Reaction Sintering of MgAlON at 1500 °C from Al2O3, MgO and AlN and Its Wettability by AlSi7Mg
Abstract
:1. Introduction
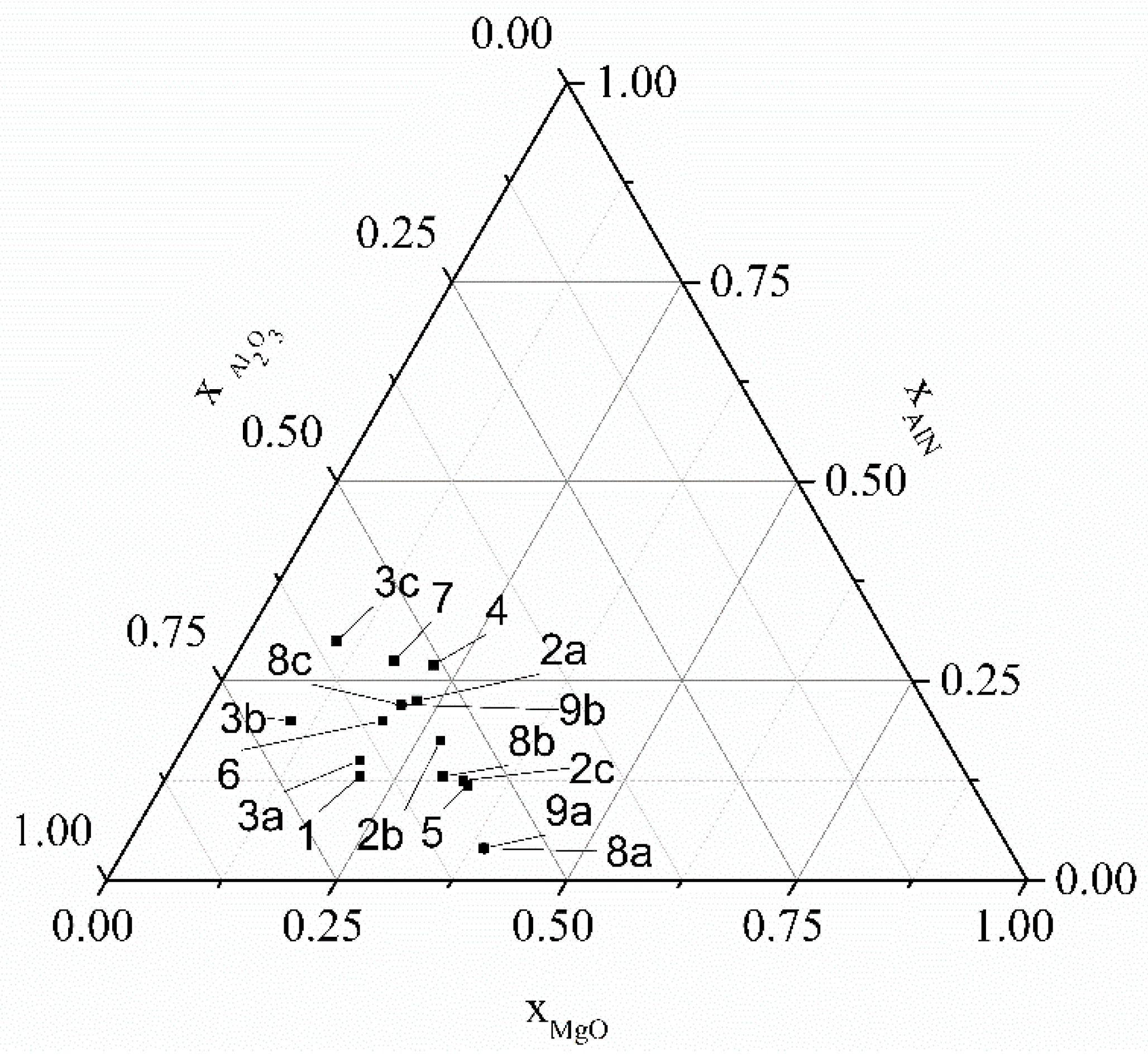
| Number (cf. Figure 1) | Source | Synthesis Temperature, Duration | |||
|---|---|---|---|---|---|
| 1 | [18] | 0.13 | 0.21 | 0.66 | 1550 °C, 6 h; 1675 °C, 6 h |
| 2a | [13] | 0.225 | 0.225 | 0.55 | 1500–1650 °C, 5 h |
| 2b | [13] | 0.175 | 0.275 | 0.55 | 1500–1650 °C, 5 h |
| 2c | [13] | 0.125 | 0.325 | 0.55 | 1500–1650 °C, 5 h |
| 3a | [26] | 0.15 | 0.2 | 0.65 | 1550–1800 °C, 3 h |
| 3b | [26] | 0.2 | 0.1 | 0.7 | 1550–1800 °C, 3 h |
| 3c | [26] | 0.3 | 0.1 | 0.6 | 1550–1800 °C, 3 h |
| 4 | [25] | 0.27 | 0.22 | 0.51 | 1300–1600 °C, 3 h |
| 5 | [16] | 0.119 | 0.333 | 0.548 | 1550–1650 °C, 5 h |
| 6 | [27] | 0.2 | 0.2 | 0.6 | Hot pressing, SPS, 1600 °C, 1 h |
| 7 | [19] | 0.275 | 0.175 | 0.55 | 1450–1700 °C, 6 h |
| 8a | [3] | 0.04 | 0.39 | 0.57 | 1450 °C, 9 h |
| 8b | [3] | 0.13 | 0.3 | 0.57 | 1450 °C, 9 h |
| 8c | [3] | 0.22 | 0.21 | 0.57 | 1450 °C, 9 h |
| 9a | [23] | 0.04 | 0.39 | 0.57 | 1400 °C, 3h |
| 9b | [23] | 0.22 | 0.21 | 0.57 | 1400 °C, 3h |
2. Theoretical and Thermodynamic Considerations
3. Methods and Experiments
3.1. Sample Preparation
3.2. Reaction Sintering
3.3. X-ray Diffraction and Chemical Analysis
| Phase | Space Group | ICSD Nr. | Original Reference |
|---|---|---|---|
| Al2O3 | 160604 | [58] | |
| MgO | 52026 | [59] | |
| AlN | 34475 | [60] | |
| MgAl2O4 | 31373 | [61] |
3.4. Raman Spectroscopic Measurements
3.5. Sessile Drop Tests on Selected Sintered Samples
4. Experimental Results and Discussion
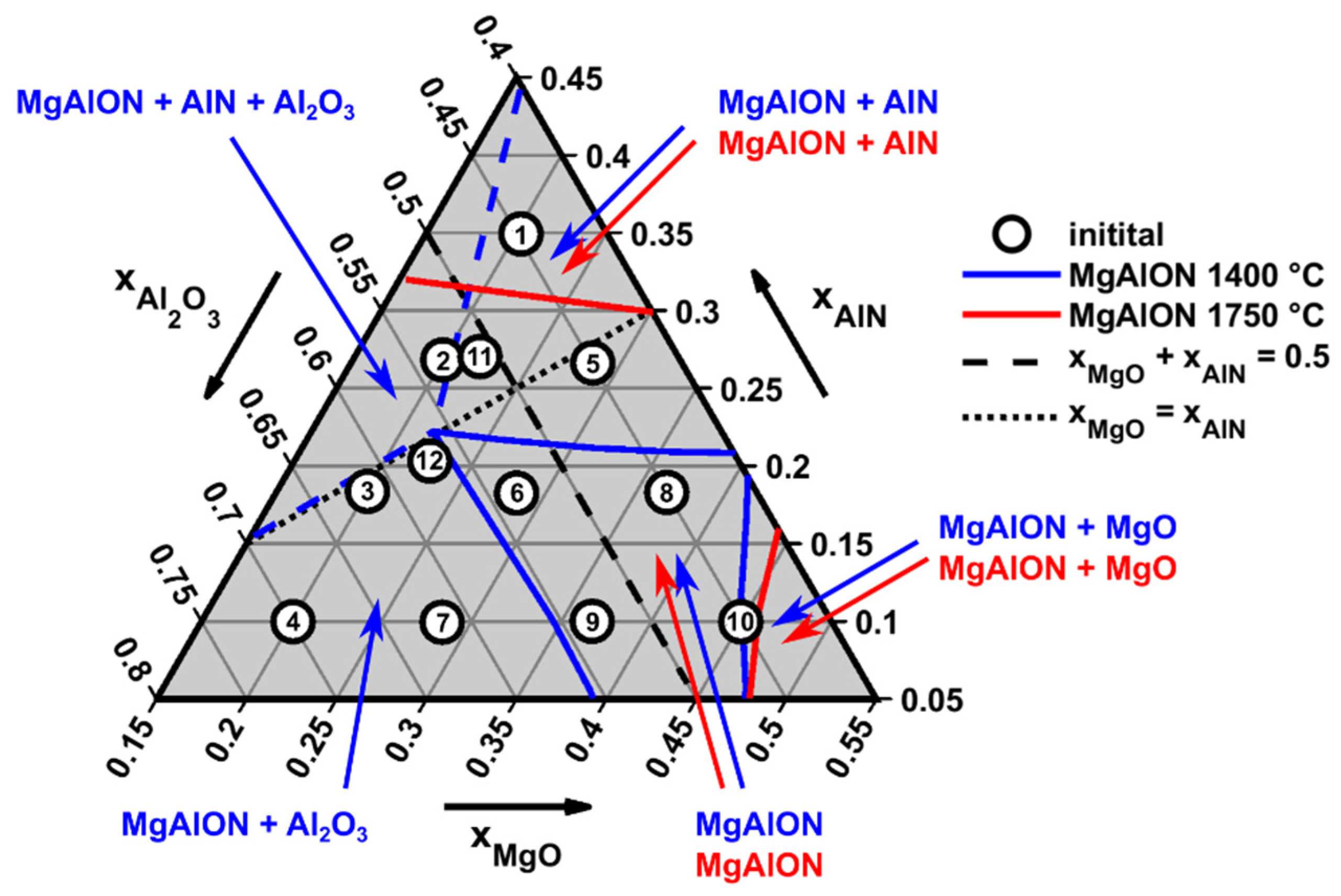
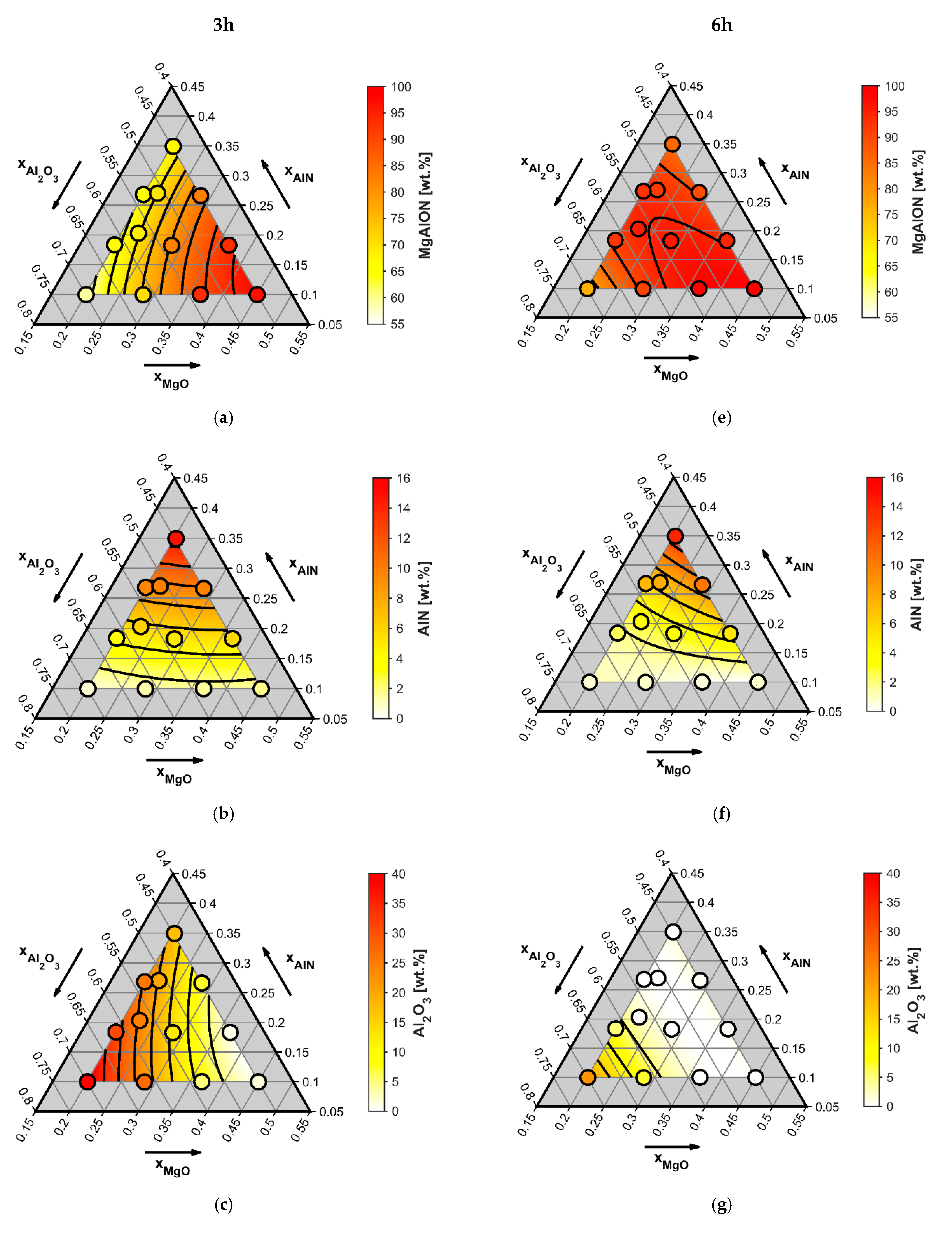
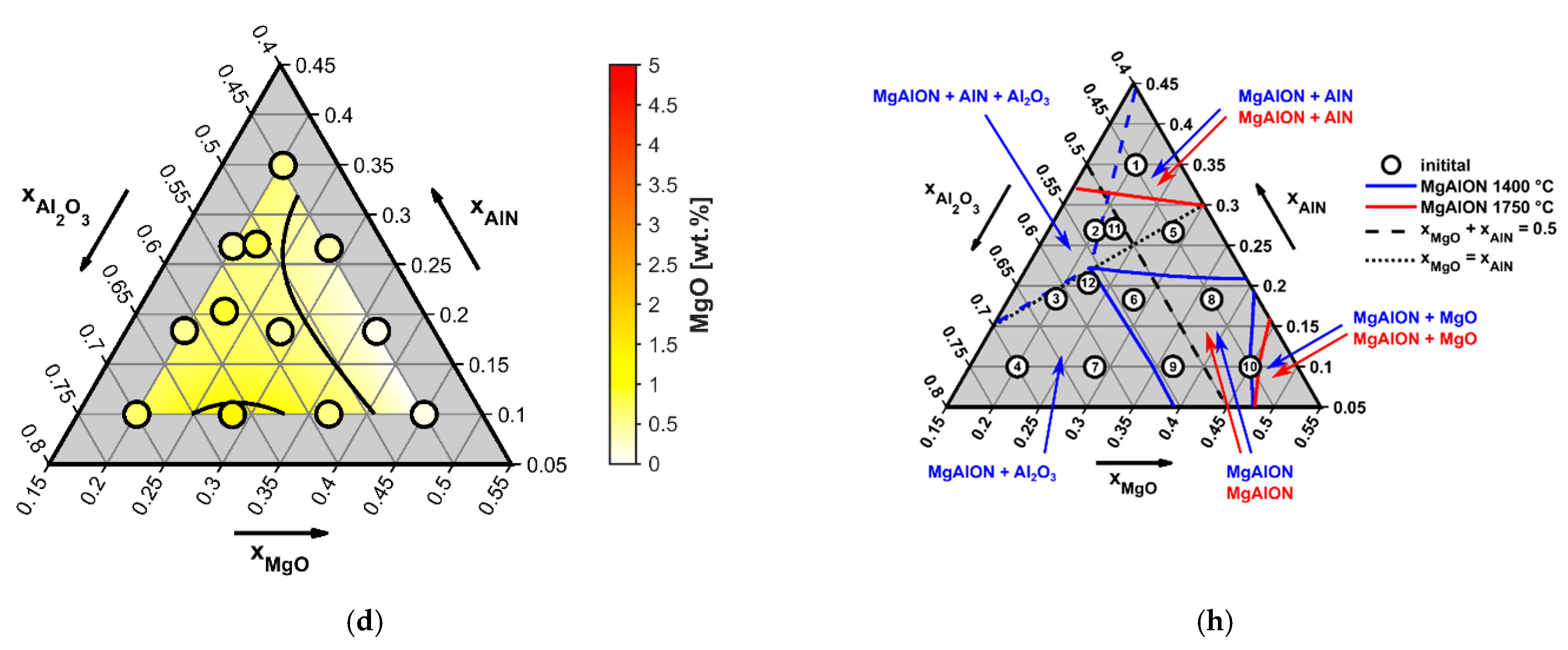
4.1. Influence of Starting Composition and Sintering Duration on the Conversion Degree
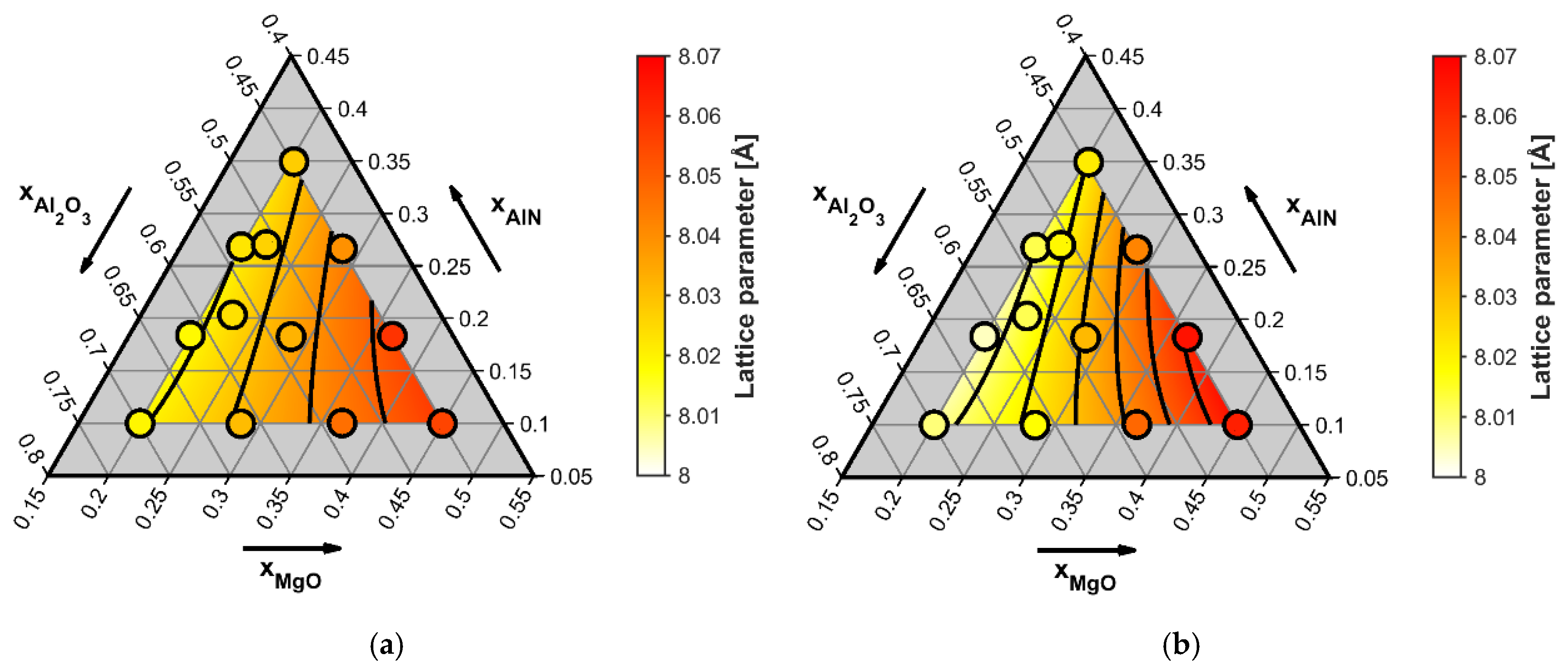
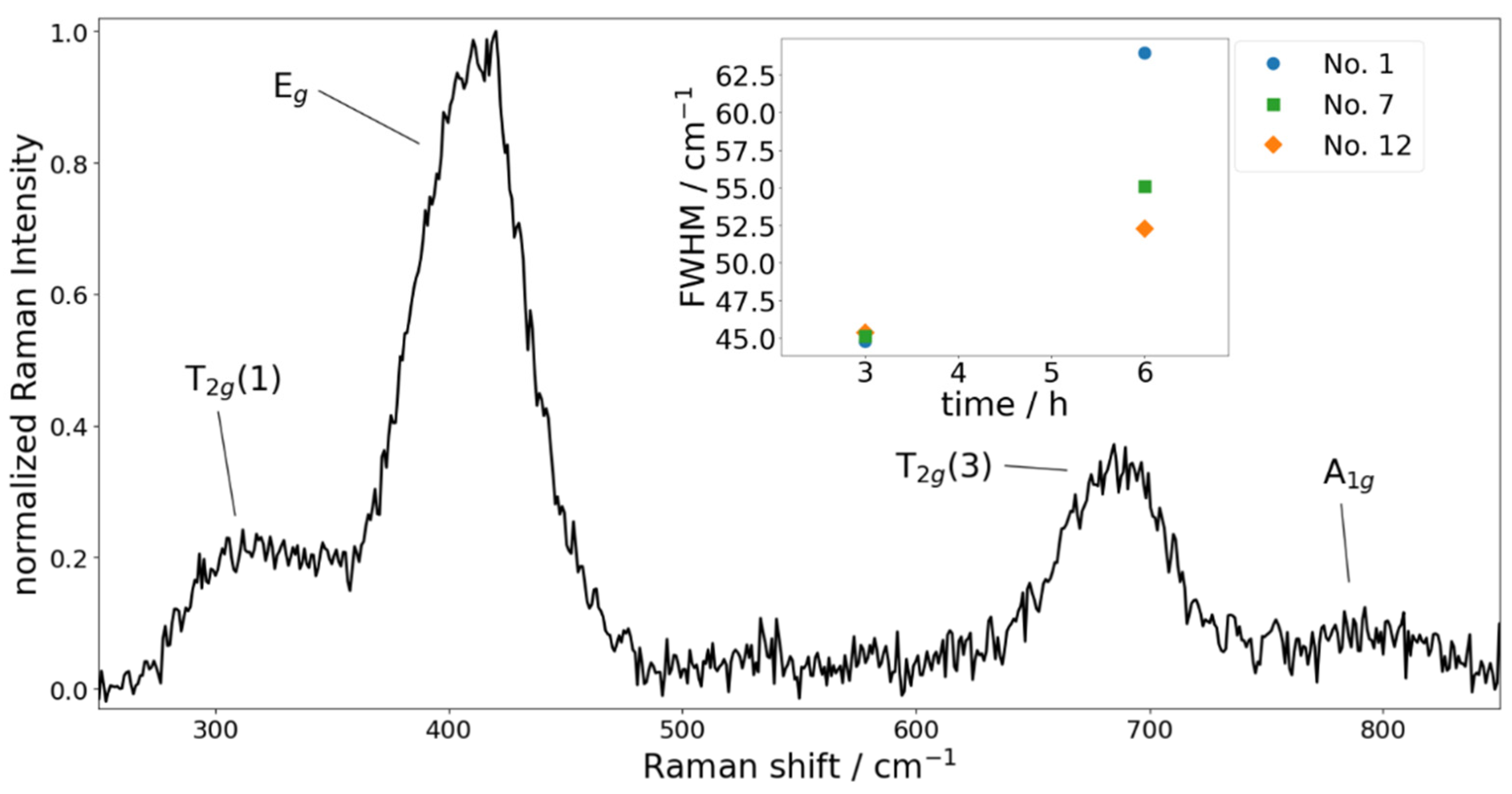
4.2. Raman Measurements
4.3. Nitrogen Content of Selected Samples
4.4. Wettability of Selected Sintered Samples by AlSi7Mg
5. Conclusions
- Pressureless reactive sintering of Al2O3-AlN-MgO mixtures at a sintering temperature of 1500 °C with a duration of 3 h did not result in a near-equilibrium state and yields an average conversion degree to MgAlON of only 76 wt%.
- Reactive sintering of the same mixtures at 1500 °C for 6 h resulted in a near-equilibrium state, reaching an average conversion degree to MgAlON of 92 wt%.
- The highest conversion degrees of 99 wt% of MgAlON were reached in the MgO-rich corner of the quasi-ternary MgO-AlN-Al2O3 system with Al2O3:AlN ratios of around 20:6.
