Gemological Characteristics and Chemical Composition of a New Type of Black Jadeite and Three Imitations
Abstract
:1. Introduction
2. Sample Description
3. Methods
3.1. Sample Composition
3.2. Chemical Analysis
4. Results
5. Discussion
5.1. Chromogenic Factor of Jadeite and Classification of Its Imitations
5.2. Implications for Identification and Evaluation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, C.; Wang, Y.; Liu, Y. Influence of Associated Minerals on Jadeite Naming. J. Gems. Gemmol. 2013, 15, 28–36, (In Chinese with English abstract). [Google Scholar]
- Yin, Z. Relationships between mineralogy, petrology and gemology of “Feicui”. Superhard Mater. Eng. 1990, 25, 16–24, (In Chinese with English abstract). [Google Scholar]
- Cheng, X.; Xiao, L. Classification and commercial evalution of “Feicui”. Superhard Mater. Eng. 1990, 25, 8–15, (In Chinese with English abstract). [Google Scholar]
- Zou, T.; Xia, F.; Chen, W.; Yu, X. Study on Clinopyroxene minerals of jadeite jade. J. Gems Gemmol. 1999, 1, 27–32, (In Chinese with English abstract). [Google Scholar]
- Zhou, S.; Lai, H.; Xu, X. The prospect of black gemstones designing and processing. J. Guilin Inst. Technol. 2002, 22, 26–29, (In Chinese with English abstract). [Google Scholar]
- Huang, D.; Xiong, W. Study on Identification and Naming of Jade Sodium Feldspathic Associated with Jade Stone. Land Resour. Shandong Prov. 2011, 27, 17–19, (In Chinese with English abstract). [Google Scholar]
- Yang, S.; Jiang, S.; Mao, Q.; Chen, Z.; Rao, C.; Li, X.L.; Li, W.C.; Yang, W.; He, P.; Li, X. Electron Probe Microanalysis in Geosciences: Analytical Procedures and Recent Advances. Atom Spectrosc. 2022, 43, 186–200. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, S. A Mathematical Model for Determining Carbon Coating Thickness and Its Application in Electron Probe Microanalysis. Microsc. Microanal. 2016, 22, 1374–1380. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, H. Reviews of recent studies on black jadeite jade. J. Gemmol. 1999, 26, 417–424. (In Chinese) [Google Scholar] [CrossRef]
- Yan, R.; Qiu, Z.; Dong, C.; Li, L. A preliminary study of typomorphic characteristics of different kinds of black jadeite jades in the world. Acta Petrol. Mineral. 2009, 28, 292–298, (In Chinese with English abstract). [Google Scholar]
- Qiu, Z. Black jadeite. Jewel. Sci. Technol. 2000, 12, 52–53. (In Chinese) [Google Scholar]
- Mao, J.; Chai, L.; Liu, X.; Fan, J.; Xu, J.; Jin, Z. Research of Genesis of Black Bands in Burma Jadeite. Spectrosc. Spect. Anal. 2013, 33, 2411–2415, (In Chinese with English abstract). [Google Scholar]
- Zhang, F. Mineralogy and Gemology Study of the Black-Jadeite. Master’s Thesis, China University of Geosciences, Beijing, China, 2013; pp. 1–40, (In Chinese with English abstract). [Google Scholar]
- Guo, F. The Study on Gemological and Mineralogical of Black-Jadeite from Myanmar. Master’s Thesis, Guilin University of Technology, Guilin, China, 2019; pp. 1–63, (In Chinese with English abstract). [Google Scholar]
- He, Y.; Zu, E. Mineralogy of Burma jade from different mines. Contrib. Geol. Miner. Resour. Res. 2017, 32, 152–160, (In Chinese with English abstract). [Google Scholar]
- Cai, S.; Zhang, E. Genesis and Formation of Amphiboles in Myanmar Raw Jadeite. Acta Mineral. Sin. 2018, 38, 50–57, (In Chinese with English abstract). [Google Scholar]
- Zou, T.; Yu, X.; Xia, F.; Chen, W. Mineralogic components of Feicui and classification of pyroxene jade. Yunan Geol. 1998, 17, 338–349, (In Chinese with English abstract). [Google Scholar]
