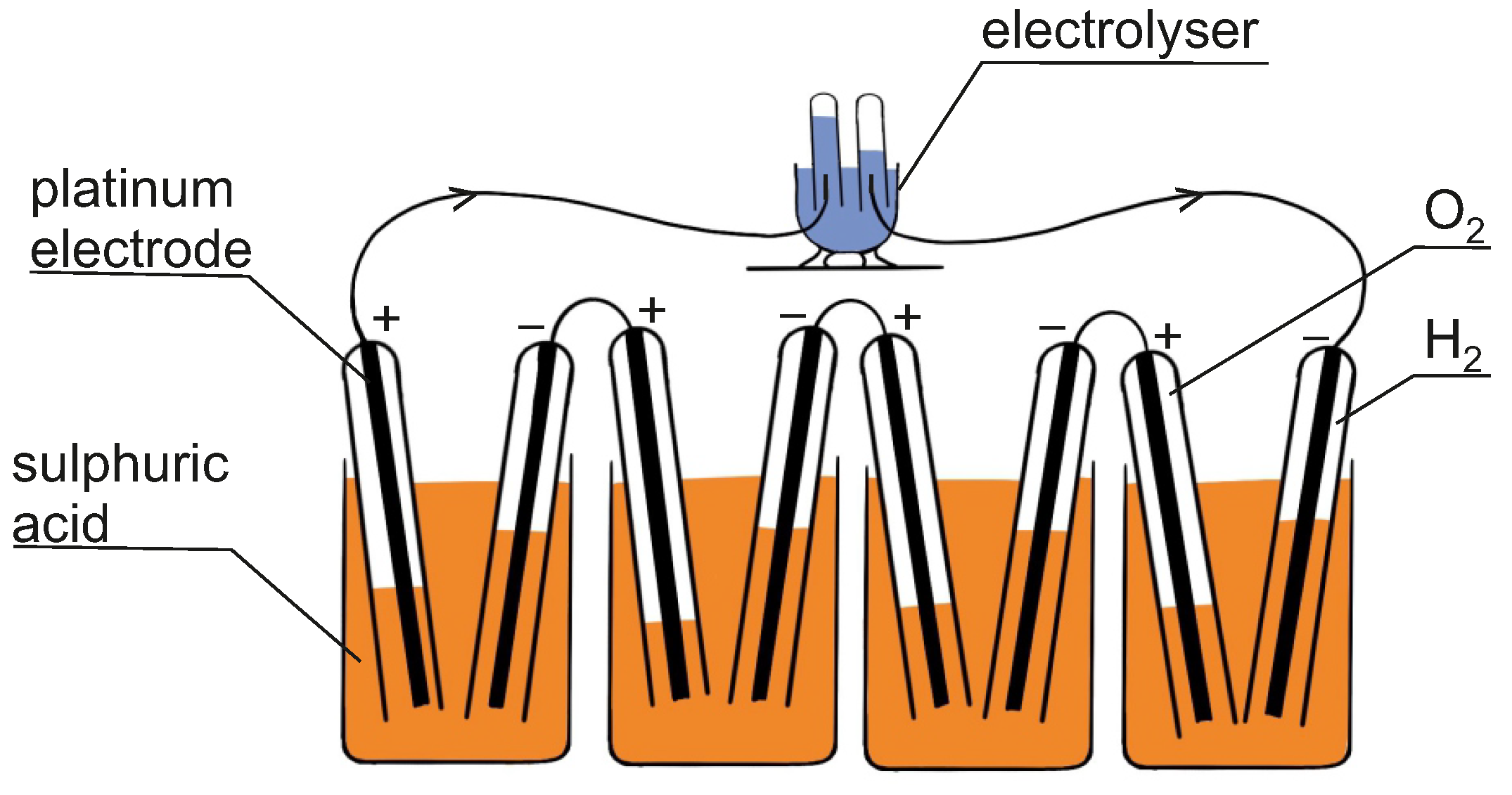
- Katz, E. Electrochemical contributions: William Nicholson (1753–1815). Electrochem. Sci. Adv. 2021, 1, e2160003. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Scholz, F. Wilhelm Ostwald’s role in the genesis and evolution of the Nernst equation. J. Solid State Electrochem. 2017, 21, 1847–1859. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Choudhury, S.; Acharya, S.K.; Khadanga, R.K.; Mohanty, S.; Arshad, J.; Ur Rehman, A.; Shafiq, M.; Choi, J.G. Harmonic Profile Enhancement of Grid Connected Fuel Cell through Cascaded H-Bridge Multi-Level Inverter and Improved Squirrel Search Optimization Technique. Energies 2021, 14, 7947. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, L.; Yuan, Q.; Yang, X.; Zhang, Q.; Xu, H.; Guo, Y.; Yang, S.; Zhou, Z.; Gu, L.; et al. Ultrathin PdAuBiTe Nanosheets as High-Performance Oxygen Reduction Catalysts for a Direct Methanol Fuel Cell Device. Adv. Mater. 2021, 33, 2103383. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, Y.C.; Wu, Z.P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.C.; et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, Y.; Yin, G.; Lin, Y. Recent progress in nanostructured electrocatalysts for PEM fuel cells. J. Mater. Chem. A 2013, 1, 4631–4641. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
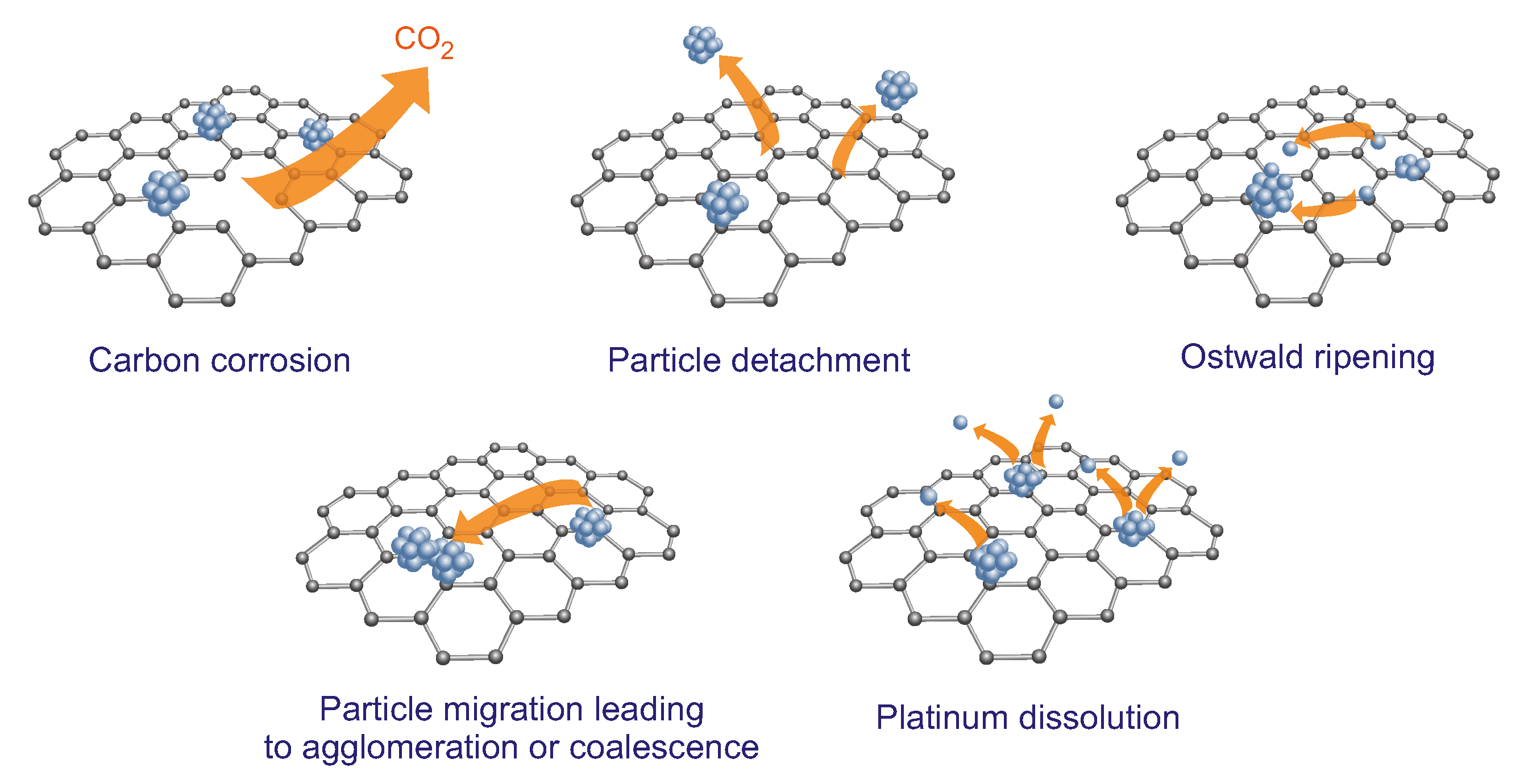
- Knights, S.D.; Colbow, K.M.; St-Pierre, J.; Wilkinson, D.P. Aging mechanisms and lifetime of PEFC and DMFC. J. Power Sources 2004, 127, 127–134. [Google Scholar] [CrossRef]
- Schultz, T.; Zhou, S.; Sundmacher, K. Current status of and recent developments in the direct methanol fuel cell. Chem. Eng. Technol. 2001, 24, 1223–1233. [Google Scholar] [CrossRef]
- Gouda, M.; Elnouby, M.; Aziz, A.N.; Youssef, M.E.; Santos, D.; Elessawy, N.A. Green and low-cost membrane electrode assembly for proton exchange membrane fuel cells: Effect of double-layer electrodes and gas diffusion layer. Front. Mater. 2020, 6, 337. [Google Scholar] [CrossRef] [Green Version]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Beermann, V.; Holtz, M.E.; Padgett, E.; de Araujo, J.F.; Muller, D.A.; Strasser, P. Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell STEM. Energy Environ. Sci. 2019, 12, 2476–2485. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tang, X.; Jia, H.; Li, H.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.J. Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 2020, 383, 164–171. [Google Scholar] [CrossRef]
- Yu, K.; Groom, D.J.; Wang, X.; Yang, Z.; Gummalla, M.; Ball, S.C.; Myers, D.J.; Ferreira, P.J. Degradation mechanisms of platinum nanoparticle catalysts in proton exchange membrane fuel cells: The role of particle size. Chem. Mater. 2014, 26, 5540–5548. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Grigoriev, S.; Millet, P.; Fateev, V. Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177, 281–285. [Google Scholar] [CrossRef]
- Edwards, P.; Thomas, J. Gold in a Metallic Divided State—From Faraday to Present-Day Nanoscience. Angew. Chem. Int. Ed. 2007, 46, 5480–5486. [Google Scholar] [CrossRef] [PubMed]
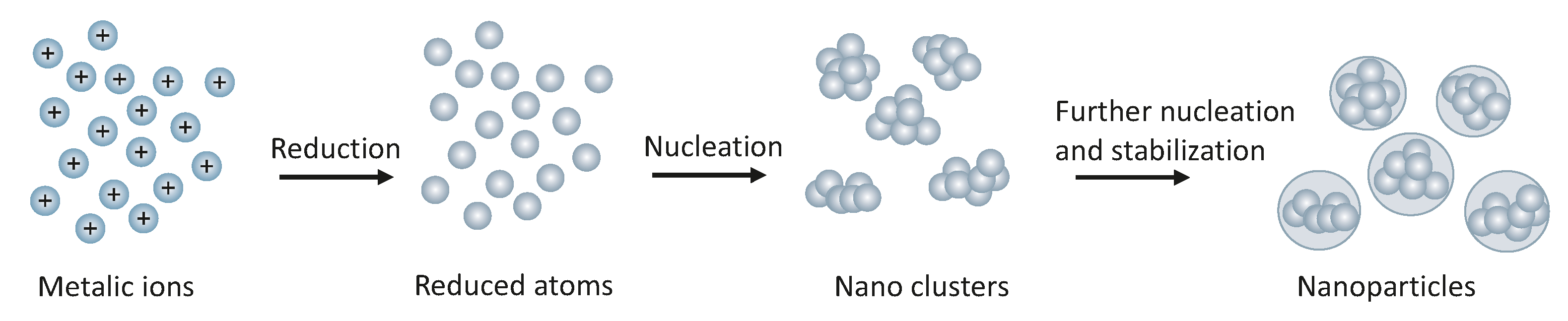
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- De Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Booth, S.G.; Uehara, A.; Chang, S.Y.; Mosselmans, J.F.W.; Schroeder, S.L.M.; Dryfe, R.A.W. Gold Deposition at a Free-Standing Liquid/Liquid Interface: Evidence for the Formation of Au(I) by Microfocus X-ray Spectroscopy (μXRF and μXAFS) and Cyclic Voltammetry. J. Phys. Chem. C 2015, 119, 16785–16792. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B. Electrochemical Synthesis of ZnO Nanoparticles. Eur. J. Inorg. Chem. 2008, 2008, 869–872. [Google Scholar] [CrossRef]
- Balachandramohan, J.; Sivasankar, T.; Sivakumar, M. Facile sonochemical synthesis of Ag2O-guar gum nanocomposite as a visible light photocatalyst for the organic transformation reactions. J. Hazard. Mater. 2020, 385, 121621. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Košević, M.G.; Zarić, M.M.; Stopić, S.R.; Stevanović, J.S.; Weirich, T.E.; Friedrich, B.G.; Panić, V.V. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Lusker, K.L.; Li, J.R.; Garno, J.C. Nanostructures of Functionalized Gold Nanoparticles Prepared by Particle Lithography with Organosilanes. Langmuir 2011, 27, 13269–13275. [Google Scholar] [CrossRef]
- Yu, X.; Pham, J.T.; Subramani, C.; Creran, B.; Yeh, Y.C.; Du, K.; Patra, D.; Miranda, O.R.; Crosby, A.J.; Rotello, V.M. Direct Patterning of Engineered Ionic Gold Nanoparticles via Nanoimprint Lithography. Adv. Mater. 2012, 24, 6330–6334. [Google Scholar] [CrossRef]
- Davies, G.L.; O’Brien, J.; Gun’ko, Y.K. Rare Earth Doped Silica Nanoparticles via Thermolysis of a Single Source Metallasilsesquioxane Precursor. Sci. Rep. 2017, 7, 45862. [Google Scholar] [CrossRef] [Green Version]
- Abedini, A.; Daud, A.R.; Abdul Hamid, M.A.; Kamil Othman, N.; Saion, E. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, A.; Kuznetsov, N.; Meshalkin, V.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Silva, L.I.; Perez-Gramatges, A.; Larrude, D.G.; Almeida, J.M.; Aucélio, R.Q.; da Silva, A.R. Gold nanoparticles produced using NaBH4 in absence and in the presence of one-tail or two-tail cationic surfactants: Characteristics and optical responses induced by aminoglycosides. Colloids Surfaces Physicochem. Eng. Asp. 2021, 614, 126174. [Google Scholar] [CrossRef]
- Moonrat, C.; Kittinaovarut, S.; Jinawath, S.; Sujaridworakun, P. The Effect of pH Values on Color Development of Silver Colloids. In Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2020; Volume 862, pp. 17–21. [Google Scholar]
- Mehr, F.P.; Khanjani, M.; Vatani, P. Synthesis of nano-Ag particles using sodium borohydride. Orient. J. Chem. 2015, 31, 1831–1833. [Google Scholar] [CrossRef]
- Sudirman, S.; Adi, W.A.; Budianto, E.; Yudianti, R. Mono-Dispersed Pt/MWNTs: Growing Directly on Multiwall Carbon Nanotubes (MWNTs) Using NaBH4 as Reducing Agent for Component of Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Chem. 2020, 12, 37–48. [Google Scholar] [CrossRef]
- Jung, S.C.; Park, Y.K.; Jung, H.Y.; Kim, S.C. Effect of Stabilizing Agents on the Synthesis of Palladium Nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 2833–2836. [Google Scholar] [CrossRef]
- Günbatar, S.; Aygun, A.; Karataş, Y.; Gülcan, M.; Şen, F. Carbon-nanotube-based rhodium nanoparticles as highly-active catalyst for hydrolytic dehydrogenation of dimethylamineborane at room temperature. J. Colloid Interface Sci. 2018, 530, 321–327. [Google Scholar] [CrossRef]
- Patharkar, R.; Nandanwar, S.; Chakraborty, M. Synthesis of colloidal ruthenium nanocatalyst by chemical reduction method. J. Chem. 2013, 2013, 831694. [Google Scholar] [CrossRef]
- He, S.B.; Zhuang, Q.Q.; Yang, L.; Lin, M.Y.; Kuang, Y.; Peng, H.P.; Deng, H.H.; Xia, X.H.; Chen, W. A Heparinase Sensor Based on a Ternary System of Hg2+–Heparin–Osmium Nanoparticles. Anal. Chem. 2019, 92, 1635–1642. [Google Scholar] [CrossRef]
- Cui, M.; Huang, X.; Zhang, X.; Xie, Q.; Yang, D. Ultra-small iridium nanoparticles as active catalysts for the selective and efficient reduction of nitroarenes. New J. Chem. 2020, 44, 18274–18280. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Wang, Z.L.; Green, T.C.; Henglein, A.; El-Sayed, M.A. Shape-Controlled Synthesis of Colloidal Platinum Nanoparticles. Science 1996, 272, 1924–1925. [Google Scholar] [CrossRef] [PubMed]
