A First-Principles Study on the Structural and Carrier Transport Properties of Inorganic Perovskite CsPbI3 under Pressure
Abstract
:1. Introduction
2. Methodology
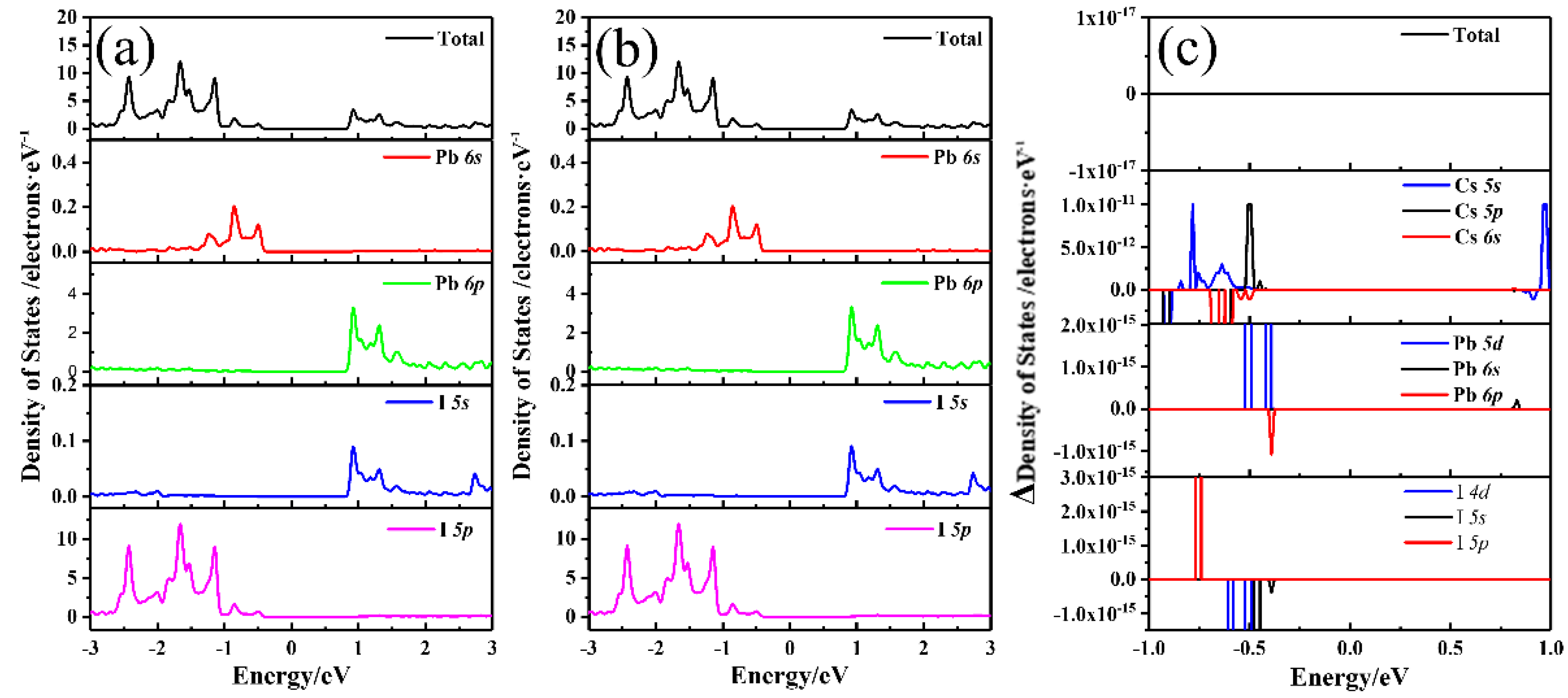
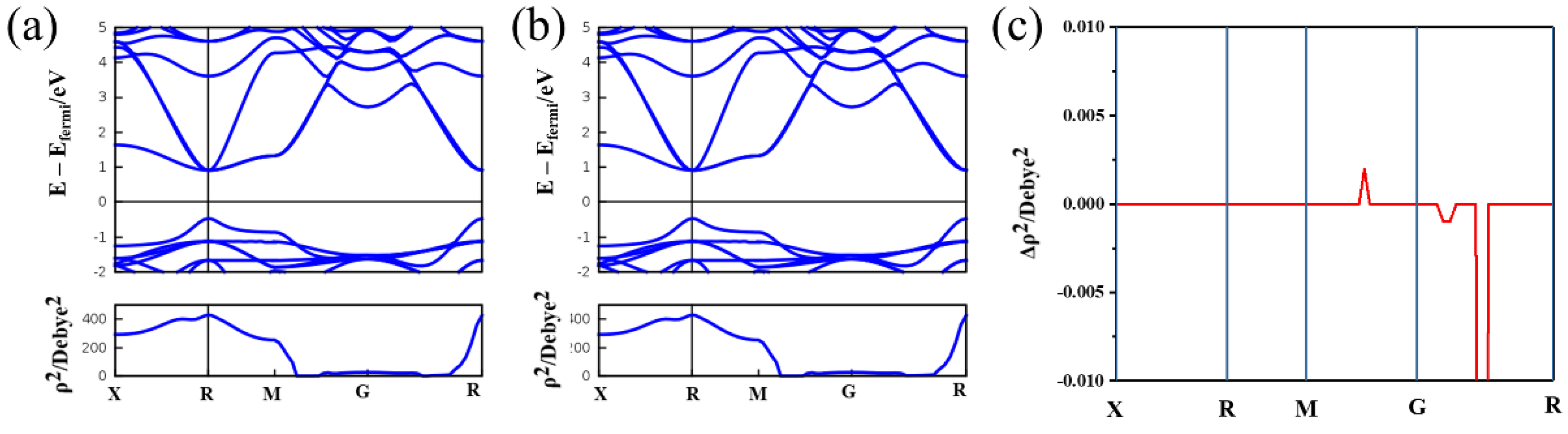
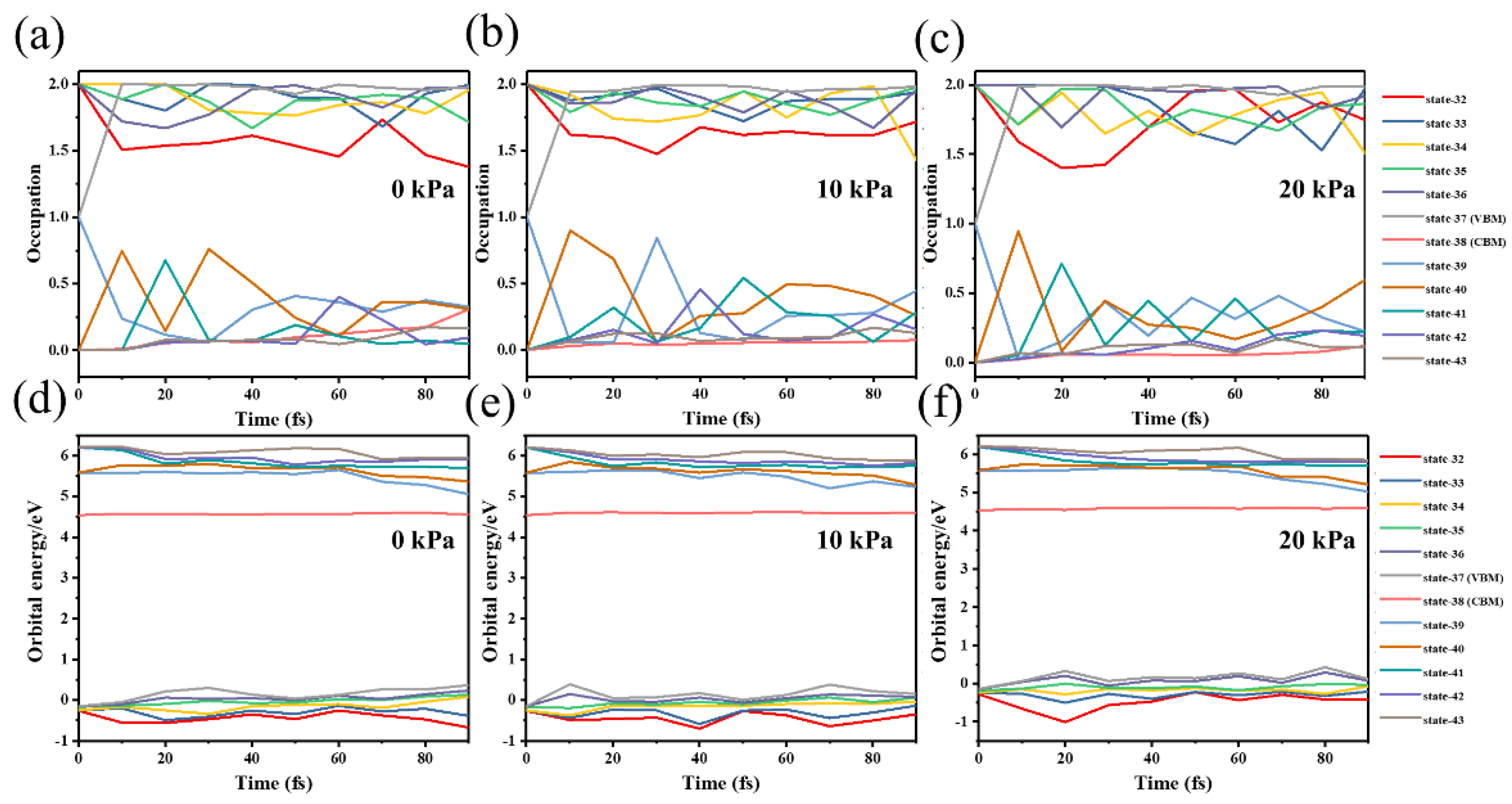
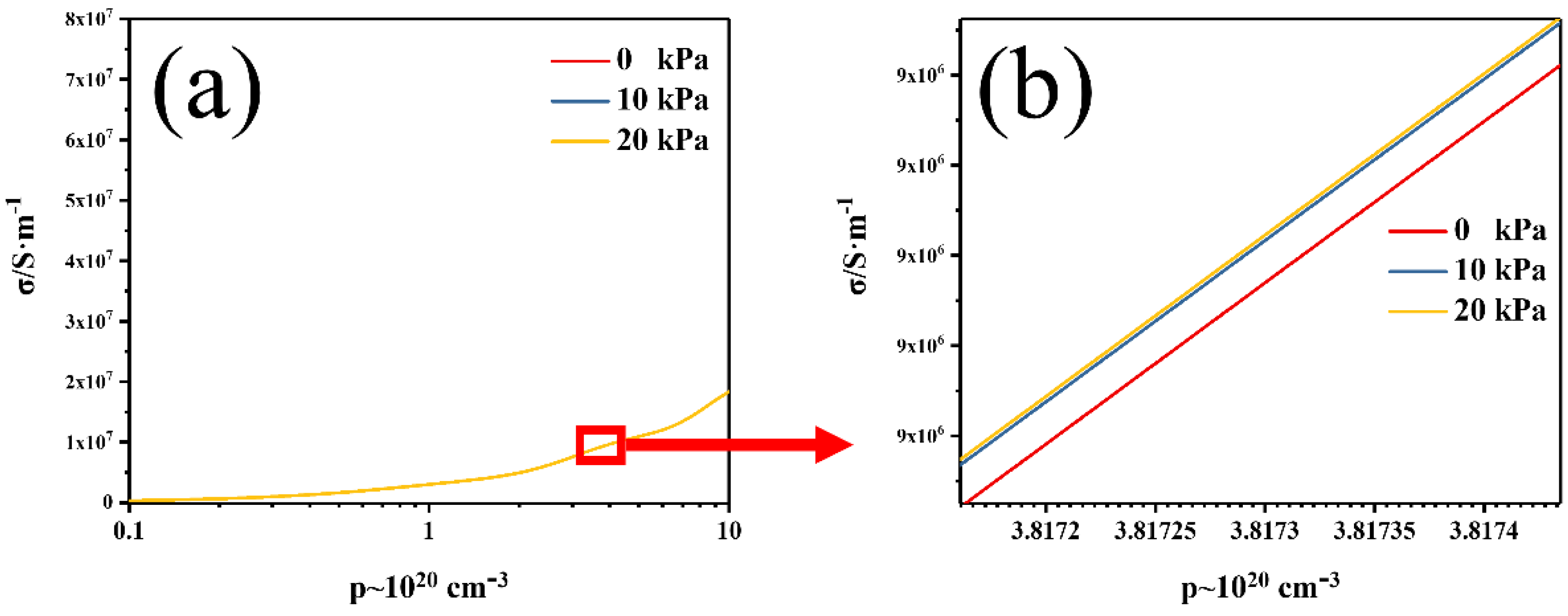
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Zhao, L.; Huang, S.; Yan, X.; Qiu, Q.; Zhao, Y.; Zhu, L.; Qiang, Y.; Li, H.; Li, G. A p-p+ homojunction-enhanced hole transfer in inverted planar perovskite solar cells. ChemSusChem 2021, 14, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Zhou, Q.; Bai, Z.; Lu, W.G.; Wang, Y.; Zou, B.; Zhong, H. In situ fabrication of halide terovskite nanocrystal-embedded polymer composite films with enhanced photoluminescence for display backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L.; et al. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tang, G.; Huang, H.; Wu, X.; Zhou, P.M.; Zou, L.; Xie, L.; Deng, J.M.; Wang, X.; Zhong, H.Z.; et al. Enhanced piezo-response in copper halide perovskites based PVDF composite films. Sci. Bull. 2018, 63, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.L.; Li, X.; Traoré, B.; Zhang, Y.; Fang, J.; Han, Y.; Even, J.; Katan, C.; Zhao, K.; Liu, S.; et al. m-Phenylenediammonium as a new spacer for Dion-Jacobson two-dimensional perovskites. J. Am. Chem. Soc. 2021, 143, 12063–12073. [Google Scholar] [CrossRef]
- Hou, Y.; Du, X.; Scheiner, S.; McMeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I.; et al. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 2017, 358, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient light-emitting diodes based on in situ fabricated FAPbBr3 nanocrystals: The enhancing role of the ligand-assisted reprecipitation process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef]