- At 1500 °C, MgAlON formed from MgAl2O4, which is a direct reaction product of MgO and Al2O3, and the subsequent, simultaneous dissolution of Al2O3 and AlN in the spinel structure. During this process, MgO was fully consumed after the first 3 h; the loss of magnesium due to evaporation or parasitic reactions was insignificant.
- The relative amount of nitrogen in the MgAlON phase of the samples with the lowest and highest conversion degree after 6 h of reactive sintering was measured to be 1.5 wt% for both samples.
- Contact angles measured between sintered MgAlON samples and AlSi7Mg via sessile drop tests at 950 °C were shown to be around 145–152°, indicating low wettability and non-reactivity, exceeding the ones measured for MgAl2O4, a material commonly used as a coating for metal melt filters. Therefore, MgAlON is regarded to be a promising ceramic material to be used in light metal melt filtration.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jack, K.H. Sialons and related nitrogen ceramics. J. Mater. Sci. 1976, 11, 1135–1158. [Google Scholar] [CrossRef]
- Gao, F.; Liu, R.; Wang, X.J. A Preliminary Study on Preparation of MgAlON Refractory. Adv. Mater. Res. 2013, 750, 2191–2195. [Google Scholar] [CrossRef]
- Morey, O. Spectroscopic analysis of “MgAlON” spinel powders: Influence of nitrogen content. Solid State Ion. 2003, 159, 381–388. [Google Scholar] [CrossRef]
- Willems, H.X. Preparation and Properties of Translucent Gamma-Aluminium Oxynitride. Ph.D. Thesis, Department of Chemical Engineering and Chemistry, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 1992. [Google Scholar] [CrossRef]
- Corbin, N.D. Aluminum oxynitride spinel: A Review. J. Eur. Ceram. Soc. 1989, 5, 143–154. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Y.; Tong, S.H.; Yue, D.D.; Ma, J.J. Effect of the Addition of Al Powder on the Microstructure and Phase Constitution of Magnesia-Spinel Composites Sintered at 1800 °C in N2. Key Eng. Mater. 2016, 697, 345–349. [Google Scholar] [CrossRef]
- Weiss, S.; Greil, P.; Gauckler, L.J. The System Al-Mg-O-N. Communications of the American Ceramic Society, 1982; Volume 65, p. C68. Available online: https://www.academia.edu/31753923/The_System_Al-Mg-O-N (accessed on 6 April 2022).
- Willems, H.X.; de With, G.; Metselaar, R. Thermodynamics of Alon III: Stabilization of Alon with MgO. J. Eur. Ceram. Soc. 1993, 12, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Ji, Z. Study on Hydration Resistance of MgAlON Composites. J. Phys. Conf. Ser. 2020, 1637, 012031. [Google Scholar] [CrossRef]
