Structural Stability, Thermodynamic and Elastic Properties of Cubic Zr0.5Nb0.5 Alloy under High Pressure and High Temperature
Abstract
:1. Introduction
2. Theoretical Calculations’ Details
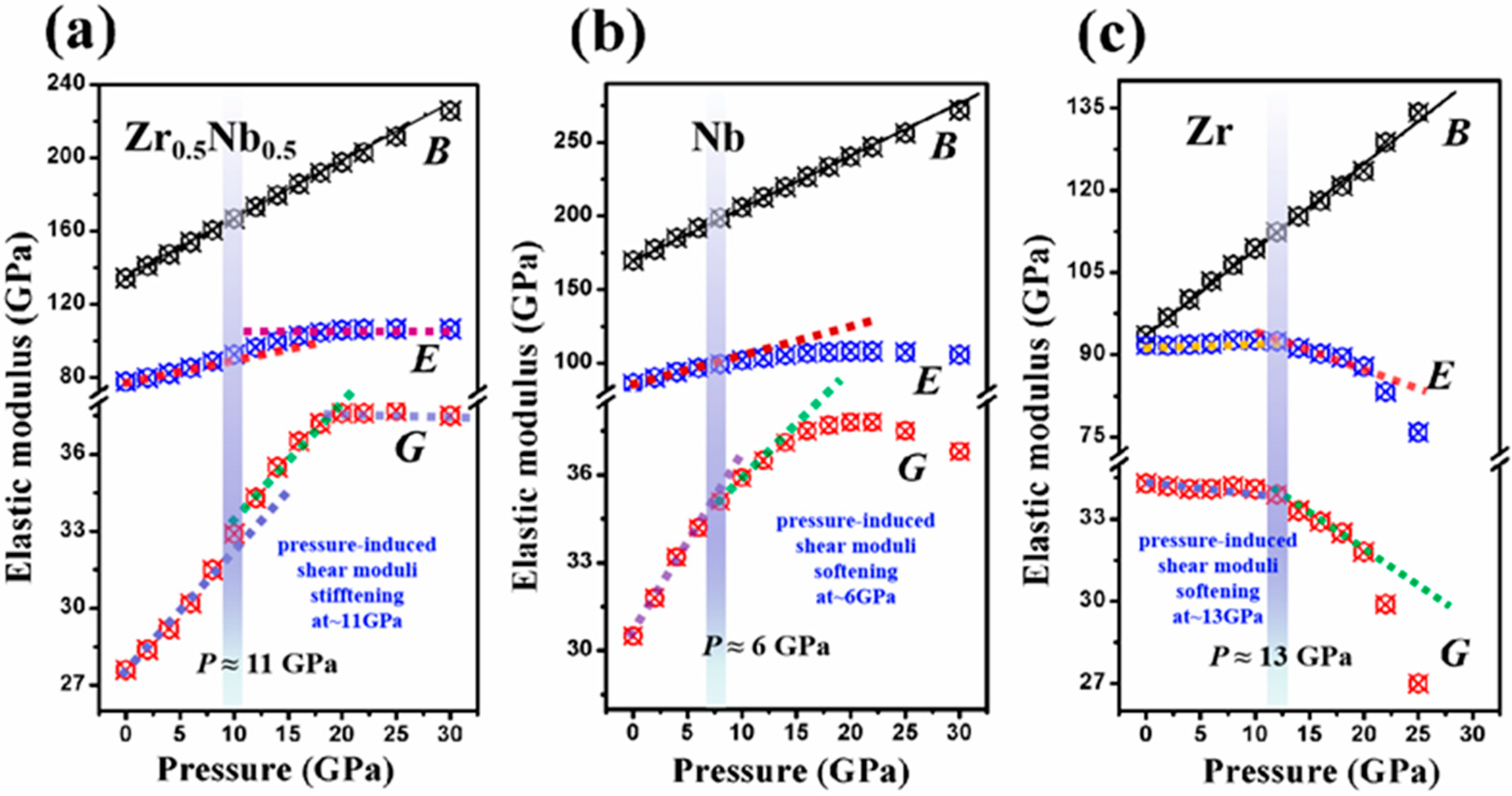
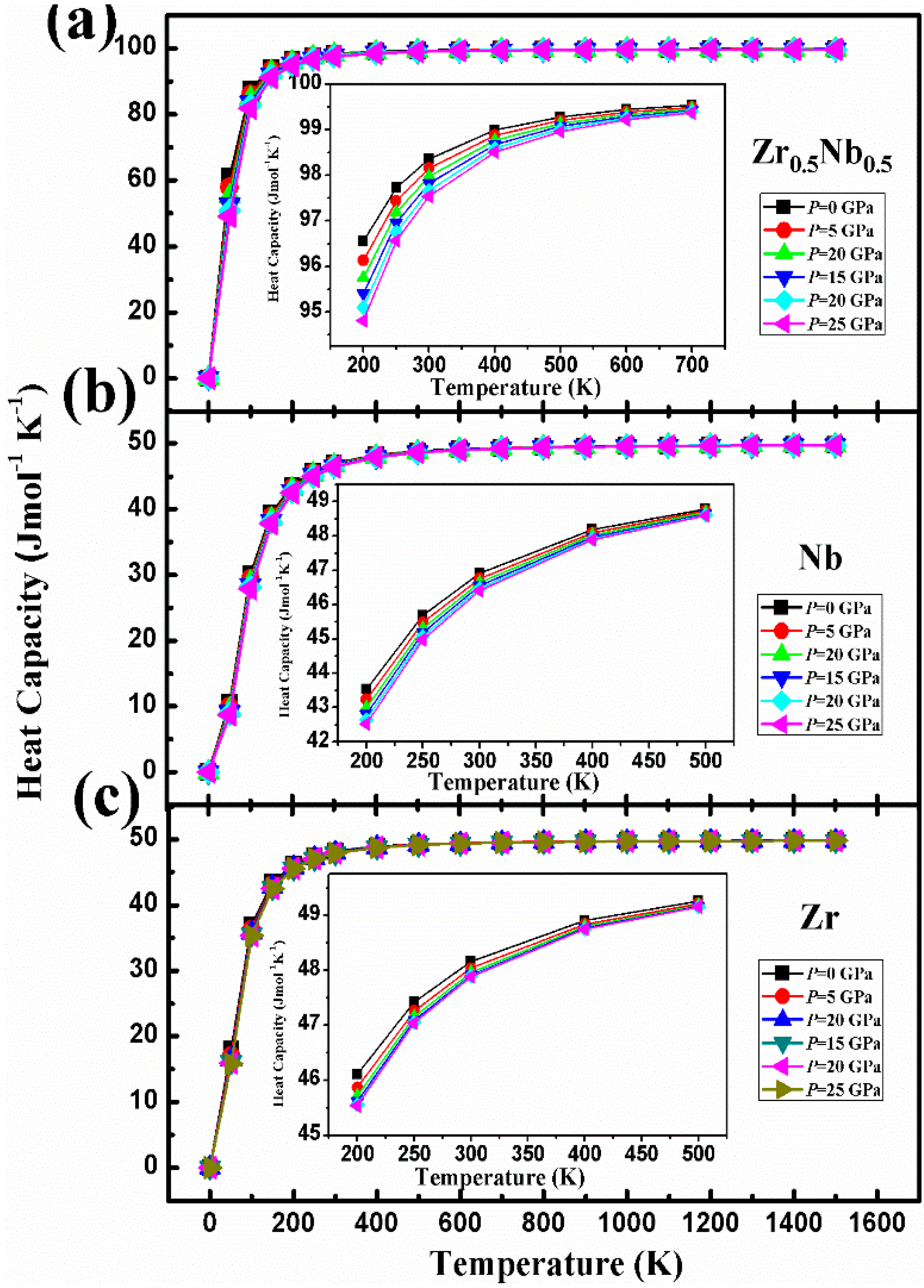
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kondo, R.; Nomura, N.; Suyalatu; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and mechanical properties of as-cast Zr-Nb alloys. Acta Biomater. 2011, 7, 4278. [Google Scholar] [CrossRef] [PubMed]
- Saldana, L.; Mendez-Vilas, A.; Jiang, L.; Multigner, M.; Gonzalez-Carrasco, J.L.; Perez-Prado, M.T.; Gonzalez-Martin, M.L.; Munuera, L.; Vilaboa, N. In vitro biocompatibility of an ultrafine grained zirconium. Biomaterials 2007, 8, 4343. [Google Scholar] [CrossRef]
- Zhilyaev, A.P.; Sabirov, I.; Gonzalez-Doncel, G.; Molina-Aldareguia, J.; Srinivasarao, B.; Perez-Prado, M.T. Effect of Nb additions on the microstructure, thermal stability and mechanical behavior of high pressure Zr phases under ambient conditions. Mater. Sci. Eng. A 2011, 528, 3496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Wang, B.; Qiu, K.; Lin, W.; Li, L.; Wang, Y.; Nie, F.; Zheng, Y. Microstructure, corrosion behavior and cytotoxicity of Zr-Nb alloys for biomedical application. Mater. Sci. Eng. C 2012, 32, 851. [Google Scholar] [CrossRef]
- Tewari, R.; Srivastava, D.; Dey, G.K.; Chakravarty, J.K.; Banerjee, S. Microstructural evolution in zirconium-based alloys. J. Nucl. Mater. 2008, 383, 153. [Google Scholar] [CrossRef]
- Anzellini, S.; Burakovsky, L.; Turnbull, R.; Bandiello, E.; Errandonea, D. P-V-T equation of state of iridium up to 80 GPa and 3100 K. Crystals 2021, 11, 452. [Google Scholar] [CrossRef]
- Baty, S.R.; Burakovsky, L.; Errandonea, D. Ab Initio phase diagram of copper. Crystals 2021, 11, 537. [Google Scholar] [CrossRef]
- Parisiades, P. A review of the melting curves of transition metals at high pressures using static compression techniques. Crystals 2021, 11, 416. [Google Scholar] [CrossRef]