- Petroski, J.M.; Wang, Z.L.; Green, T.C.; El-Sayed, M.A. Kinetically Controlled Growth and Shape Formation Mechanism of Platinum Nanoparticles. J. Phys. Chem. B 1998, 102, 3316–3320. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.T.; Xu, B.Q. Shape-controlled synthesis of Pt nanocrystals: An evolution of the tetrahedral shape. Appl. Organomet. Chem. 2006, 20, 638–647. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Role of ions in the colloidal synthesis of gold nanowires. Philos. Mag. 2007, 87, 2143–2158. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhou, H.; Zhu, Y.; Sutter, E.; Ji, Y.; Rafailovich, M.H.; Sokolov, J.C. Seedless and Templateless Synthesis of Rectangular Palladium Nanoparticles. Chem. Mater. 2007, 19, 2065–2070. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yoshimori, K.; Tomita, K.; Sakai, M.; Sawada, T.; Niidome, Y.; Yamada, S.; Shosenji, H. Novel effects of twin-tailed cationic surfactants on the formation of gold nanorods. Chem. Lett. 2007, 36, 1230–1231. [Google Scholar] [CrossRef]
- Sui, Z.; Chen, X.; Wang, L.; Xu, L.; Zhuang, W.; Chai, Y.; Yang, C. Capping effect of CTAB on positively charged Ag nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 308–314. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Rogach, A.L.; Shevchenko, G.P.; Afanas’ev, Z.M.; Sviridov, V.V. Changes in the Morphology and Optical Absorption of Colloidal Silver Reduced with Formic Acid in the Polymer Matrix under UV Irradiation. J. Phys. Chem. B 1997, 101, 8129–8132. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, S.; Yang, Z.; Tsung, C.K.; Stucky, G.D.; Sun, L.; Wang, J.; Yan, C. Glutathione- and Cysteine-Induced Transverse Overgrowth on Gold Nanorods. J. Am. Chem. Soc. 2007, 129, 6402–6404. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Sachar, S.; Kaur, G.; Bhandari, P.; Kaur, G.; Biesinger, M.C.; Possmayer, F.; Petersen, N.O. Dependence of crystal growth of gold nanoparticles on the capping behavior of surfactant at ambient conditions. Cryst. Growth Des. 2008, 8, 1713–1719. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission Electron Microscopy of Shape-Controlled Nanocrystals and Their Assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Arán Ais, R.M.; Vidal-Iglesias, F.; Solla-Gullon, J.; Herrero, E.; Feliu, J. Electrochemical Characterization of Clean Shape-Controlled Pt Nanoparticles Prepared in Presence of Oleylamine/Oleic Acid. Electroanalysis 2015, 27, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Montiel, M.; Vidal-Iglesias, F.; Montiel, V.; Solla-Gullón, J. Electrocatalysis on shape-controlled metal nanoparticles: Progress in surface cleaning methodologies. Curr. Opin. Electrochem. 2017, 1, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Ward, C.J.; Tronndorf, R.; Eustes, A.S.; Auad, M.L.; Davis, E.W. Seed-mediated growth of gold nanorods: Limits of length to diameter ratio control. J. Nanomater. 2014, 2014, 47. [Google Scholar] [CrossRef] [Green Version]
- Mondal, J.; Trinh, Q.T.; Jana, A.; Ng, W.K.H.; Borah, P.; Hirao, H.; Zhao, Y. Size-Dependent Catalytic Activity of Palladium Nanoparticles Fabricated in Porous Organic Polymers for Alkene Hydrogenation at Room Temperature. ACS Appl. Mater. Interfaces 2016, 8, 15307–15319. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chen, H.; Ni, B.; Wang, K.; He, T.; Wu, Y.; Wang, X. Mesoporous ZrO2 Nanoframes for Biomass Upgrading. ACS Appl. Mater. Interfaces 2017, 9, 26897–26906. [Google Scholar] [CrossRef]
- Xu, G.R.; Bai, J.; Jiang, J.X.; Lee, J.M.; Chen, Y. Polyethyleneimine functionalized platinum superstructures: Enhancing hydrogen evolution performance by morphological and interfacial control. Chem. Sci. 2017, 8, 8411–8418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.R.; Bai, J.; Yao, L.; Xue, Q.; Jiang, J.X.; Zeng, J.H.; Chen, Y.; Lee, J.M. Polyallylamine-Functionalized Platinum Tripods: Enhancement of Hydrogen Evolution Reaction by Proton Carriers. ACS Catal. 2017, 7, 452–458. [Google Scholar] [CrossRef]
- Xu, G.R.; Wang, B.; Zhu, J.Y.; Liu, F.Y.; Chen, Y.; Zeng, J.H.; Jiang, J.X.; Liu, Z.H.; Tang, Y.W.; Lee, J.M. Morphological and Interfacial Control of Platinum Nanostructures for Electrocatalytic Oxygen Reduction. ACS Catal. 2016, 6, 5260–5267. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, D.; Shi, Y.; Guo, S.; Wang, Q.; Zhang, G.; Cui, P.; Cheng, S. Highly Porous Pt2Ir Alloy Nanocrystals as a Superior Catalyst with High-Efficiency C–C Bond Cleavage for Ethanol Electrooxidation. J. Phys. Chem. Lett. 2021, 12, 6773–6780. [Google Scholar] [CrossRef]
- Sadek, M.; Abd El-Lateef, H.M.; Mohran, H.S.; Elrouby, M. A promising star-like PtNi and coral reefs-like PtCo nano-structured materials for direct methanol fuel cell application. Electrochim. Acta 2021, 399, 139370. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, F.; Wei, J.; Sun, H.; Yuan, Y.; Bao, H.; Li, F.; Zhang, Z.; Han, S.; Niu, W. Designer Gold-Framed Palladium Nanocubes for Plasmon-Enhanced Electrocatalytic Oxidation of Ethanol. Chem.-Eur. J. 2022. [Google Scholar] [CrossRef]
- Hyun-Jung, C.; Kang, J.Y.; Jeon, I.Y.; Eo, S.M.; Tan, L.S.; Baek, J.B. Immobilization of platinum nanoparticles on 3,4-diaminobenzoyl-functionalized multi-walled carbon nanotube and its electrocatalytic activity. J. Nanopart. Res. 2012, 14, 704. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.C.; Mitra, M.D.; Shukla, S.; Narayan, R.J. Organotrialkoxysilane-Functionalized Noble Metal Monometallic, Bimetallic, and Trimetallic Nanoparticle Mediated Non-Enzymatic Sensing of Glucose by Resonance Rayleigh Scattering. Biosensors 2021, 11, 122. [Google Scholar] [CrossRef]
- Ali, S.; Sharma, A.S.; Ahmad, W.; Zareef, M.; Hassan, M.M.; Viswadevarayalu, A.; Jiao, T.; Li, H.; Chen, Q. Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Crit. Rev. Anal. Chem. 2021, 51, 454–481. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Li, Y.; Yu, H.; Yin, S.; Xue, H.; Li, X.; Xu, Y.; Wang, L. Tri-metallic PtPdAu mesoporous nanoelectrocatalysts. Nanotechnology 2018, 29, 255404. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef] [Green Version]
- González, A.L.; Reyes-Esqueda, J.A.; Noguez, C. Optical Properties of Elongated Noble Metal Nanoparticles. J. Phys. Chem. C 2008, 112, 7356–7362. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.U.; Jang, S.P.; Lee, S.Y. Production of aqueous spherical gold nanoparticles using conventional ultrasonic bath. Nanoscale Res. Lett. 2012, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal Gold and Silver Triangular Nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Mulder, D.W.; Phiri, M.M.; Jordaan, A.; Vorster, B.C. Modified HEPES one-pot synthetic strategy for gold nanostars. R. Soc. Open Sci. 2019, 6, 190160. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, Y.; Huang, C.Z.; Xia, Y. Synthesis of Ag Nanocubes 18–32 nm in Edge Length: The Effects of Polyol on Reduction Kinetics, Size Control, and Reproducibility. J. Am. Chem. Soc. 2013, 135, 1941–1951. [Google Scholar] [CrossRef] [Green Version]
- Jiu, J.; Murai, K.; Kim, D.; Kim, K.; Suganuma, K. Preparation of Ag nanorods with high yield by polyol process. Mater. Chem. Phys. 2009, 114, 333–338. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Zaheer, Z.; Rafiuddin. Multi-branched flower-like silver nanoparticles: Preparation and characterization. Colloids Surf. Physicochem. Eng. Asp. 2011, 384, 427–431. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Nagao, H.; Ichiji, M.; Hirasawa, I. Synthesis of Platinum Nanoparticles by Reductive Crystallization Using Polyethyleneimine. Chem. Eng. Technol. 2017, 40, 1242–1246. [Google Scholar] [CrossRef]
- Herricks, T.; Chen, J.; Xia, Y. Polyol Synthesis of Platinum Nanoparticles: Control of Morphology with Sodium Nitrate. Nano Lett. 2004, 4, 2367–2371. [Google Scholar] [CrossRef]
- Walbrück, K.; Kuellmer, F.; Witzleben, S.; Guenther, K. Synthesis and Characterization of PVP-Stabilized Palladium Nanoparticles by XRD, SAXS, SP-ICP-MS, and SEM. J. Nanomater. 2019, 2019, 4758108. [Google Scholar] [CrossRef]