- Dai, W.; Yamaguchi, A.; Lin, W.; Ommyoji, J.; Yu, J.; Zou, Z. Oxidation Behavior of Magnesium Aluminum Oxynitride. J. Ceram. Soc. Jpn. 2007, 115, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-Q. Study on the Preparation and Mechanical Properties of MgAlON Composites. Kem. U Ind. 2016, 65, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, W.; Seetharaman, S. Kinetic studies of oxidation of MgAlON and a comparison of the oxidation behaviour of AlON, MgAlON, O’SiAlON-ZrO2, and BN–ZCM ceramics. Int. J. Mater. Res. 2002, 93, 545–553. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, J.L.; Li, F.S.; Chen, Z.Y.; Wan, L.G. Synthesis of MgAlON-Bonded MgO Refractory. Adv. Mater. Res. 2010, 105, 769–772. [Google Scholar] [CrossRef]
- Schramm, A.; Bock, B.; Schmidt, A.; Zienert, T.; Ditze, A.; Scharf, C.; Aneziris, C.G. Interface reactions of differently coated car-bon-bonded alumina filters with an AZ91 magnesium alloy melt. Ceram. Int. 2018, 44, 17415–17424. [Google Scholar] [CrossRef]
- Schramm, A.; Recksiek, V.; Dudczig, S.; Scharf, C.; Aneziris, C.G. Immersion Testing of Variously Coated Ceramic Foam Filters in an AZ91 Magnesium Melt. Adv. Eng. Mater. 2021, 24, 2100519. [Google Scholar] [CrossRef]
- Pichlbauer, S.; Harmuth, H.; Lenčéš, Z.; Šajgalík, P. Preliminary investigations of the production of MgAlON bonded refrac-tories. J. Eur. Ceram. Soc. 2012, 32, 2013–2018. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Tian, M.; Wang, Y.; Liu, M.; Wang, H.; Zhang, G. Pressureless reaction sintering and hot isostatic pressing of transparent MgAlON ceramic with high strength. Ceram. Int. 2018, 44, 17383–17390. [Google Scholar] [CrossRef]
- Granon, A.; Goeuriot, P.; Thevenot, F. Aluminum magnesium oxynitride: A new transparent spinel ceramic. J. Eur. Ceram. Soc. 1995, 15, 249–254. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Rixecker, G.; Aldinger, F.; Maiti, H.S. Effect of Controlling Parameters on the Reaction Sequences of Formation of Nitrogen-Containing Magnesium Aluminate Spinel from MgO, Al2O3, and AlN. J. Am. Ceram. Soc. 2004, 87, 480–482. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, W.; Wang, Y.; Song, H.; Xie, X.; Zhang, Z.; Yao, C.; Luo, H.; Niu, R. Fabrication and nanoindentation characterization of MgAlON transparent ceramics. Opt. Mater. 2018, 84, 714–721. [Google Scholar] [CrossRef]