- Kharchenko, V.O.; Kharchenko, D.O. Ab-initio calculations for the structural properties of Zr-Nb alloys. Condens. Matter Phys. 2013, 16, 13801. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, J.; Pantea, C.; Qian, J.; Daemen, L.L.; Rigg, P.A.; Hixson, R.S.; Gray, G.T.; Yang, Y.; Wang, L.; et al. Thermal equations of state of the α, β, and ω phases of zirconium. Phys. Rev. B 2005, 71, 184119. [Google Scholar] [CrossRef]
- Moorehead, M.; Yu, Z.; Borrel, L.; Hu, J.; Cai, Z.; Couet, A. Comprehensive investigation of the role of Nb on the oxidation kinetics of Zr-Nb alloys. Corros. Sci. 2019, 155, 173–181. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, M.K. Nuclear applications: Zirconium alloys. In Encyclopedia of Materials: Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2016; pp. 350–363. [Google Scholar]
- Benites, G.M.; Guillermet, A.F.; Cuello, G.J.; Campo, J. Structural properties of metastable phases in Zr-Nb alloys I. Neutron diffraction study and analysis of lattice parameters. J. Alloys Compd. 2000, 299, 183. [Google Scholar] [CrossRef]
- Grad, G.B.; Pieres, J.J.; Guillermet, A.F.; Cuello, G.J.; Granada, J.R.; Mayer, R.E. Systematics of lattice parameters and bonding distances of the omega phase in Zr-Nb alloys. Phys. B 1995, 213–214, 433. [Google Scholar] [CrossRef]
- Landa, A.; Söderlind, P. First-principles phase stability at high temperatures and pressure in Nb90Zr10 alloy. J. Alloys Compd. 2017, 690, 647. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, L.; Wang, M.; Shi, X.; Huang, G.; Zhang, L. Computational modeling of elastic constants as a function of temperature and composition in Zr-Nb alloys. Calphad 2015, 48, 89. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, X.; Northwood, D.O. Investigation of Plasma Electrolytic Oxidation (PEO) coatings on a Zr-2.5Nb alloy using high temperature/pressure autoclave and tribological tests. Surf. Coat. Technol. 2010, 205, 1774. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Huang, Y. The structure, mechanical, electronic and thermodynamic properties of bcc Zr-Nb alloy: A first principles study. J. Alloys Compd. 2021, 862, 158029. [Google Scholar] [CrossRef]