- Iben Ayad, A.; Belda Marín, C.; Colaco, E.; Lefevre, C.; Méthivier, C.; Ould Driss, A.; Landoulsi, J.; Guénin, E. “Water soluble” palladium nanoparticle engineering for C–C coupling, reduction and cyclization catalysis. Green Chem. 2019, 21, 6646–6657. [Google Scholar] [CrossRef]
- Wang, Y.; Du, M.; Xu, J.; Yang, P.; Du, Y. Size-Controlled Synthesis of Palladium Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 891–894. [Google Scholar] [CrossRef]
- Alsalahi, W.; Tylus, W.; Trzeciak, A.M. Green synthesis of rhodium nanoparticles, catalytically active in benzene hydrogenation and 1-hexene hydroformylation. ChemCatChem 2018, 10, 2051–2058. [Google Scholar] [CrossRef]
- Fernández, G.; Pleixats, R. Rhodium Nanoparticles Stabilized by PEG-Tagged Imidazolium Salts as Recyclable Catalysts for the Hydrosilylation of Internal Alkynes and the Reduction of Nitroarenes. Catalysts 2020, 10, 1195. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Schaak, R.E. Ligand-Induced Fate of Embryonic Species in the Shape-Controlled Synthesis of Rhodium Nanoparticles. ACS Nano 2015, 9, 1707–1720. [Google Scholar] [CrossRef]
- Yu, M.; Diao, X.; Huang, T.; Liu, H.; Li, J. Shape-controlled Synthesis of Ruthenium Nanoparticles. Funct. Mater. Lett. 2011, 4, 337–340. [Google Scholar] [CrossRef]
- Yan, X.; Liu, H.; Liew, K.Y. Size control of polymer-stabilized ruthenium nanoparticles by polyol reduction. J. Mater. Chem. 2001, 11, 3387–3391. [Google Scholar] [CrossRef]
- Kusada, K.; Kobayashi, H.; Yamamoto, T.; Matsumura, S.; Sumi, N.; Sato, K.; Nagaoka, K.; Kubota, Y.; Kitagawa, H. Discovery of Face-Centered-Cubic Ruthenium Nanoparticles: Facile Size-Controlled Synthesis Using the Chemical Reduction Method. J. Am. Chem. Soc. 2013, 135, 5493–5496. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhao, Y.; Wang, C.; Song, Q. Synthesis of 2.5 nm colloidal iridium nanoparticles with strong surface enhanced Raman scattering activity. Microchim. Acta 2016, 183, 2047–2053. [Google Scholar] [CrossRef]
- Bonet, F.; Delmas, V.; Grugeon, S.; Herrera Urbina, R.; Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct. Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Chakrapani, K.; Sampath, S. Interconnected, ultrafine osmium nanoclusters: Preparation and surface enhanced Raman scattering activity. Chem. Commun. 2013, 49, 6173–6175. [Google Scholar] [CrossRef]
- Wakisaka, T.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Ibrahima, G.; Seo, O.; Kim, J.; Hiroi, S.; Sakata, O.; et al. Discovery of face-centred cubic Os nanoparticles. Chem. Commun. 2020, 56, 372–374. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Huang, Y.; Li, S.; Xue, C.; Gan, C.L.; Boey, F.; Zhang, H. Photochemically Controlled Synthesis of Anisotropic Au Nanostructures: Platelet-like Au Nanorods and Six-Star Au Nanoparticles. ACS Nano 2010, 4, 6196–6202. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Xing, L.; Xiahou, Y.; Zhang, P.; Du, W.; Xia, H. Size control synthesis of monodisperse, quasi-spherical silver nanoparticles to realize surface-enhanced Raman scattering uniformity and reproducibility. ACS Appl. Mater. Interfaces 2019, 11, 17637–17646. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Ayoko, G.A. Fabrication of nanostructured SERS substrates on conductive solid platforms for environmental application. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1294–1329. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Liu, J. Bioapplications of renal-clearable luminescent metal nanoparticles. Biomater. Sci. 2017, 5, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All that glitters is not silver—A new look at microbiological and medical applications of silver nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 1–23. [Google Scholar] [CrossRef]
- Bai, Q.; Li, D.; He, L.; Xiao, H.; Sui, N.; Liu, M. Solvent-free selective hydrogenation of o-chloronitrobenzene to o-chloroaniline over alumina supported Pt nanoparticles. Prog. Nat. Sci. Mater. Int. 2015, 25, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef]
- Li, N.; Tang, S.; Meng, X. Preparation of Pt–GO composites with high-number-density Pt nanoparticles dispersed uniformly on GO nanosheets. Prog. Nat. Sci. Mater. Int. 2016, 26, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Rong, H.; Zhang, S.; Muhammad, S.; Zhang, J. Noble metal-based nanocomposites for fuel cells. In Novel Nanomaterials; IntechOpen: London, UK, 2018; pp. 291–310. [Google Scholar]
- Chaturvedi, S.; Dave, P.N. A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2013, 17, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold-and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Cuenya, B.R.; Behafarid, F. Nanocatalysis: Size-and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 2015, 70, 135–187. [Google Scholar] [CrossRef]
- Bond, G.C. Supported metal catalysts: Some unsolved problems. Chem. Soc. Rev. 1991, 20, 441–475. [Google Scholar] [CrossRef]
- Geonmonond, R.; Marques da Silva, A.; Camargo, P. Controlled synthesis of noble metal nanomaterials: Motivation, principles, and opportunities in nanocatalysis. An. Acad. Bras. Ciências 2018, 90, 719–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Lee, S.; Fan, C.; Wu, T.; Anderson, S.L. CO Oxidation on Aun/TiO2 Catalysts Produced by Size-Selected Cluster Deposition. J. Am. Chem. Soc. 2004, 126, 5682–5683. [Google Scholar] [CrossRef]
- Yu, K.; Yeung, C.; Tsang, S. Carbon Dioxide Fixation into Chemicals (Methyl Formate) at High Yields by Surface Coupling over a Pd/Cu/ZnO Nanocatalyst. J. Am. Chem. Soc. 2007, 129, 6360–6361. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lu, G.; Zhou, X.; Cao, X.; Boey, F.; Zhang, H. Controlled Assembly of Gold Nanoparticles and Graphene Oxide Sheets on Dip Pen Nanolithography-Generated Templates. Langmuir 2009, 25, 10455–10458. [Google Scholar] [CrossRef]
- Li, L.; Xing, Y. Pt-Ru Nanoparticles Supported on Carbon Nanotubes as Methanol Fuel Cell Catalysts. J. Phys. Chem. C 2007, 111, 2803–2808. [Google Scholar] [CrossRef]
- Zhou, W.; Du, G.; Hu, P.; Yin, Y.; Li, J.; Yu, J.; Wang, G.; Wang, J.; Liu, H.; Wang, J.; et al. Nanopaper based on Ag/TiO2 nanobelts heterostructure for continuous-flow photocatalytic treatment of liquid and gas phase pollutants. J. Hazard. Mater. 2011, 197, 19–25. [Google Scholar] [CrossRef]
- Ishida, H.; Campbell, S.; Blackwell, J. General Approach to Nanocomposite Preparation. Chem. Mater. 2000, 12, 1260–1267. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Amani Babadi, A.; Akmal Che Mansor, M.S.; Julkapli, N.M.; Hessel, V.; Ostrikov, K.K. Gold–Carbon Nanocomposites for Environmental Contaminant Sensing. Micromachines 2021, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Hu, P. Understanding supported noble metal catalysts using first-principles calculations. J. Chem. Phys. 2019, 151, 180902. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Meng, Y.; Klasen, T.; Sahraoui, L.; Schüth, F. Structural mimicking of inorganic catalyst supports with polydivinylbenzene to improve performance in the selective aerobic oxidation of ethanol and glycerol in water. J. Catal. 2013, 308, 341–351. [Google Scholar] [CrossRef]
- Martinuzzi, S.; Cozzula, D.; Centomo, P.; Zecca, M.; Müller, T.E. The distinct role of the flexible polymer matrix in catalytic conversions over immobilised nanoparticles. RSC Adv. 2015, 5, 56181–56188. [Google Scholar] [CrossRef] [Green Version]
- Koga, H.; Tokunaga, E.; Hidaka, M.; Umemura, Y.; Saito, T.; Isogai, A.; Kitaoka, T. Topochemical synthesis and catalysis of metal nanoparticles exposed on crystalline cellulose nanofibers. Chem. Commun. 2010, 46, 8567–8569. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.; Vaughan, O.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.; Johnson, B.; Lambert, R. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef]