- Dai, W.; Yamaguchi, A.; Lin, W.; Ommyoji, J.; Yu, J.; Zou, Z. Preparation and Properties of Spark Plasma Sintered Magnesium Aluminum Oxynitride. J. Ceram. Soc. Jpn. 2007, 115, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.C.; Yang, D.Y.; Qu, Y.C.; Wang, T.; Zhang, C.B. Current Distribution Model and Control Principle of MgAlON Composites by Spark Plasma Sintering (SPS). Adv. Mater. Res. 2013, 833, 111–114. [Google Scholar] [CrossRef]
- Granon, A.; Goeuriot, P.; Thevenot, F.; Guyader, J.; L’Haridon, P.; Laurent, Y. Reactivity in the Al2O3-AlN-MgO system. The MgAlON spinel phase. J. Eur. Ceram. Soc. 1994, 13, 365–370. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Zhang, W.; Chen, Q. Pressureless sintering and fabrication of highly transparent MgAlON ceramic from the carbothermal powder. J. Alloys Compd. 2018, 745, 617–623. [Google Scholar] [CrossRef]
- Wang, X.T.; Wang, H.Z.; Zhang, W.J.; Sun, J.L.; Hong, Y.R. Synthesis of MgAlON from Al-Al2O3-MgO by Reaction Sintering. Key Eng. Mater. 2002, 224, 373–378. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Liu, C.; Yang, P.; Mu, P.; Wen, J.; Chen, S.; Li, Y. Preparation and characterization of novel nonstoichiometric magnesium aluminate spinels. Ceram. Int. 2018, 44, 15104–15109. [Google Scholar] [CrossRef]
- McCanley, J.W. A Simple Model for Aluminum Oxynitride Spinels. J. Am. Ceram. Soc. 1978, 61, 372–373. [Google Scholar] [CrossRef]
- Morey, O.; Goeuriot, P. “MgAlON” spinel structure: A new crystallographic model of solid solution as suggested by 27Al solid state NMR. J. Eur. Ceram. Soc. 2005, 25, 501–507. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, Y.; Huang, Y.; Sun, J. Precise Determination of the MgAlON/(AlN + MgAlON) Phase Boundries in the MgO-AlN-Al2O3 Ternary System at 1600 °C and 1700 °C. J. Mater. Sci. Technol. 2005, 21, 277–280. [Google Scholar]
- Hallstedt, B. Thermodynamic assessment of the system MgO-Al2O3. J. Am. Ceram. Soc. 1992, 75, 1497–1507. [Google Scholar] [CrossRef]
- Zienert, T.; Fabrichnaya, O. Thermodynamic assessment and experiments in the system MgO-Al2O3. Calphad 2013, 40, 1–9. [Google Scholar] [CrossRef]
- Sheldon, R.I.; Hartmann, T.; Sickafus, K.E.; Ibarra, A.; Scott, B.L.; Argyriou, D.N.; Larson, A.C.; Von Dreele, R.B. Cation Disorder and Vacancy Distribution in Nonstoichiometric Magnesium Aluminate Spinel, MgO·xAl2O3. J. Am. Ceram. Soc. 2004, 82, 3293–3298. [Google Scholar] [CrossRef]
- Dupree, R.; Lewis, M.H.; Smith, M.E. A study of the vacancy distribution in non-stoichiometric spinels by magic-angle spinning NMR. Philos. Mag. A 1986, 53, L17–L20. [Google Scholar] [CrossRef]
- Willems, H.X.; de With, G.; Metselaar, R.; Helmholdt, R.B.; Petersen, K.K. Neutron diffraction of γ-aluminium oxynitride. J. Mat. Sci. Lett. 1993, 12, 1470–1472. [Google Scholar] [CrossRef]