- Struzhkin, V.V.; Timofeev, Y.A.; Hemley, R.J.; Mao, H.-K. Superconducting Tc and electron-phonon coupling in Nb to 132 GPa: Magnetic susceptibility at megabar pressures. Phys. Rev. Lett. 1997, 79, 4262. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Qi, X.; Wang, X.; Chen, T.; Li, X.; Welch, D.; Li, B. High-pressure behavior and thermoelastic properties of niobium studied by in situ x-ray diffraction. J. Appl. Phys. 2014, 116, 013516. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Chen, H.; Welch, D.; Zhao, Y.; Li, B. Thermoelasticity and anomalies in the pressure dependence of phonon velocities in niobium. Appl. Phys. Lett. 2018, 112, 011901. [Google Scholar] [CrossRef]
- Liu, W.; Li, B.; Wang, L.; Zhang, J.; Zhao, Y. Simultaneous ultrasonic and synchrotron x-ray studies on pressure induced α-ω phase transition in zirconium. J. Appl. Phys. 2008, 104, 076102. [Google Scholar] [CrossRef]
- Liu, W.; Li, B.; Wang, L.; Zhang, J.; Zhao, Y. Elasticity of ω-phase zirconium. Phys. Rev. B 2007, 76, 144107. [Google Scholar] [CrossRef]
- Yang, H.L.; Kano, S.; McGrady, J.; Chen, D.Y.; Murakami, K.; Abe, H. Microstructural evolution and hardening effect in low-dose self-ion irradiated Zr–Nb alloys. J. Nucl. Mater. 2020, 542, 152523. [Google Scholar] [CrossRef]
- Yang, H.; Kano, S.; Shen, J.; McGrady, J.; Zhao, Z.; Duan, Z.; Abe, H. On the strength-hardness relationships in a Zr-Nb alloy plate with bimodal basal texture microstructure. Mater. Sci. Eng. A 2018, 732, 333. [Google Scholar] [CrossRef]
- Greeff, C.W. Phase changes and the equation of state of Zr. Model. Simul. Mater. Sci. Eng. 2005, 13, 1015. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Mater. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Liu, K.; Dong, B.; Zhou, X.; Wang, S.; Zhao, Y.; Chang, J. Structural, elastic, and thermodynamic properties of hexagonal molybdenum nitrides under high pressure from first principles. J. Alloys Compd. 2015, 632, 830. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Zheng, Z.; Peng, S. The elastic and thermodynamic properties of ZrMo2 from first principles calculations. J. Alloys Compd. 2014, 615, 975. [Google Scholar] [CrossRef]