- Yamada, Y.; Tsung, C.K.; Huang, W.; Huo, Z.; Habas, S.; Soejima, T.; Aliaga, C.; Somorjai, G.; Yang, P. Nanocrystal bilayer for tandem catalysis. Nat. Chem. 2011, 3, 372–376. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Alayoglu, S.; Musselwhite, N.; Na, K.; Somorjai, G.A. Designed Catalysts from Pt Nanoparticles Supported on Macroporous Oxides for Selective Isomerization of n-Hexane. J. Am. Chem. Soc. 2014, 136, 6830–6833. [Google Scholar] [CrossRef]
- Kuo, C.H.; Tang, Y.; Chou, L.Y.; Sneed, B.T.; Brodsky, C.N.; Zhao, Z.; Tsung, C.K. Yolk–Shell Nanocrystal@ZIF-8 Nanostructures for Gas-Phase Heterogeneous Catalysis with Selectivity Control. J. Am. Chem. Soc. 2012, 134, 14345–14348. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.; Tsung, C.K.; Yamada, Y.; Yang, P.; Somorjai, G. Thermally Stable Pt/Mesoporous Silica Core-shell Nanocatalysts for High-Temperature Reactions. Nat. Mater. 2008, 8, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Zhao, Q.; Li, D.; Wang, J.Q.; Cho, M.; Cho, H.; Terasaki, O.; Chen, S.; Wan, Y. Highly Active Heterogeneous 3 nm Gold Nanoparticles on Mesoporous Carbon as Catalysts for Low-Temperature Selective Oxidation and Reduction in Water. ACS Catal. 2015, 5, 797–802. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Zhou, R.; Qiao, S. Silver/Nitrogen-Doped Graphene Interaction and Its Effect on Electrocatalytic Oxygen Reduction. Chem. Mater. 2014, 26, 5868–5873. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, R.; Xu, Y.; Zhang, L.; Liu, P.; Chen, Q. Adsorbate-Mediated Deposition of Noble-Metal Nanoparticles on Carbon Substrates for Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 6460–6465. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Yang, T.; Ling, H.; Lamonier, J.F.; Jaroniec, M.; Huang, J.; Monteiro, M.J.; Liu, J. A synthetic strategy for carbon nanospheres impregnated with highly monodispersed metal nanoparticles. NPG Asia Mater. 2016, 8, e240. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials: Carbon nanomaterials for advanced energy conversion and storage (small 8/2012). Small 2012, 8, 1122. [Google Scholar] [CrossRef]
- Noor, T.; Yaqoob, L.; Iqbal, N. Recent Advances in Electrocatalysis of Oxygen Evolution Reaction using Noble-Metal, Transition-Metal, and Carbon-Based Materials. ChemElectroChem 2021, 8, 447–483. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Uchida, M.; Aoyama, Y.; Tanabe, M.; Yanagihara, N.; Eda, N.; Ohta, A. Influences of both carbon supports and heat-treatment of supported catalyst on electrochemical oxidation of methanol. J. Electrochem. Soc. 1995, 142, 2572. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Effects of catalyst carbon support on proton conduction and cathode performance in PEM fuel cells. J. Electrochem. Soc. 2011, 158, B614. [Google Scholar] [CrossRef]
- Qu, W.L.; Wang, Z.B.; Jiang, Z.Z.; Gu, D.M.; Yin, G.P. Investigation on performance of Pd/Al2O3-C catalyst synthesized by microwave assisted polyol process for electrooxidation of formic acid. Rsc Adv. 2012, 2, 344–350. [Google Scholar] [CrossRef]
- Moore, A.D.; Holmes, S.M.; Roberts, E.P. Evaluation of porous carbon substrates as catalyst supports for the cathode of direct methanol fuel cells. RSC Adv. 2012, 2, 1669–1674. [Google Scholar] [CrossRef]
- Vogel, W. Size contraction in Pt/C and PtRu/C commercial E-TEK electrocatalysts: An in situ X-ray diffraction study. J. Phys. Chem. C 2008, 112, 13475–13482. [Google Scholar] [CrossRef]
- Chen, T.W.; Kalimuthu, P.; Veerakumar, P.; Lin, K.C.; Chen, S.M.; Ramachandran, R.; Mariyappan, V.; Chitra, S. Recent Developments in Carbon-Based Nanocomposites for Fuel Cell Applications: A Review. Molecules 2022, 27, 761. [Google Scholar] [CrossRef]
- Li, J.; Stephanopoulos, M.F.; Xia, Y. Introduction: Heterogeneous single-atom catalysis. Chem. Rev. 2020, 120, 11699–11702. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Wu, B.; Kuang, Y.; Zhang, X.; Chen, J. Noble metal nanoparticles/carbon nanotubes nanohybrids: Synthesis and applications. Nano Today 2011, 6, 75–90. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–gold nanoparticles hybrid—Synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Ham, K.; Kang, S.; Ju, H.; Lee, J. Enhanced corrosion tolerance and highly durable ORR activity by low Pt electrocatalyst on unique pore structured CNF in PEM fuel cell. Electrochim. Acta 2020, 348, 136346. [Google Scholar] [CrossRef]
- Jeon, Y.; Ji, Y.; Cho, Y.I.; Lee, C.; Park, D.H.; Shul, Y.G. Oxide–Carbon Nanofibrous Composite Support for a Highly Active and Stable Polymer Electrolyte Membrane Fuel-Cell Catalyst. ACS Nano 2018, 12, 6819–6829. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mardle, P.; Ji, X.; Wu, J.; Guan, S.; Dong, H.; Du, S. Thin film electrodes from Pt nanorods supported on aligned N-CNTs for proton exchange membrane fuel cells. Appl. Catal. B Environ. 2020, 260, 118031. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El-Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H.A. The Key to High Performance Low Pt Loaded Electrodes. J. Electrochem. Soc. 2017, 164, F418–F426. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Lu, Y.; Steinberger-Wilckens, R. PtPd nanowire arrays supported on reduced graphene oxide as advanced electrocatalysts for methanol oxidation. Carbon 2014, 79, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, P.; Jiang, S.P. Pd nanoparticles assembled on Ni- and N-doped carbon nanotubes towards superior electrochemical activity. Int. J. Hydrog. Energy 2021, 46, 2065–2074. [Google Scholar] [CrossRef]
- Tetrahydrofuran-functionalized multi-walled carbon nanotubes as effective support for Pt and PtSn electrocatalysts of fuel cells. Electrochim. Acta 2010, 55, 2964–2971. [CrossRef]
- Alegre, C.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Pastor, E.; Lázaro, M.J. Oxygen-Functionalized Highly Mesoporous Carbon Xerogel Based Catalysts for Direct Methanol Fuel Cell Anodes. J. Phys. Chem. C 2013, 117, 13045–13058. [Google Scholar] [CrossRef]
- La-Torre-Riveros, L.; Guzman-Blas, R.; Méndez-Torres, A.E.; Prelas, M.; Tryk, D.A.; Cabrera, C.R. Diamond Nanoparticles as a Support for Pt and PtRu Catalysts for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2012, 4, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.; Flores-Hernández, J.; Botte, G.; Pérez-Sicairos, S.; Romero-Castañon, T.; Reynoso-Soto, E.; Félix-Navarro, R. Pt-Au nanoparticles on graphene for oxygen reduction reaction: Stability and performance on proton exchange membrane fuel cell. Energy 2019, 181, 1225–1234. [Google Scholar] [CrossRef]
- Şanlı, L.I.; Yarar, B.; Bayram, V.; Gürsel, S.A. Electrosprayed catalyst layers based on graphene–carbon black hybrids for the next-generation fuel cell electrodes. J. Mater. Sci. 2017, 52, 2091–2102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hu, X.; Guo, S.; Yu, C.; Zhong, S.; Liu, X. Multi-functional graphene/carbon nanotube aerogels for its applications in supercapacitor and direct methanol fuel cell. Electrochim. Acta 2018, 264, 12–19. [Google Scholar] [CrossRef]
- Chen, D.J.; Zhang, Q.; Feng, J.X.; Ju, K.J.; Wang, A.J.; Wei, J.; Feng, J.J. One-pot wet-chemical co-reduction synthesis of bimetallic gold–platinum nanochains supported on reduced graphene oxide with enhanced electrocatalytic activity. J. Power Sources 2015, 287, 363–369. [Google Scholar] [CrossRef]
- Pan, D.; Li, X.; Zhang, A. Platinum assisted by carbon quantum dots for methanol electro-oxidation. Appl. Surf. Sci. 2018, 427, 715–723. [Google Scholar] [CrossRef]
- Deming, C.P.; Mercado, R.; Lu, J.E.; Gadiraju, V.; Khan, M.; Chen, S. Oxygen Electroreduction Catalyzed by Palladium Nanoparticles Supported on Nitrogen-Doped Graphene Quantum Dots: Impacts of Nitrogen Dopants. ACS Sustain. Chem. Eng. 2016, 4, 6580–6589. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Chen, J.; Yi, L.; Shao, P.; Xu, C.Y.; Wen, Z. Nitrogen-doped graphite encapsulating RuCo nanoparticles toward high-activity catalysis of water oxidation and reduction. Chem. Eng. J. 2021, 422, 130077. [Google Scholar] [CrossRef]