- Fang, Y.; Dong, Q.; Shao, Y.; Yuan, Y.; Huang, J.S. Highly narrowband perovskite single-crystal photodetectors enabled by surface-charge recombination. Nat. Photonics 2015, 9, 679–686. [Google Scholar] [CrossRef]
- Wang, L.; Meng, L.; Chen, L.; Huang, S.; Wu, X.; Dai, G.; Deng, L.; Han, J.; Zou, B.; Zhang, C.; et al. Ultralow-threshold and color-tunable continuous-wave lasing at room-temperature from in situ fabricated perovskite quantum dots. J. Phys. Chem. Lett. 2019, 10, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Draxl, C. Hybrid organic-inorganic perovskites as promising substrates for Pt single-atom catalysts. Phys. Rev. Lett. 2018, 122, 46101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Li, G.; Wang, S.; Liu, S.; Sun, W.; Huang, C.; Wang, Y.; Song, Q.; Xiao, S. Dark-field sensors based on organometallic halide perovskite microlasers. Adv. Mater. 2018, 30, 1801481. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Lu, S.; Wang, L.; Feng, X.; Yang, D.; Wang, K.; Xiao, G.; Zhang, L.; Redfern, S.A.T.; et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals. Nat. Commun. 2018, 9, 4506. [Google Scholar] [CrossRef]
- Xia, M.; Yuan, J.H.; Luo, J.; Pan, W.; Wu, H.; Chen, Q.; Xue, K.H.; Miao, X.; Niu, G.; Tang, J. Two-dimensional perovskites as sensitive strain sensors. J. Mater. Chem. C 2020, 8, 3814–3820. [Google Scholar] [CrossRef]
- Dai, G.; Wang, L.; Cheng, S.; Chen, Y.; Liu, X.; Deng, L.; Zhong, H. Perovskite quantum dots based optical Fabry-Pérot pressure sensor. ACS Photonics 2020, 7, 2390–2394. [Google Scholar] [CrossRef]