- Hill, R. The elastic behavior of a crystalline aggregate. Proc. Phys. Soc. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Blanco, M.A.; Francisco, E.; Luaña, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, L.; Chen, X.-R.; Li, Y.; He, H. Phase transition and elastic constants of zirconium from first-principles calculations. J. Phys. Condens. Matter. 2008, 20, 235230. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Z.; Liu, H.; Li, W.; Zhang, P. First-principles calculations of phase transition, elastic modulus, and superconductivity under pressure for zirconium. J. Appl. Phys. 2011, 109, 174113. [Google Scholar] [CrossRef] [Green Version]
- Ikehata, H.; Nagasako, N.; Furuta, T.; Fukumoto, A.; Miwa, K.; Saito, T. First-principles calculations for development of low elastic modulus Ti alloys. Phys. Rev. B 2004, 70, 174113. [Google Scholar] [CrossRef]
- Errandonea, D.; Burakovsky, L.; Preston, D.L.; MacLeod, S.G.; Santamaría-Perez, D.; Chen, S.; Cynn, H.; Simak, S.I.; McMahon, M.I.; Proctor, J.E.; et al. Experimental and theoretical confirmation of an orthorhombic phase transition in niobium at high pressure and temperature. Commun. Mater. 2020, 1, 60. [Google Scholar] [CrossRef]
- Wallace, D.C. Thermoelasticity of Stressed Materials and Comparison of Various Elastic Constants. Phys. Rev. 1967, 162, 776–789. [Google Scholar] [CrossRef]
- Grimvall, G.; Magyari-Köpe, B.; Ozoliņš, V.; Persson, K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012, 84, 945–986. [Google Scholar] [CrossRef] [Green Version]
- Olijnyk, H.; Nakano, S.; Jephcoat, A.P.; Takemura, K. Unusual pressure response of the E2g mode and elastic shear modulus C44 in hcp scandium. J. Phys. Condens. Matter 2006, 18, 10971–10976. [Google Scholar] [CrossRef]
- Olijnyk, H.; Jephcoat, A.P. Optical zone-centre phonon modes and macroscopic elasticity in hcp metals. Solid State Commun. 2000, 115, 335. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 2009, 45, 823. [Google Scholar] [CrossRef]
- Steinle-Neumann, G.; Stixrude, L.; Cohen, R.E. First-principles elastic constants for the hcp transition metals Fe, Co, and Re at high pressure. Phys. Rev. B 1999, 60, 791. [Google Scholar] [CrossRef] [Green Version]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909. [Google Scholar] [CrossRef]