- Mondal, S.K. Synthesis of Mesoporous Fullerene and its Platinum Composite: A Catalyst for PEMFc. J. Electrochem. Soc. 2012, 159, K156–K160. [Google Scholar] [CrossRef]
- McPherson, I.J.; Ash, P.A.; Jones, L.; Varambhia, A.; Jacobs, R.M.J.; Vincent, K.A. Electrochemical CO Oxidation at Platinum on Carbon Studied through Analysis of Anomalous in Situ IR Spectra. J. Phys. Chem. C 2017, 121, 17176–17187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, J.W.; Xiang, P.H.; Qiao, J. Fabrication of graphene-fullerene hybrid by self-assembly and its application as support material for methanol electrocatalytic oxidation reaction. Appl. Surf. Sci. 2018, 440, 477–483. [Google Scholar] [CrossRef]
- Gu, K.; Kim, E.; Sharma, S.; Sharma, P.; Bliznakov, S.; Hsiao, B.; Rafailovich, M. Mesoporous carbon aerogel with tunable porosity as the catalyst support for enhanced proton-exchange membrane fuel cell performance. Mater. Today Energy 2021, 19, 100560. [Google Scholar] [CrossRef]
- Kin, Y.; Saito, K.; Oda, H.; Ando, T.; Nakagawa, K. Development of Direct Methanol Fuel Cell Catalyst Using Marimo Nano Carbon. Catal. Lett. 2019, 149, 1–6. [Google Scholar] [CrossRef]
- Bak, J.; Kim, H.; Lee, S.; Kim, M.; Kim, E.J.; Roh, J.; Shin, J.; Choi, C.H.; Cho, E. Boosting the Role of Ir in Mitigating Corrosion of Carbon Support by Alloying with Pt. ACS Catal. 2020, 10, 12300–12309. [Google Scholar] [CrossRef]
- Luo, F.; Hu, H.; Zhao, X.; Yang, Z.; Zhang, Q.; Xu, J.; Kaneko, T.; Yoshida, Y.; Zhu, C.; Cai, W. Robust and Stable Acidic Overall Water Splitting on Ir Single Atoms. Nano Lett. 2020, 20, 2120–2128. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Pramanik, H. Addition of rhenium (Re) to Pt-Ru/f-MWCNT anode electrocatalysts for enhancement of ethanol electrooxidation in half cell and single direct ethanol fuel cell. Int. J. Hydrog. Energy 2020, 45, 13300–13321. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yeh, T.K.; Tsai, C.H. Electrodeposition of platinum-ruthenium nanoparticles on carbon nanotubes directly grown on carbon cloths for methanol oxidation. Mater. Chem. Phys. 2008, 109, 422–428. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Chen, J.J.; Wang, F.b.; Sheng, Z.H.; Xia, X.H. A facile approach to the synthesis of highly electroactive Pt nanoparticles on graphene as an anode catalyst for direct methanol fuel cells. Chem. Commun. 2010, 46, 5951–5953. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Zhu, M.; Jiang, F.; Du, Y.; Wang, C.; Yang, P. Highly efficient electrocatalytic performance based on Pt nanoflowers modified reduced graphene oxide/carbon cloth electrode. J. Mater. Chem. 2012, 22, 13707–13713. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, I.; Kwon, O.J.; Kim, J. One-step co-electrodeposition of Pt-Ru electrocatalysts on carbon paper for direct methanol fuel. Chem. Eng. J. 2012, 181, 276–280. [Google Scholar] [CrossRef]
- Gao, L.; Ding, L.; Fan, L. Pt nanoflower/graphene-layered composites by ZnO nanoparticle expansion of graphite and their enhanced electrocatalytic activity for methanol oxidation. Electrochim. Acta 2013, 106, 159–164. [Google Scholar] [CrossRef]
- Gao, F.; Yang, N.; Smirnov, W.; Obloh, H.; Nebel, C. Size-controllable and homogeneous platinum nanoparticles on diamond using wet chemically assisted electrodeposition. Electrochim. Acta 2013, 90, 445–451. [Google Scholar] [CrossRef]
- Mavrokefalos, C.; Hasan, M.; Rohan, J.; Foord, J. Enhanced Mass Activity and Stability of Bimetallic Pd-Ni Nanoparticles on Boron-Doped Diamond for Direct Ethanol Fuel Cell Applications. ChemElectroChem 2017, 5, 455–463. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Lokesh, K.; Ojha, P.; Sahu, A.; Bhat, S.; Kalpana, D. Electrochemical deposition of three-dimensional platinum nanoflowers for high-performance polymer electrolyte fuel cells. J. Colloid Interface Sci. 2020, 572, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.A.; Chen, X.; Yasin, F.M.; Eggers, P.K.; Boulos, R.A.; Wang, X.; Chua, H.T.; Raston, C.L. Shear flow assisted decoration of carbon nano-onions with platinum nanoparticles. Chem. Commun. 2013, 49, 5171–5173. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, B.; Tao, B. A facile room temperature chemical route to Pt nanocube/carbon nanotube heterostructures with enhanced electrocatalysis. J. Power Sources 2011, 196, 191–195. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Wang, H.; Cao, Y.; He, J. Facile Deposition of Pd Nanoparticles on Carbon Nanotube Microparticles and Their Catalytic Activity for Suzuki Coupling Reactions. J. Phys. Chem. C 2008, 112, 8172–8176. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L. Substrate-Enhanced Electroless Deposition of Metal Nanoparticles on Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef]
- Ming, M.; Zhang, Y.; He, C.; Zhao, L.; Niu, S.; Fan, G.; Hu, J.S. Room-Temperature Sustainable Synthesis of Selected Platinum Group Metal (PGM = Ir, Rh, and Ru) Nanocatalysts Well-Dispersed on Porous Carbon for Efficient Hydrogen Evolution and Oxidation. Small 2019, 15, 1903057. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, H.; Gao, Z.; Hu, J.; Liu, D.; Dai, L. A facile approach to high-performance trifunctional electrocatalysts by substrate-enhanced electroless deposition of Pt/NiO/Ni on carbon nanotubes. Nanoscale 2020, 12, 14615–14625. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Covalent chemistry of single-wall carbon nanotubes. J. Mater. Chem. 2002, 12, 1952–1958. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Modified carbon nanotubes: An effective way to selective attachment of gold nanoparticles. Carbon 2003, 41, 2923–2929. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon–carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Olin, H. Gold-carbon nanotube nanocomposites: Synthesis and applications. Int. J. Biomed. Nanosci. Nanotechnol. 2011, 2, 112–135. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Li, H.; Chang, G.; Zhang, Y.; Tian, J.; Liu, S.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Photocatalytic synthesis of highly dispersed Pd nanoparticles on reduced graphene oxide and their application in methanol electro-oxidation. Catal. Sci. Technol. 2012, 2, 1153–1156. [Google Scholar] [CrossRef]
- Fan, J.J.; Fan, Y.J.; Wang, R.X.; Xiang, S.; Tang, H.G.; Sun, S.G. A novel strategy for the synthesis of sulfur-doped carbon nanotubes as a highly efficient Pt catalyst support toward the methanol oxidation reaction. J. Mater. Chem. A 2017, 5, 19467–19475. [Google Scholar] [CrossRef]
- Lu, R.; Zang, J.; Wang, Y.; Zhao, Y. Microwave synthesis and properties of nanodiamond supported PtRu electrocatalyst for methanol oxidation. Electrochim. Acta 2012, 60, 329–333. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Hung, W.M.; Chen, W.Y.; Lin, J.Y. Microwave-assisted polyol synthesis of Pt–Zn electrocatalysts on carbon nanotube electrodes for methanol oxidation. Int. J. Hydrog. Energy 2011, 36, 2765–2772. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Wei, J.M.; Hsiao, H.T.; Chen, W.Y. Fabrication of flower-like platinum clusters onto graphene sheets by pulse electrochemical deposition. Electrochim. Acta 2012, 64, 177–182. [Google Scholar] [CrossRef]
- He, Z.; Chen, J.; Liu, D.; Zhou, H.; Kuang, Y. Electrodeposition of Pt-Ru nanoparticles on carbon nanotubes and their electrocatalytic properties for methanol electrooxidation. Diam. Relat. Mater. 2004, 13, 1764–1770. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, M.; Bangsaruntip, S.; Dai, H. Spontaneous Reduction of Metal Ions on the Sidewalls of Carbon Nanotubes. J. Am. Chem. Soc. 2002, 124, 9058–9059. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xia, X.; Du, Y.; Wang, C. Electroless deposition of Au nanoparticles on reduced graphene oxide/polyimide film for electrochemical detection of hydroquinone and catechol. Front. Mater. Sci. 2017, 11, 262–270. [Google Scholar] [CrossRef]