- Kargin, F.Y.; Akhmadullina, N.S.; Lysenkov, A.S.; Sirotinkin, V.P.; Shamrai, V.F. Synthesis and X-ray Diffraction Study of Aluminum γ-Oxonitride Solid Solutions. Russ. J. Inorg. Chem. 2020, 65, 1320–1325. [Google Scholar] [CrossRef]
- SGTE (Scientific Group Thermodata Europe) Substance Database (SGSUB). Available online: https://www.sgte.net/en/neu (accessed on 20 April 2022).
- Mao, H.; Selleby, M. Thermodynamic reassessment of the Si3N4–AlN–Al2O3–SiO2 system—Modeling of the SiAlON and liquid phases. Calphad 2007, 31, 269–280. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Sundman, B. A thermodynamic reassessment of the Si-Al-O-N system. J. Eur. Ceram. Soc. 1995, 15, 239–247. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; De Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the exoscale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Corso, A. Pseudopotentials periodic table: From H to Pu. Comput. Mater. Sci. 2014, 95, 337–350. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Standards and Technology, NIST-JANAF Thermochemical Tables, NIST Standard Reference Database 13. Available online: https://janaf.nist.gov (accessed on 29 March 2022).
- Wang, X.; Li, W.; Seetharaman, S. Synthesis and characterisation of MgAlON. Int. J. Mater. Res. 2002, 93, 540–544. [Google Scholar] [CrossRef]
- Fruhstorfer, J.; Schafföner, S.; Aneziris, C.G. Dry ball mixing and deagglomeration of alumina and zirconia composite fine powders using a bimodal ball size distribution. Ceram. Int. 2014, 40, 15293–15302. [Google Scholar] [CrossRef]
- Fukumoto, S.; Hookabe, T.; Tsubakino, H. Hydrolysis behavior of aluminum nitride in various solutions. J. Mater. Sci. 2000, 35, 2743–2748. [Google Scholar] [CrossRef]
- Mathers, J.P.; Frey, R.G. Transparent Aluminum Oxynitride-Based Ceramic Article. U.S. Patent 5,231,062, 9 August 1990. [Google Scholar]
- Bond, F.C. Grinding ball size selection. Min. Eng. 1958, 10, 592–595. [Google Scholar]
- Schubert, H. (Ed.) Handbuch der mechanischen Verfahrenstechnik; John Wiley & Sons: Weinheim, Germany, 2003. [Google Scholar]
- Fruhstorfer, J.; Kerber, F.; Weigelt, C.; Moritz, K.; Aneziris, C.G. Activated reaction synthesis of silicon oxynitride from silica and silicon nitride. Ceram. Int. 2018, 44, 8467–8475. [Google Scholar] [CrossRef]
- Karlsruhe, F.I.Z. Inorganic Crystal Structure Database. Available online: https://icsd.fiz-karlsruhe.de/search/basic.xhtml;jsessionid=AB09DC1F362105E8C821E3DC5F0FF2B5 (accessed on 25 February 2021).