- Schreiber, E.; Anderson, O.L. Elastic constants and their measurement. J. Appl. Mech. 1973, 42, 747. [Google Scholar] [CrossRef] [Green Version]






| Material | a (Å) | c (Å) | V0 (Å3) | B0 (GPa) | B0′ | Ref. |
|---|---|---|---|---|---|---|
| Nb0.5Zr0.5 (cubic) | 3.419 | 39.957 | 134.2 | 3.3 | This work | |
| 3.432 | 40.424 | Zhao et al. a (Theor.) | ||||
| 3.447 | 40.957 | Ikehata et al. b (Theor.) | ||||
| Nb (cubic) | 3.308 | 36.306 | 169.8 | 3.7 | This work | |
| 3.307 | 36.184 | 174.9(3.2) | 3.97 | Zou et al. c,d (Exp.) | ||
| 3.308 | 36.199 | 170.5 | 3.85 | Zhao a; Daniel e et al. (Theor.) | ||
| hcp-Zr (α phase) | 3.231 | 5.168 | 46.953 | 93.6 | 1.6 | This work |
| 3.233 | 5.146 | 46.57 | 95.3 | 3.0(10) | Zhao et al. f (Exp.); Liu et al. g (Exp.) |
| Material | B0 GPa) | G0 (GPa) | B0′ | G0′ | Ref. |
|---|---|---|---|---|---|
| Nb0.5Zr0.5 (cubic) | 134.2 | 27.6 | 3.3 | 0.48 | This work |
| Nb (cubic) | 169.8 | 30.5 | 3.7 | 0.63 | This work |
| 174.9 | 37.1 | 3.97 | 0.83 | Zou et al. a (Exp.) | |
| hcp-Zr (α phase) | 93.6 | 34.3 | 1.6 | −0.05 | This work |
| 95.3 | 36.3 | 3.0 (10) | −0.1 (2) | Liu et al. b (Exp.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, S.; Zhu, H.; Tao, P.; Huang, L.; Li, M.; Zhang, W.; Li, Y.; Zhou, C.; Zou, Y. Structural Stability, Thermodynamic and Elastic Properties of Cubic Zr0.5Nb0.5 Alloy under High Pressure and High Temperature. Crystals 2022, 12, 631. https://doi.org/10.3390/cryst12050631
Yang X, Zhang S, Zhu H, Tao P, Huang L, Li M, Zhang W, Li Y, Zhou C, Zou Y. Structural Stability, Thermodynamic and Elastic Properties of Cubic Zr0.5Nb0.5 Alloy under High Pressure and High Temperature. Crystals. 2022; 12(5):631. https://doi.org/10.3390/cryst12050631
Chicago/Turabian StyleYang, Xiuxiu, Shihao Zhang, Hang Zhu, Peidong Tao, Lili Huang, Mu Li, Wei Zhang, Ying Li, Cangtao Zhou, and Yongtao Zou. 2022. "Structural Stability, Thermodynamic and Elastic Properties of Cubic Zr0.5Nb0.5 Alloy under High Pressure and High Temperature" Crystals 12, no. 5: 631. https://doi.org/10.3390/cryst12050631