- Liu, X.W.; Mao, J.J.; Liu, P.D.; Wei, X.W. Fabrication of metal-graphene hybrid materials by electroless deposition. Carbon 2011, 49, 477–483. [Google Scholar] [CrossRef]
- Qi, J.; Jiang, L.; Tang, Q.; Zhu, S.; Wang, S.; Yi, B.; Sun, G. Synthesis of graphitic mesoporous carbons with different surface areas and their use in direct methanol fuel cells. Carbon 2012, 50, 2824–2831. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; Garcia, A.; Caméan, I.; Lazaro, M. Comparative study of Pt catalysts supported on different high conductive carbon materials for methanol and ethanol oxidation. Electrochim. Acta 2013, 102, 19–27. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Z.; Xu, J.; Wang, W.; Chen, Z. Mesoporous carbon synthesized from dual colloidal silica/block copolymer template approach as the support of platinum nanoparticles for direct methanol fuel cells. Electrochim. Acta 2013, 88, 184–192. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Maiyalagan, T.; Alaje, T.O.; Scott, K. Highly stable Pt–Ru nanoparticles supported on three-dimensional cubic ordered mesoporous carbon (Pt–Ru/CMK-8) as promising electrocatalysts for methanol oxidation. J. Phys. Chem. C 2012, 116, 2630–2638. [Google Scholar] [CrossRef]
- Su, F.; Poh, C.K.; Tian, Z.; Xu, G.; Koh, G.; Wang, Z.; Liu, Z.; Lin, J. Electrochemical behavior of Pt nanoparticles supported on meso-and microporous carbons for fuel cells. Energy Fuels 2010, 24, 3727–3732. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. One-step synthesis of sodium carboxymethyl cellulose-derived carbon aerogel/nickel oxide composites for energy storage. Chem. Eng. J. 2017, 324, 287–295. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and facile fabrication of carbon aerogels from cellulose-based waste newspaper for solving organic pollution. Carbohydr. Polym. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Antolini, E. Lignocellulose, cellulose and lignin as renewable alternative fuels for direct biomass fuel cells. ChemSusChem 2021, 14, 189–207. [Google Scholar] [CrossRef]
- Cheng, S.; Rettew, R.E.; Sauerbrey, M.; Alamgir, F.M. Architecture-dependent surface chemistry for Pt monolayers on carbon-supported Au. ACS Appl. Mater. Interfaces 2011, 3, 3948–3956. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.J.; Lee, S.Y. Effect of surface modification of mesoporous carbon supports on the electrochemical activity of fuel cells. J. Colloid Interface Sci. 2013, 405, 150–156. [Google Scholar] [CrossRef]
- Su, F.; Tian, Z.; Poh, C.K.; Wang, Z.; Lim, S.H.; Liu, Z.; Lin, J. Pt nanoparticles supported on nitrogen-doped porous carbon nanospheres as an electrocatalyst for fuel cells. Chem. Mater. 2010, 22, 832–839. [Google Scholar] [CrossRef]
- Harzer, G.S.; Orfanidi, A.; El-Sayed, H.; Madkikar, P.; Gasteiger, H.A. Tailoring catalyst morphology towards high performance for low Pt loaded PEMFC cathodes. J. Electrochem. Soc. 2018, 165, F770. [Google Scholar] [CrossRef] [Green Version]
- Saifuddin, N.; Raziah, A.; Junizah, A. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Planeix, J.; Coustel, N.; Coq, B.; Brotons, V.; Kumbhar, P.; Dutartre, R.; Geneste, P.; Bernier, P.; Ajayan, P. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. [Google Scholar] [CrossRef]
- Brandao, A.T.; Rosoiu, S.; Costa, R.; Lazar, O.A.; Silva, A.F.; Anicai, L.; Pereira, C.M.; Enachescu, M. Characterization and electrochemical studies of MWCNTs decorated with Ag nanoparticles through pulse reversed current electrodeposition using a deep eutectic solvent for energy storage applications. J. Mater. Res. Technol. 2021, 15, 342–359. [Google Scholar] [CrossRef]
- Ohtaka, A.; Sansano, J.M.; Nájera, C.; Miguel-García, I.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Palladium and Bimetallic Palladium–Nickel Nanoparticles Supported on Multiwalled Carbon Nanotubes: Application to Carbon-Carbon Bond-Forming Reactions in Water. ChemCatChem 2015, 7, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fu, Y.; Lai, C.; Qin, L.; Li, B.; Liu, S.; Yi, H.; Xu, F.; Li, L.; Zhang, M.; et al. Porous materials confining noble metals for the catalytic reduction of nitroaromatics: Controllable synthesis and enhanced mechanism. Environ. Sci.-Nano 2021, 8, 3067–3097. [Google Scholar] [CrossRef]
- Guo, D.J.; Cui, S.K. A composite strategy to prepare high active Pt-WO3/MWCNT catalysts for methanol electro-oxidation. J. Phys. Chem. Solids 2021, 159, 110293. [Google Scholar] [CrossRef]
- Lee, K.M.; Li, L.; Dai, L. Asymmetric end-functionalization of multi-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 4122–4123. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, F.P.; Wang, X.; Shen, P.K. Anchoring metal nanoparticles on hydrofluoric acid treated multiwalled carbon nanotubes as stable electrocatalysts. Electrochem. Commun. 2008, 10, 1101–1104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Kuehner, D.; Ivaska, A.; Niu, L. Green synthesis of 1–2 nm gold nanoparticles stabilized by amine-terminated ionic liquid and their electrocatalytic activity in oxygen reduction. Green Chem. 2008, 10, 907–909. [Google Scholar] [CrossRef]
- Tunckol, M.; Fantini, S.; Malbosc, F.; Durand, J.; Serp, P. Effect of the synthetic strategy on the non-covalent functionalization of multi-walled carbon nanotubes with polymerized ionic liquids. Carbon 2013, 57, 209–216. [Google Scholar] [CrossRef]
- Zheng, M.; Li, P.; Fu, G.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Efficient anchorage of highly dispersed and ultrafine palladium nanoparticles on the water-soluble phosphonate functionalized multiwall carbon nanotubes. Appl. Catal. B Environ. 2013, 129, 394–402. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Garcia-Bernabé, A.; Compañ, V. Bimetallic Pt-M electrocatalysts supported on single-wall carbon nanotubes for hydrogen and methanol electrooxidation in fuel cells applications. Int. J. Hydrog. Energy 2018, 43, 872–884. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Zhang, W.; Ma, Q.; Xu, Q.; Khotseng, L.; Su, H. Highly efficient methanol oxidation on durable PtxIr/MWCNT catalysts for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2020, 45, 6447–6460. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Y.; Dai, L. Vertically aligned carbon nanotube arrays co-doped with phosphorus and nitrogen as efficient metal-free electrocatalysts for oxygen reduction. J. Phys. Chem. Lett. 2012, 3, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Nie, H.; Yang, Z.; Zhang, J.; Liu, Z.; Xu, X.; Huang, S. Metal-free selenium doped carbon nanotube/graphene networks as a synergistically improved cathode catalyst for oxygen reduction reaction. Nanoscale 2012, 4, 6455–6460. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, B.G.; Meunier, V.; Romo-Herrera, J.M.; Cruz-Silva, E.; Cullen, D.A.; Terrones, H.; Smith, D.J.; Terrones, M. Nitrogen-mediated carbon nanotube growth: Diameter reduction, metallicity, bundle dispersability, and bamboo-like structure formation. ACS Nano 2007, 1, 369–375. [Google Scholar] [CrossRef]
- Chizari, K.; Janowska, I.; Houllé, M.; Florea, I.; Ersen, O.; Romero, T.; Bernhardt, P.; Ledoux, M.J.; Pham-Huu, C. Tuning of nitrogen-doped carbon nanotubes as catalyst support for liquid-phase reaction. Appl. Catal. A Gen. 2010, 380, 72–80. [Google Scholar] [CrossRef]
- Chen, G.X.; Zhang, J.M.; Wang, D.D.; Xu, K.W. First-principles study of palladium atom adsorption on the boron- or nitrogen-doped carbon nanotubes. Phys. B Condens. Matter 2009, 404, 4173–4177. [Google Scholar] [CrossRef]