- Almassri, A.M.; Wan Hasan, W.Z.; Ahmad, S.A.; Ishak, A.J.; Ghazali, A.M.; Talib, D.N.; Wada, C. Pressure sensor: State of the art, design, and application for robotic hand. J. Sens. 2015, 12, 846487. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Khan, J.; Niu, G.; Tang, J. Inorganic CsPbI3 perovskite-based solar cells: A choice for a tandem device. Solar RRL 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Huang, S.; Shan, H.; Xuan, W.; Xu, W.; Hu, D.; Zhu, L.; Huang, C.; Sui, W.; Xiao, C.; Zhao, Y.; et al. High-performance humidity sensor based on CsPdBr3 nanocrystals for noncontact sensing of hydromechanical characteristics of unsaturated soil. Phys. Status Solidi RRL 2022, 2200017. [Google Scholar] [CrossRef]
- Ke, F.; Wang, C.; Jia, C.; Wolf, N.R.; Yan, J.; Niu, S.; Devereaus, T.P.; Karunadasa, H.I.; Mao, W.L.; Lin, Y. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt. Nat. Commun. 2021, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, X.; Huang, Y.; Wang, X.; Li, F.; Wang, Y.; Tian, F.; Liu, B.; Shen, Z.X.; Cui, T. New metallic ordered phase of perovskite CsPbI3 under pressure. Adv. Sci. 2019, 6, 1900399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satpathy, S.; Christensen, N.E.; Jepsen, O. Metallization of CsI under pressure: Theoretical results. Phys. Rev. B 1985, 32, 6793. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Maines, R.G.; Chattopadhyay, T. Pressure-induced structural transitions in PbI2: A high-pressure Raman and optical absorption study. Pramana 1986, 27, 449–457. [Google Scholar] [CrossRef]