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Tateishi, K.; Ishizawa, N. Structural Evolution of Corundum at High Temperatures. Jpn. J. Appl. Phys. 2008, 47, 616–619. [Google Scholar] [CrossRef]
- Tsirelson, V.G.; Avilov, A.S.; Abramov, Y.A.; Belokoneva, E.L.; Kitaneh, R.; Feil, D. X-ray and Electron Diffraction Study of MgO. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 8–17. [Google Scholar] [CrossRef]
- Schulz, H.; Thiemann, K. Crystal structure refinement of AlN and GaN. Solid State Commun. 1977, 23, 815–819. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takéuchi, Y. Order-disorder transition in MgAl2O4 spinel at high temperatures up to 1700 °C. Z. Für Krist.-Cryst. Mater. 1983, 165, 65–78. [Google Scholar] [CrossRef]
- Wondratschek, H.; Müller, U. Volume C: Mathematical, Physical and Chemical Tables. In International Tables for Crystallography, 3rd ed.; Kluwer Academic: Dordrecht, The Netherlands, 2004. [Google Scholar]
- National Institute of Standards and Technology. X-ray Form Factor, Attenuation and Scattering Tables. Available online: https://physics.nist.gov/PhysRefData/FFast/html/form.html (accessed on 3 October 2021).
- Zong, X.; Ren, L.; Wang, H.; Tu, B.; Wang, W.; Fu, Z. Structural Study of MgyAl(8 + x − 2y)/3O4−xNx (0 <x <0.5, 0 <y <1) Spinel Probed by X-ray Diffraction, 27Al MAS NMR, and First-Principles Calculations. Inorg. Chem. 2020, 59, 17009–17017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Chen, S.; Liang, Y.-Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef]
- Fankhänel, B.; Stelter, M.; Voigt, C.; Aneziris, C.G. Interaction of AlSi7Mg with Oxide Ceramics. Adv. Eng. Mater. 2017, 19, 1700084. [Google Scholar] [CrossRef] [Green Version]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures. In Pergamon Materials Series; Cahn, R.W., Ed.; Pergamon: Oxford, UK, 1999; Volume 3, ISBN 0-080-54378-2. [Google Scholar]
- Yan, M.; Li, Y.; Li, L.; Sun, Y.; Tong, S.; Sun, J. In-situ synthesis and reaction mechanism of MgAlON in Al2O3-MgO composites produced in flowing nitrogen. Ceram. Int. 2017, 43, 14791–14797. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, L.; Qiu, G.; Bai, Z.; Guo, M.; Cheng, F.; Zhang, M. Low temperature and pressureless synthesis of MgAlON: Qualitative analysis and formation evolution. Int. J. Mater. Res. 2020, 111, 537–545. [Google Scholar] [CrossRef]
- Willems, H.X.; Hendrix, M.R.M.; de With, G.; Metselaar, R. Thermodynamics of Alon II: Phase relations. J. Eur. Ceram. Soc. 1992, 10, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Li, Y.; Yan, M.; Jiang, P.; Ma, J.; Yue, D. In situ reaction mechanism of MgAlON in Al–Al2O3–MgO composites at 1700 °C under flowing N2. Int. J. Min. Met. Mater 2017, 24, 1061–1066. [Google Scholar] [CrossRef] [Green Version]