- Rajala, T.; Kronberg, R.; Backhouse, R.; Buan, M.E.M.; Tripathi, M.; Zitolo, A.; Jiang, H.; Laasonen, K.; Susi, T.; Jaouen, F.; et al. A platinum nanowire electrocatalyst on single-walled carbon nanotubes to drive hydrogen evolution. Appl. Catal. B Environ. 2020, 265, 118582. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yeh, T.K.; Tsai, C.H. An improved electrodeposition technique for preparing platinum and platinum–ruthenium nanoparticles on carbon nanotubes directly grown on carbon cloth for methanol oxidation. Electrochem. Commun. 2006, 8, 1445–1452. [Google Scholar] [CrossRef]
- Zhang, l.; Fang, Z.; Zhao, G.C.; Wei, X.W. Electrodeposited Platinum Nanoparticles on the Multi-Walled Carbon Nanotubes and its Electrocatalytic for Nitric Oxide. Int. J. Electrochem. Sci. 2008, 3, 746–754. [Google Scholar]
- Chen, X.; Li, N.; Eckhard, K.; Stoica, L.; Xia, W.; Assmann, J.; Muhler, M.; Schuhmann, W. Pulsed electrodeposition of Pt nanoclusters on carbon nanotubes modified carbon materials using diffusion restricting viscous electrolytes. Electrochem. Commun. 2007, 9, 1348–1354. [Google Scholar] [CrossRef]
- Xiao, F.; Mo, Z.; Zhao, F.; Zeng, B. Ultrasonic-electrodeposition of gold–platinum alloy nanoparticles on multi-walled carbon nanotubes–ionic liquid composite film and their electrocatalysis towards the oxidation of nitrite. Electrochem. Commun. 2008, 10, 1740–1743. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Q.; Tan, Y.; Fu, Y.; Su, Z.; Huang, Y.; Yao, S. Preparation of Pt/multiwalled carbon nanotubes modified Au electrodes via Pt–Cu co-electrodeposition/Cu stripping protocol for high-performance electrocatalytic oxidation of methanol. Mater. Chem. Phys. 2009, 118, 371–378. [Google Scholar] [CrossRef]
- Lorençon, E.; Ferlauto, A.S.; de Oliveira, S.; Miquita, D.R.; Resende, R.R.; Lacerda, R.G.; Ladeira, L.O. Direct production of carbon nanotubes/metal nanoparticles hybrids from a redox reaction between metal ions and reduced carbon nanotubes. ACS Appl. Mater. Interfaces 2009, 1, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Ding, F.; Yakobson, B.I.; Lu, P.; Qi, L.; Li, J. In situ observation of graphene sublimation and multi-layer edge reconstructions. Proc. Natl. Acad. Sci. USA 2009, 106, 10103–10108. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Correction: Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 5226. [Google Scholar] [CrossRef]
- Liu, M.; Hof, F.; Moro, M.; Valenti, G.; Paolucci, F.; Pénicaud, A. Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting. Nanoscale 2020, 12, 20165–20170. [Google Scholar] [CrossRef]
- Yang, N.; Swain, G.M.; Jiang, X. Nanocarbon electrochemistry and electroanalysis: Current status and future perspectives. Electroanalysis 2016, 28, 27–34. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqui, U.; Ahmad, A.; Hamid, N. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: An efficient anode catalyst for direct methanol fuel cells. J. Alloy. Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Su, C.; Loh, K.P. Carbocatalysts: Graphene Oxide and Its Derivatives. Acc. Chem. Res. 2013, 46, 2275–2285. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, J.; Wang, L.; Tian, C.; Jiang, B.; Fu, H. Fabrication of a palladium nanoparticle/graphene nanosheet hybrid via sacrifice of a copper template and its application in catalytic oxidation of formic acid. Chem. Commun. 2011, 47, 2014–2016. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Kitta, M.; Xu, Q. Monodispersed Pt nanoparticles on reduced graphene oxide by a non-noble metal sacrificial approach for hydrolytic dehydrogenation of ammonia borane. Nano Res. 2017, 10, 3811–3816. [Google Scholar] [CrossRef]
- Jeong, D.W.; Park, S.; Choi, W.J.; Bae, G.; Chung, Y.J.; Yang, C.S.; Lee, Y.K.; Kim, J.J.; Park, N.; Lee, J.O. Electron-transfer transparency of graphene: Fast reduction of metal ions on graphene-covered donor surfaces. Phys. Status Solidi RRL 2015, 9, 180–186. [Google Scholar] [CrossRef]
- Iqbal, M.; Li, C.; Jiang, B.; Hossain, M.S.A.; Islam, M.T.; Henzie, J.; Yamauchi, Y. Tethering mesoporous Pd nanoparticles to reduced graphene oxide sheets forms highly efficient electrooxidation catalysts. J. Mater. Chem. A 2017, 5, 21249–21256. [Google Scholar] [CrossRef]
- Li, S.S.; Zheng, J.N.; Ma, X.; Hu, Y.Y.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of hierarchical dendritic PtPd nanogarlands supported on reduced graphene oxide with enhanced electrocatalytic properties. Nanoscale 2014, 6, 5708–5713. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Hwang, S.K.; Kwak, C.H.; Oh, S.Y.; Kim, C.Y.; Lee, G.w.; Lee, J.B.; Huh, Y.S.; Han, Y.K. Pt-Au bimetallic nanoparticles decorated on reduced graphene oxide as an excellent electrocatalysts for methanol oxidation. Synth. Met. 2016, 219, 52–59. [Google Scholar] [CrossRef]
- Hassan, H.M.; Abdelsayed, V.; Abd El Rahman, S.K.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Zhou, M.; Ashokkumar, M.; Huang, N.M.; Manickam, S. Sonochemical and sustainable synthesis of graphene-gold (G-Au) nanocomposites for enzymeless and selective electrochemical detection of nitric oxide. Biosens. Bioelectron. 2017, 87, 622–629. [Google Scholar]
- Huang, Y.X.; Xie, J.F.; Zhang, X.; Xiong, L.; Yu, H.Q. Reduced graphene oxide supported palladium nanoparticles via photoassisted citrate reduction for enhanced electrocatalytic activities. ACS Appl. Mater. Interfaces 2014, 6, 15795–15801. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.C.; Yan, Q.; Chen, P.; Zhang, H. In Situ Synthesis of Metal Nanoparticles on Single-Layer Graphene Oxide and Reduced Graphene Oxide Surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar] [CrossRef]
- Qin, X.; Li, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. One-pot synthesis of Au nanoparticles/reduced graphene oxide nanocomposites and their application for electrochemical H2O2, glucose, and hydrazine sensing. Gold Bull. 2014, 47, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.Q.; Pan, X.; Zhang, N.; Xu, Y.J. A facile one-step way to anchor noble metal (Au, Ag, Pd) nanoparticles on a reduced graphene oxide mat with catalytic activity for selective reduction of nitroaromatic compounds. CrystEngComm 2013, 15, 6819–6828. [Google Scholar] [CrossRef]
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef]
- Liu, S.; Tian, N.; Xie, A.Y.; Du, J.H.; Xiao, J.; Liu, L.; Sun, H.Y.; Cheng, Z.Y.; Zhou, Z.Y.; Sun, S.G. Electrochemically seed-mediated synthesis of sub-10 nm tetrahexahedral Pt nanocrystals supported on graphene with improved catalytic performance. J. Am. Chem. Soc. 2016, 138, 5753–5756. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wei, M.; D’Aloia, A.; Wu, G. Heteroatom polymer-derived 3D high-surface-area and mesoporous graphene sheet-like carbon for supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30212–30224. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Fu, C.; Yang, F.; Li, X.; Jiang, F.; Guo, Y.; Zhu, F.; Yang, L.; Shen, S.; Zhang, J. Composition-graded Cu–Pd nanospheres with Ir-doped surfaces on N-doped porous graphene for highly efficient ethanol electro-oxidation in alkaline media. ACS Catal. 2019, 10, 1171–1184. [Google Scholar] [CrossRef]
- Qiu, X.; Yan, X.; Cen, K.; Sun, D.; Xu, L.; Tang, Y. Achieving highly electrocatalytic performance by constructing holey reduced graphene oxide hollow nanospheres sandwiched by interior and exterior platinum nanoparticles. ACS Appl. Energy Mater. 2018, 1, 2341–2349. [Google Scholar] [CrossRef]