- Jia, W.; Cao, Z.; Wang, L.; Fu, J.; Chi, X.; Gao, W.; Wang, L.W. The analysis of a plane wave pseudopotential density functional theory code on a GPU machine. Comput. Phys. Commun. 2013, 184, 9–18. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xi, L.; Zhang, W.; Chen, L.D.; Yang, J. Electrical transport properties of filled CoSb3 skutterudites: A theoretical study. J. Electron. Mater. 2009, 38, 1397–1401. [Google Scholar] [CrossRef]
- Xi, L.; Pan, S.; Li, X.; Xu, Y.; Ni, J.; Sun, X.; Yang, J.; Luo, J.; Xi, J.; Zhu, W.; et al. Discovery of high-performance thermoelectric chalcogenides through reliable high-throughput material screening. J. Am. Chem. Soc. 2018, 140, 10785–10793. [Google Scholar] [CrossRef]
- Jia, W.; Fu, J.; Cao, Z.; Wang, L.; Chi, X.; Gao, W.; Wang, L.W. Fast plane wave density functional theory molecular dynamics calculations on multi-GPU machines. J. Comput. Phys. 2013, 251, 102–115. [Google Scholar] [CrossRef]
- Wei, L.; Chen, J.F.; Qinyu, H.; Teng, W. Electronic and elastic properties of MoS2. Phys. B Condens. Matter 2010, 405, 2498–2502. [Google Scholar] [CrossRef]
- Cherniukh, I.; Rainò, G.; Stöferle, T.; Burian, M.; Travesset, A.; Naumenko, D.; Amenitsch, H.; Erni, R.; Mahrt, R.F.; Bodnarchuk, M.I. Perovskite-type superlattices from lead halide perovskite nanocubes. Nature 2021, 593, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Gandini, M.; Villa, I.; Beretta, M.; Gotti, C.; Imran, M.; Carulli, F.; Fantuzzi, E.; Sassi, M.; Zaffalon, M.; Brofferio, C.; et al. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 2020, 15, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.B.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef]