- O’Horo, M.P.; Frisillo, A.L.; White, W.B. Lattice vibrations of MgAl2O4 spinel. J. Phys. Chem. Solids 1973, 34, 23–28. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman fingerprint of chromate, aluminate and ferrite spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Slotznick, S.P.; Shim, S.-H. In situ Raman spectroscopy measurements of MgAl2O4 spinel up to 1400 °C. Am. Miner. 2008, 93, 470–476. [Google Scholar] [CrossRef]
- Van Minh, N.; Yang, I.-S. A Raman study of cation-disorder transition temperature of natural MgAl2O4 spinel. Vib. Spectrosc. 2004, 35, 93–96. [Google Scholar] [CrossRef]
- Schramm, A.; Nowak, R.; Bruzda, G.; Polkowski, W.; Fabrichnaya, O.; Aneziris, C.G. High temperature wettability and corrosion of ZrO2, Al2O3, Al2O3-C, MgO and MgAlON ceramic substrates by an AZ91 magnesium alloy melt. J. Eur. Ceram. Soc. 2022, 42, 3023–3035. [Google Scholar] [CrossRef]
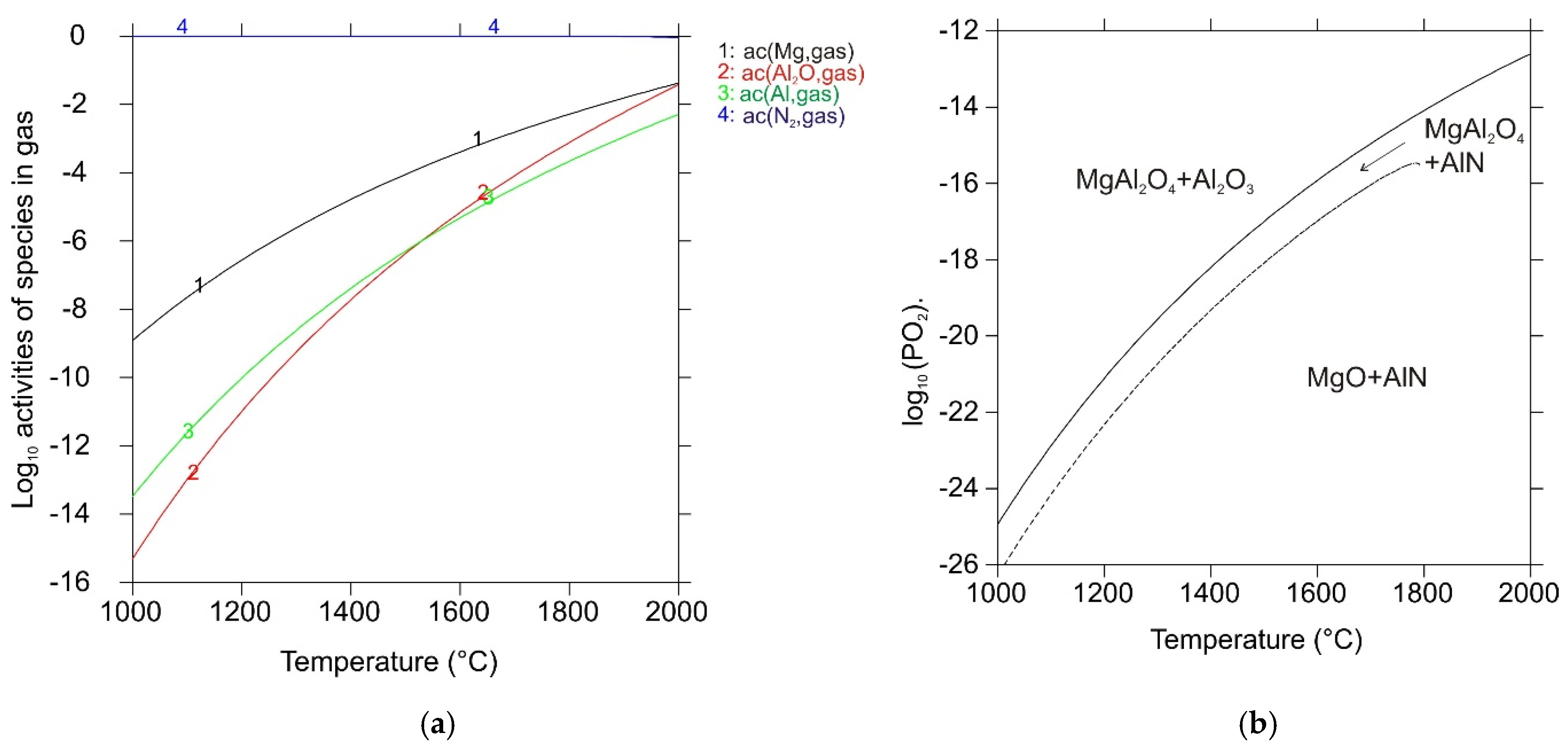
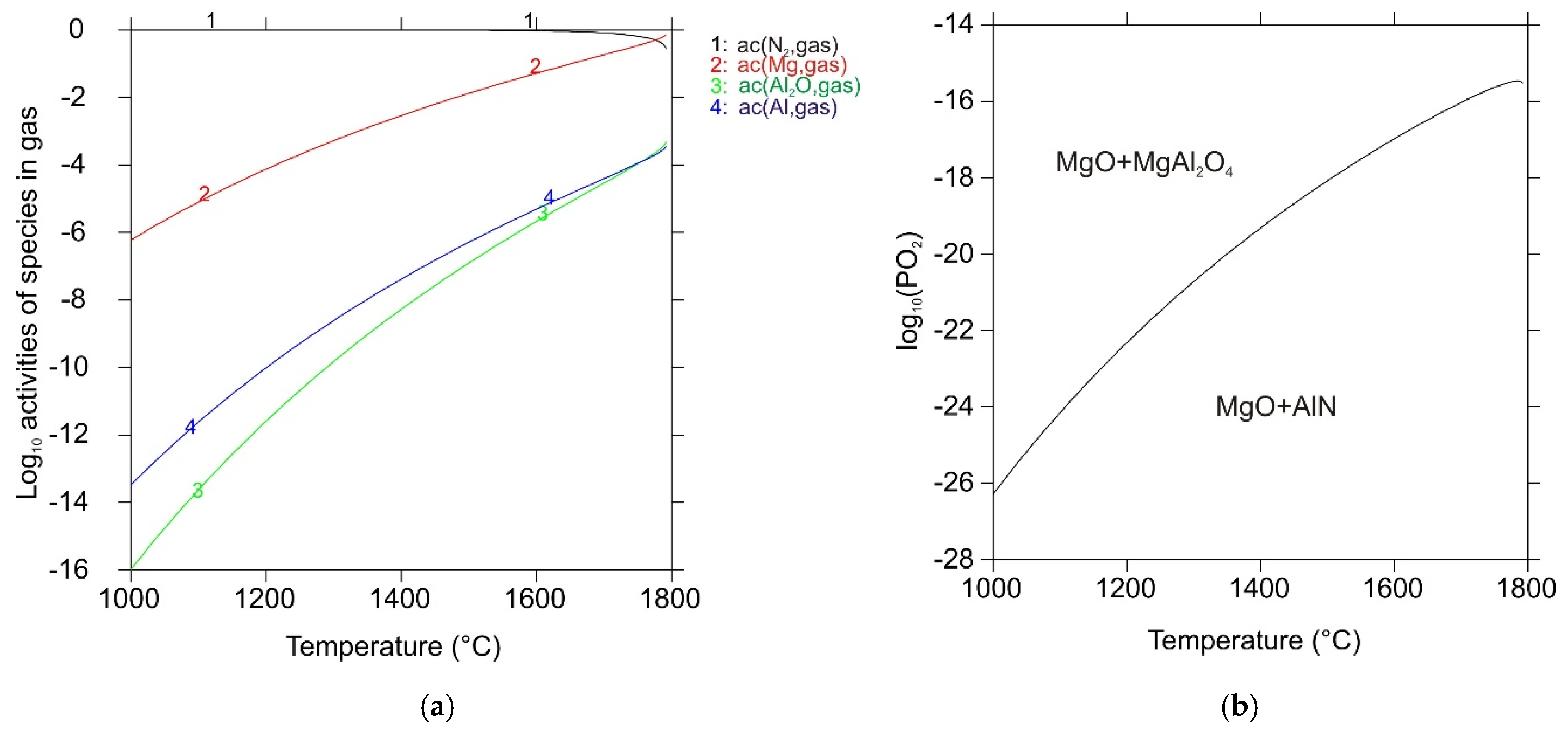
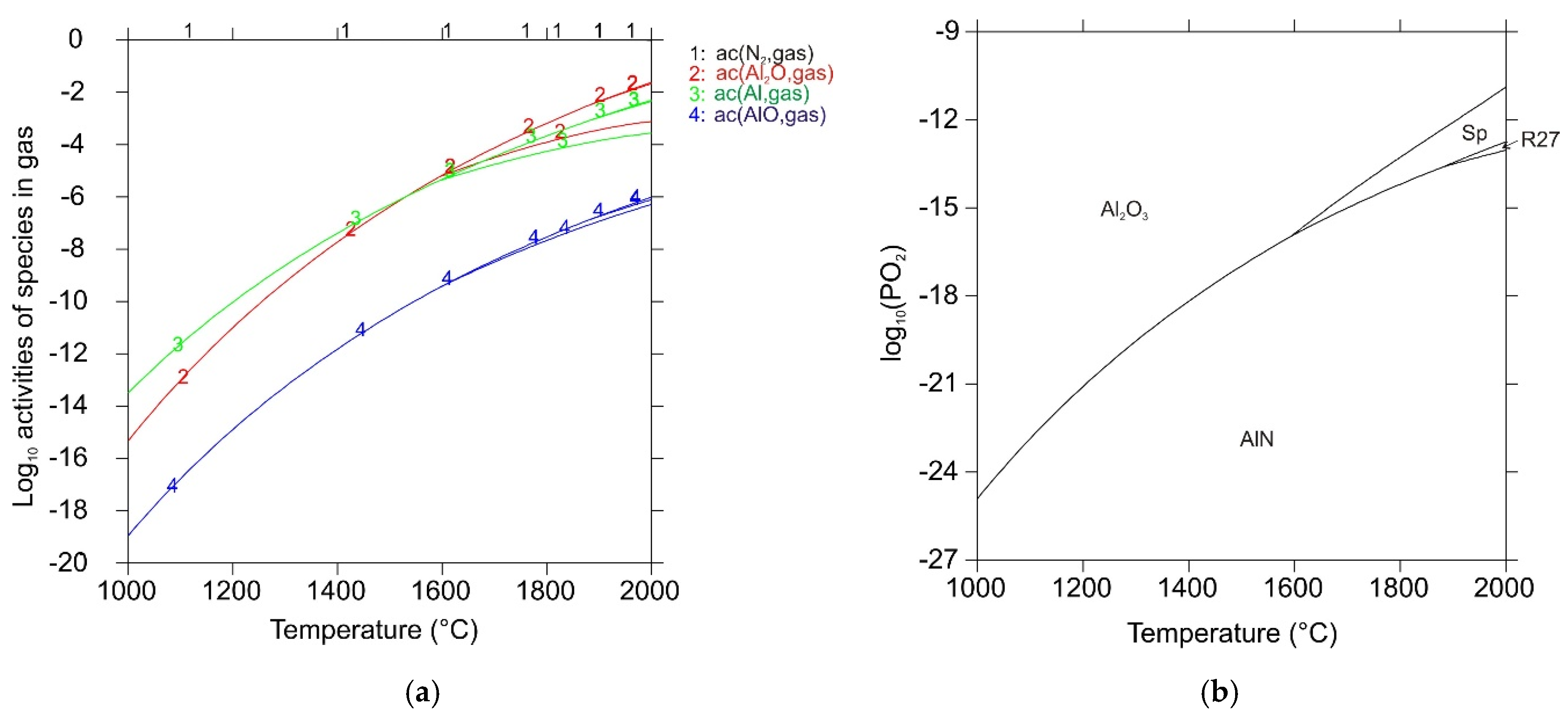

| Reaction No. | Chemical Equation | ΔrG°DFT (kJ mol−1) | ΔrG°ref (kJ mol−1) |
|---|---|---|---|
| 1 | MgO(s) → Mg(g) + ½ O2(g) | 325.4 | 366.3 |
| 2 | MgAl2O4(s) → Al2O3(s) + Mg(g) + ½ O2(g) | 351.6 | 408.3 |
| 3 | Al2O3(s) + 4 AlN(s) → 3 Al2O(g) + 2 N2(g) | 681.9 | 793.4 |
| 4 | MgAl2O4(s) + 4 AlN(s) → 3 Al2O(g) + Mg(g)+ ½ O2(g) + 2 N2(g) | 1033.4 | 1201.7 |
| 5 | AlN(s) → Al(g) + ½ N2(g) | 247.0 | 230.8 |
| 6 | 2 Al(g) + ½ O2(g) → Al2O(g) | −529.3 | −485.5 |
| Mix No. | ρmix (Ø) (t m−3) | d80 (Ø) (µm) | |||
|---|---|---|---|---|---|
| 1 | 0.35 | 0.2 | 0.45 | 3.769 | 10.8 |
| 2 | 0.267 | 0.2 | 0.533 | 3.85 | 9.97 |
| 3 | 0.183 | 0.2 | 0.617 | 3.899 | 9.37 |
| 4 | 0.1 | 0.2 | 0.7 | 3.942 | 8.85 |
| 5 | 0.267 | 0.283 | 0.45 | 3.815 | 14.16 |
| 6 | 0.183 | 0.283 | 0.533 | 3.868 | 13.19 |
| 7 | 0.1 | 0.283 | 0.617 | 3.916 | 12.38 |
| 8 | 0.183 | 0.367 | 0.45 | 3.834 | 17.6 |
| 9 | 0.1 | 0.367 | 0.533 | 3.886 | 16.42 |
| 10 | 0.1 | 0.45 | 0.45 | 3.852 | 21.034 |
| 11 [48] | 0.27 | 0.22 | 0.51 | 3.841 | 10.91 |
| 12 [23] | 0.21 | 0.22 | 0.57 | 3.879 | 10.62 |
| Mix No. | Starting Composition (Mole Fraction) (x) | Conversion Degree to MgAlON Spinel (wt%) after: | |||
|---|---|---|---|---|---|
| AlN | MgO | Al2O3 | 3 h of Sintering | 6 h of Sintering | |
| 1 | 0.35 | 0.2 | 0.45 | 67 | 86 |
| 2 | 0.267 | 0.2 | 0.533 | 65 | 92 |
| 3 | 0.183 | 0.2 | 0.617 | 65 | 92 |
| 4 | 0.1 | 0.2 | 0.7 | 59 | 76 |
| 5 | 0.267 | 0.283 | 0.45 | 82 | 89 |
| 6 | 0.183 | 0.283 | 0.533 | 82 | 96 |
| 7 | 0.1 | 0.283 | 0.617 | 71 | 90 |
| 8 | 0.183 | 0.367 | 0.45 | 93 | 94 |
| 9 | 0.1 | 0.367 | 0.533 | 93 | 99 |
| 10 | 0.1 | 0.45 | 0.45 | 97 | 99 |
| 11 | 0.27 | 0.22 | 0.51 | 70 | 92 |
| 12 | 0.21 | 0.22 | 0.57 | 68 | 96 |