- Kumar, R.; Oh, J.H.; Kim, H.J.; Jung, J.H.; Jung, C.H.; Hong, W.G.; Kim, H.J.; Park, J.Y.; Oh, I.K. Nanohole-structured and palladium-embedded 3D porous graphene for ultrahigh hydrogen storage and CO oxidation multifunctionalities. ACS Nano 2015, 9, 7343–7351. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-assembled and one-step synthesis of interconnected 3D network of Fe3O4/reduced graphene oxide nanosheets hybrid for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Qiu, X.; Li, T.; Deng, S.; Cen, K.; Xu, L.; Tang, Y. A General Strategy for the Synthesis of PtM (M= Fe, Co, Ni) Decorated Three-Dimensional Hollow Graphene Nanospheres for Efficient Methanol Electrooxidation. Chem.–A Eur. J. 2018, 24, 1246–1252. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, Q.; Su, Y.; Xu, L.; Wang, H.; Liu, J.; Hou, S. Palladium nanoparticles encapsulated into hollow N-doped graphene microspheres as electrocatalyst for ethanol oxidation reaction. ACS Appl. Nano Mater. 2019, 2, 1898–1908. [Google Scholar] [CrossRef]
- Yu, K.; Lin, Y.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Ternary N, S, and P-doped hollow carbon spheres derived from polyphosphazene as Pd supports for ethanol oxidation reaction. Catalysts 2019, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Q.; Huang, Z.; Liu, G.; Zhang, H. Recent progress in graphene-based noble-metal nanocomposites for electrocatalytic applications. Adv. Mater. 2019, 31, 1800696. [Google Scholar] [CrossRef] [PubMed]
- Stambula, S.; Gauquelin, N.; Bugnet, M.; Gorantla, S.; Turner, S.; Sun, S.; Liu, J.; Zhang, G.; Sun, X.; Botton, G.A. Chemical structure of nitrogen-doped graphene with single platinum atoms and atomic clusters as a platform for the PEMFC electrode. J. Phys. Chem. C 2014, 118, 3890–3900. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1, 3-butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef] [PubMed]
- Detavernier, C.; Dendooven, J.; Sree, S.P.; Ludwig, K.F.; Martens, J.A. Tailoring nanoporous materials by atomic layer deposition. Chem. Soc. Rev. 2011, 40, 5242–5253. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Lin, Y.; Wu, H.; Zhang, W.; Sun, Z.; Cheng, H.; Liu, W.; Wang, C.; Li, J.; Huang, X.; et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Lisitsyn, A.S.; Podyacheva, O.Y.; Hage, F.S.; Ramasse, Q.M.; Bangert, U.; Bulusheva, L.G. Single atoms of Pt-group metals stabilized by N-doped carbon nanofibers for efficient hydrogen production from formic acid. ACS Catal. 2016, 6, 3442–3451. [Google Scholar] [CrossRef]
- Zhang, C.; Sha, J.; Fei, H.; Liu, M.; Yazdi, S.; Zhang, J.; Zhong, Q.; Zou, X.; Zhao, N.; Yu, H.; et al. Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 2017, 11, 6930–6941. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Kim, M.; Kwon, H.C.; Cho, S.J.; Yun, S.; Kim, H.T.; Mayrhofer, K.J.; Kim, H.; Choi, M. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 2016, 7, 10922. [Google Scholar] [CrossRef] [Green Version]


| Metal | Shape | Size [nm] | Precursor | Reductant | Stabilizer | References |
|---|---|---|---|---|---|---|
| Au | Spherical | 20–50 | HAuCl4 | SC | - | [99] |
| Spherical | 3.5–4 | HAuCl4 | NaBH4 | SC | [100] | |
| Nanoprisms | 144 ± 30 (edge length) | HAuCl4 | NaBH4 | CTAB and AA | [101] | |
| Nanostars | 37 ± 2 | HAuCl4 | HEPES | PVP | [102] | |
| Ag | Cubic | 18–32 | CF3COOAg | DEG | PVP | [103] |
| Nanorods | 80–100 (diameter) | AgNO3 | EG | PVP | [104] | |
| Nanostars | ~300 | AgNO3 | HA | CCA | [105] | |
| Flower-like | ~450 | AgNO3 | CCA | CTAB | [106] | |
| Spherical | 10–200 (tunable) | AgNO3 | SC and TA | - | [107] | |
| Pt | Spherical | 4.9 | H2PtCl6/K2PtCl4 | NaBH4 | PEI | [108] |
| Cubic Tetrahedral | 5.2 | H2PtCl6/K2PtCl4 | NaBH4 | PEI | [108] | |
| Spherical Tetrahedral Octahedral (tunable) | 3–30 | H2PtCl6 | EG | PVP | [109] | |
| Pd | Spherical | 5–15 | PdCl2 | TEG | PVP | [110] |
| Nanodendrites | 50 | Na2PdCl4 | SA | Phosphonic acids with aromatic side chains | [111] | |
| Spherical | 1.5–23.3 (tunable) | PdCl2 | NaBH4 | PVP | [112] | |
| Rh | Spherical | 1.5–4 | Rh(acac)(CO)2 | - | PVP/PVA | [113] |
| Spherical | 2.9–5.6 | RhCl3 | NaBH4 | PEG-tagged imidazolium salts | [114] | |
| Triangular | 10 | RhCl3 | TREG | PVP | [115] | |
| Ru | Pompon-like | ~148 | RuCl3 | TREG | PVP | [116] |
| Spherical | 1.4–7.4 | RuCl3 | EG/DEG/TEG | PVP | [117] | |
| Cubic | 2.4–5 | Ru(acac)3/RuCl3 | EG/TEG | PVP | [118] | |
| Ir | Spherical | 2.5 ± 0.5 | IrCl3 | NaBH4 | SC | [119] |
| Spherical | ~3 | H2IrCl6 | EG | - | [120] | |
| Spherical | 3–4 | IrCl3 | NaBH4 | TA | [65] | |
| Os | Spherical NPs forming nanochains | 1–1.5 (single NP) | OsCl3 | AA | AA | [121] |
| Cubic | 0.7–1.8 | Os(acac)3/OsCl3 | EG | PVP | [122] |
| Synthesis Method | Precursors | Type of NMNPs | NM Particle Size [nm] | References |
|---|---|---|---|---|
| Surface functionalization | CNF, NaOH, H2SO4, H2PtCl6 | Pt | 6 | [178] |
| CNF/TiO2, EG, NaOH, H2PtCl6 | Pt | 3.8 | [179] | |
| Ketjen Black, HNO3, H2PtCl6 | Pt | 2.5 | [180] | |
| N-CNT, HCOOH, H2PtCl6 | Pt (nanorods) | 3–4 × 10 | [181] | |
| Vulcan XC72, HNO3, H2PtCl6 | Pt | 2.2 ± 0.4 | [182] | |
| rGO, HCOOH, H2PtCl6, PdCl2 | PtPd | 4 × 20–200 | [183] | |
| Pt nanowires | ||||
| Pd nanoparticles | 5 | |||
| Ni-N-CNTs, PDDA, PdCl2 | Pd | 2–5 | [184] | |
| MWCNTs, THF, H2PtCl6, SnCl4 | PtSn | ~4 | [185] | |
| CX, HNO3, H2PtCl6, RuCl3 | PtRu | 3.8–4.4 | [186] | |
| BDD, NaOH, SDBS, RuCl3, H2PtCl6 | PtRu | 2–5 | [187] | |
| rGO, KAuCl4, K2PtCl6 | Pt | 2.34 ± 0.52 | [188] | |
| Pt-Au | 2.86 ± 1.30 | |||
| rGO, Vulcan XC-72, H2PtCl6 | Pt | 0.5–2.5 | [189] | |
| G-CNTs, KOH, H2PtCl6 | Pt | 4.3–6.8 | [190] | |
| rGO, EG, HAuCl4, H2PtCl6 | Pt-Au | [191] | ||
| CQD, Vulcan XC-72, H2PtCl6, NaBH4 | Pt | [192] | ||
| N-GQD, Na2CO3, HCl, PdCl2 | Pd | [193] | ||
| N-GN, CoCl2, RuCl3 | RuCo | 6.2 | [194] | |
| C60, H2PtCl6, | Pt | <5 | [195] | |
| C60, NaOH, H2PtCl6 | Pt | 3.93–4.20 | [196] | |
| GO-PyrC60, PdCl2 | Pd | 10 | [197] | |
| CA, Vulcan XC-72R, H2PtCl6, NaBH4 | Pt | 3.24 | [198] | |
| MNC, CCA, NaOH, H2PtCl6, NaBH4 | Pt | 3.1 | [199] | |
| Vulcan XC-72R, NaOH, H2PtCl6, H2IrCl6 | PtIr | 3.6–3.9 | [200] | |
| CNT, H2SO4, IrCl3 | Ir | ~1 | [201] | |
| MWCNT, EG, HNO3, H2SO4, H2PtCl6, RuCl3, ReCl3 | Pt-Ru | 2.79 ± 0.58 | [202] | |
| Pt-Re | 3.53 ± 0.80 | |||
| Pt-Ru-Re | 2.88 ± 0.64 | |||
| Pt-Ru-Re | 2.68 ± 0.55 | |||
| Pt-Ru-Re | 3.19 ± 0.54 | |||
| Electrochemical deposition | CNT, EG, H2PtCl6, RuCl3 | Pt-Ru | 3.1–5.6 | [203] |
| GO, H2PtCl6, KH2PO4 | Pt | 10 | [204] | |
| GO, CNF, H2PtCl6, H2SO4 | Pt | 350–500 | [205] | |
| CP, H2PtCl6, RuCl3, HCl, KOH | Pt-Ru | 52.9 ± 9.2 | [206] | |
| GR, ZnO, K2PtCl6, H2SO4 | Pt | 250 | [207] | |
| BDD, NaBH4, NaOH, H2PtCl6 | Pt | 15 ± 5 | [208] | |
| BDD, Ni(NO3)2, HCl, PdCl2 | Pd | ~13 | [209] | |
| Vulcan XC-72, H2PtCl6, H2SO4 | Pt | 1–4 | [210] | |
| Electroless deposition | CNO, H2PtCl6 | Pt | 20 | [211] |
| CNT, CoCl2, H2PtCl6 | Pt | 30–40 | [212] | |
| CNT, SDS, EG, Na2PdCl4 | Pd | 2–5 | [213] | |
| MWCNT, HAuCl4 | Au | 10 | [214] | |
| PC, C6H5K3O7, RhCl3, RuCl3, IrCl3 | Ir | 0.96 ± 0.13 | [215] | |
| Rh | 1.11 ± 0.31 | |||
| Ru | 1.37 ± 0.39 | |||
| NiO/Ni/CNTs, K2PtCl6 | Pt | ~2 | [216] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczmarska, A.; Adamek, M.; El Houbbadi, S.; Kowalczyk, P.; Laskowska, M. Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals 2022, 12, 584. https://doi.org/10.3390/cryst12050584
Karczmarska A, Adamek M, El Houbbadi S, Kowalczyk P, Laskowska M. Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals. 2022; 12(5):584. https://doi.org/10.3390/cryst12050584
Chicago/Turabian StyleKarczmarska, Agnieszka, Michał Adamek, Sara El Houbbadi, Paweł Kowalczyk, and Magdalena Laskowska. 2022. "Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review" Crystals 12, no. 5: 584. https://doi.org/10.3390/cryst12050584
APA StyleKarczmarska, A., Adamek, M., El Houbbadi, S., Kowalczyk, P., & Laskowska, M. (2022). Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals, 12(5), 584. https://doi.org/10.3390/cryst12050584