- Li, J.; Jing, Q.; Xiao, S.; Gao, Y.; Wang, Y.; Zhang, W.; Sun, X.W.; Wang, K.; He, T. Spectral dynamics and multiphoton Aabsorption properties of all-inorganic perovskite nanorods. J. Phys. Chem. Lett. 2020, 11, 4817–4825. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.W. The nature of electron mobility in hybrid perovskite CH3NH3PbI3. Nano Lett. 2017, 17, 3646–3654. [Google Scholar] [CrossRef]
- Zhang, H.; Debroye, E.; Steele, J.A.; Roeffaers, M.B.J.; Hofkens, J.; Wang, H.I.; Bonn, M. Highly mobile large polarons in black phase CsPbI3. ACS. Energy Lett. 2021, 6, 568–573. [Google Scholar] [CrossRef]
- Mastrikov, Y.; Chuklina, N.G.; Sokolov, M.; Popov, A.; Gryaznov, D.; Kotomin, E.A. Small radius electron and hole polarons in PbX2 (X = F, Cl, Br) crystals: A computational study. J. Mater. Chem. C 2021, 9, 16536–16544. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jiao, M.; Wang, X.; He, X. A First-Principles Study on the Structural and Carrier Transport Properties of Inorganic Perovskite CsPbI3 under Pressure. Crystals 2022, 12, 648. https://doi.org/10.3390/cryst12050648
Huang S, Jiao M, Wang X, He X. A First-Principles Study on the Structural and Carrier Transport Properties of Inorganic Perovskite CsPbI3 under Pressure. Crystals. 2022; 12(5):648. https://doi.org/10.3390/cryst12050648
Chicago/Turabian StyleHuang, Sheng, Mingzhi Jiao, Xi Wang, and Xinjian He. 2022. "A First-Principles Study on the Structural and Carrier Transport Properties of Inorganic Perovskite CsPbI3 under Pressure" Crystals 12, no. 5: 648. https://doi.org/10.3390/cryst12050648
APA StyleHuang, S., Jiao, M., Wang, X., & He, X. (2022). A First-Principles Study on the Structural and Carrier Transport Properties of Inorganic Perovskite CsPbI3 under Pressure. Crystals, 12(5), 648. https://doi.org/10.3390/cryst12050648