| Average conversion degree: | 76 | 92 | |||
| Substrate | Overall Nitrogen Content (wt%) | Nitrogen Content, Expected (wt%) | Ratio (Measured/Expected) | Nitrogen Content in Remaining AlN (wt%) | Absolute Nitrogen Content in MgAlON (wt%) | Relative Nitrogen Content in MgAlON (w/w%) |
|---|---|---|---|---|---|---|
| MgAlON-containing sample No. 4 | 1.48 ± 0.17 | 1.67 | 0.89 ± 0.11 | 0.31 | 1.17 ± 0.17 | 1.5 ± 0.3 |
| MgAlON-containing sample No. 9 | 1.64 ± 0.15 | 1.91 | 0.86 ± 0.08 | 0.17 | 1.47 ± 0.15 | 1.5 ± 0.2 |
| Substrate | Ø Sa (μm) |
|---|---|
| As-sintered MgAlON-containing sample, composition No. 4 I | 4.2 ± 1.7 |
| As-sintered MgAlON-containing sample, composition No. 4 II | 4.8 ± 2.2 |
| As-sintered MgAlON-containing sample, composition No. 9 I | 4.0 ± 0.5 |
| As-sintered MgAlON-containing sample, composition No. 9 II | 3.8 ± 1.0 |
| As-fired alumina substrate, MgAl2O4 coating [66] | 3.6 ± 0.1 |
| As-fired alumina substrate, Al2O3 coating [66] | 1.7 ± 0.2 |
| Substrate | 90 Min of Contact Time | 180 Min of Contact Time | Overall Ø Contact Angle | ||||
|---|---|---|---|---|---|---|---|
| Left | Right | Ø | Left | Right | Ø | ||
| As-sintered MgAlON-containing sample, composition No. 4 I | 150.9° | 151.0° | 150.9° | 150.7° | 150.7° | 150.7° | 150 ± 0.5° |
| As-sintered MgAlON-containing sample, composition No. 4 II | 153.9° | 153.9° | 153.9° | 151.2° | 151.2° | 151.2° | 152 ± 0.5° |
| As-sintered MgAlON-containing sample, composition No. 9 I | 149.2° | 149.2° | 149.2° | 145.7° | 145.6° | 145.6° | 146 ± 0.5° |
| As-sintered MgAlON-containing sample, composition No. 9 II | 147.7° | 147.7° | 147.7° | 144.4° | 144.4° | 144.4° | 145 ± 0.6° |
| As-fired alumina substrate, MgAl2O4 coating [66] | 129 ± 1° | 122 ± 1° | |||||
| As-fired alumina substrate, Al2O3 coating [66] | 103 ± 1° | 100 ± 1° | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schramm, A.; Thümmler, M.; Fabrichnaya, O.; Brehm, S.; Kraus, J.; Kortus, J.; Rafaja, D.; Scharf, C.; Aneziris, C.G. Reaction Sintering of MgAlON at 1500 °C from Al2O3, MgO and AlN and Its Wettability by AlSi7Mg. Crystals 2022, 12, 654. https://doi.org/10.3390/cryst12050654
Schramm A, Thümmler M, Fabrichnaya O, Brehm S, Kraus J, Kortus J, Rafaja D, Scharf C, Aneziris CG. Reaction Sintering of MgAlON at 1500 °C from Al2O3, MgO and AlN and Its Wettability by AlSi7Mg. Crystals. 2022; 12(5):654. https://doi.org/10.3390/cryst12050654
Chicago/Turabian StyleSchramm, Alina, Martin Thümmler, Olga Fabrichnaya, Simon Brehm, Jakob Kraus, Jens Kortus, David Rafaja, Christiane Scharf, and Christos G. Aneziris. 2022. "Reaction Sintering of MgAlON at 1500 °C from Al2O3, MgO and AlN and Its Wettability by AlSi7Mg" Crystals 12, no. 5: 654. https://doi.org/10.3390/cryst12050654