- Liu, X.; Wang, L.; LI, H.; Liu, J.; Zhao, F.; Lu, Q.; Hu, L. Systematic mineralogical classifications and nomenclatures of amphibole and pyroxene group minerals. Acta Mineral. Sin. 2015, 35, 20–28, (In Chinese with English abstract). [Google Scholar]
- Philpotts, J.A. Redox estimation from a calculation of Eu2+ and Eu3+ concentrations in natural phases. Earth Planet Sci. Lett. 1970, 9, 257–268. [Google Scholar] [CrossRef]
- Lv, H. Study on Mineralogical Character of Tinea on the Weathering Crust of Feicui; China University of Geosciences: Wuhan, China, 2008; pp. 1–59, (In Chinese with English abstract). [Google Scholar]
- Li, Y. Gemmological Characteristic and identification of aventurine. Superhard Mater Eng. 1997, 2, 21–22. (In Chinese) [Google Scholar]
- Han, D.; Liu, X.; Liu, Y.; Zheng, F.; Mai, A.; Zhang, H.; Wen, Z. Genesis of dolomite-related nephrite from Hetian and color-forming factors of typical nephrite in Hetian, Xinjiang. Acta Petrol. Mineral. 2018, 37, 1011–1026, (In Chinese with English abstract). [Google Scholar]
- Guo, J.; Zhang, N.; Zhu, W.; Li, J.; Zhang, X.; Qu, J. A tentative discussion on coloration mechanism of Changhua Chicken-Blood Stone. Acta Petrol. Mineral. 2004, 23, 54–56, (In Chinese with English abstract). [Google Scholar]
- Cai, J.; Yu, X.; Liu, C.; Yin, J. Study on Microstructure Characteristics and Crystal Chemistry of a Diopside Glass Ceramic. J. Synth. Cryst. 2010, 39, 1586–1591. [Google Scholar]
- Li, H.; Chen, M. Experiment Study on Synthetic Jadeite Jade. J. Gems Gemmol. 2009, 11, 34–40, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.; Liu, Y.; Chen, H.; Lu, T.; Ke, J.; Zhang, Y. Experimental Study and Identification of “New Type” Wax-permeated Jadeite. J. Gems Gemmol. 2013, 15, 26–31, (In Chinese with English abstract). [Google Scholar]
- Mu, H.; Lin, J.; Zhang, T.; Xin, C.; Li, Y. Application of Coating technology on Gemstone Optimization. Superhard Mater. Eng. 2020, 32, 59–65, (In Chinese with English abstract). [Google Scholar]
- Zhu, H.; Liu, H.; Du, R.; Zhao, X.; Huang, Z.; Ding, X.; Chen, S. Geommological Characteristics of a Feicui Jade Imitation. Superhard Mater. Eng. 2020, 32, 59–63, (In Chinese with English abstract). [Google Scholar]
- Li, R.; Xu, Z.; Xiong, Y.; Zhang, P.; Zhang, Y. Gemmological Characteristics of Dark Green Jadeite Imitations. J. Gems Gemmol. 2014, 16, 49–54, (In Chinese with English abstract). [Google Scholar]
- Liu, J.; Li, R.; Xie, M.; Xu, Z. Gemmological Characteristics of Grossular Jade as Jadeite Imitation. J. Gems Gemmol. 2014, 16, 47–50, (In Chinese with English abstract). [Google Scholar]
- Li, X.; Xu, Z.; Guo, J.; Liu, J. Zoisite Jade: One Fake Black Jadeite. J. Gems Gemmol. 2013, 15, 38–42, (In Chinese with English abstract). [Google Scholar]
- Peng, J.; Wang, D. Preliminary Study on Idocrase Jade. J. Gems Gemmol. 2010, 12, 29–31, (In Chinese with English abstract). [Google Scholar]
- China Association for Quality Inspection. Gem Testing–Infrared Spectroscopy Method: T/CAQI 73-2019; China Standard Press: Beijing, China, 2019; pp. 1–104. (In Chinese) [Google Scholar]
- Wang, L.; Di, J. Spectroscopic Study on the Main Mineral of DuShan Yu from Henan Province, China and the Philippines. J. Gems Gemmol. 2021, 23, 39–45, (In Chinese with English abstract). [Google Scholar]
- Luo, Y. Gemmological Characteristics of Black Hornblende Jade. J. Gems Gemmol. 2007, 9, 59–62, (In Chinese with English abstract). [Google Scholar]

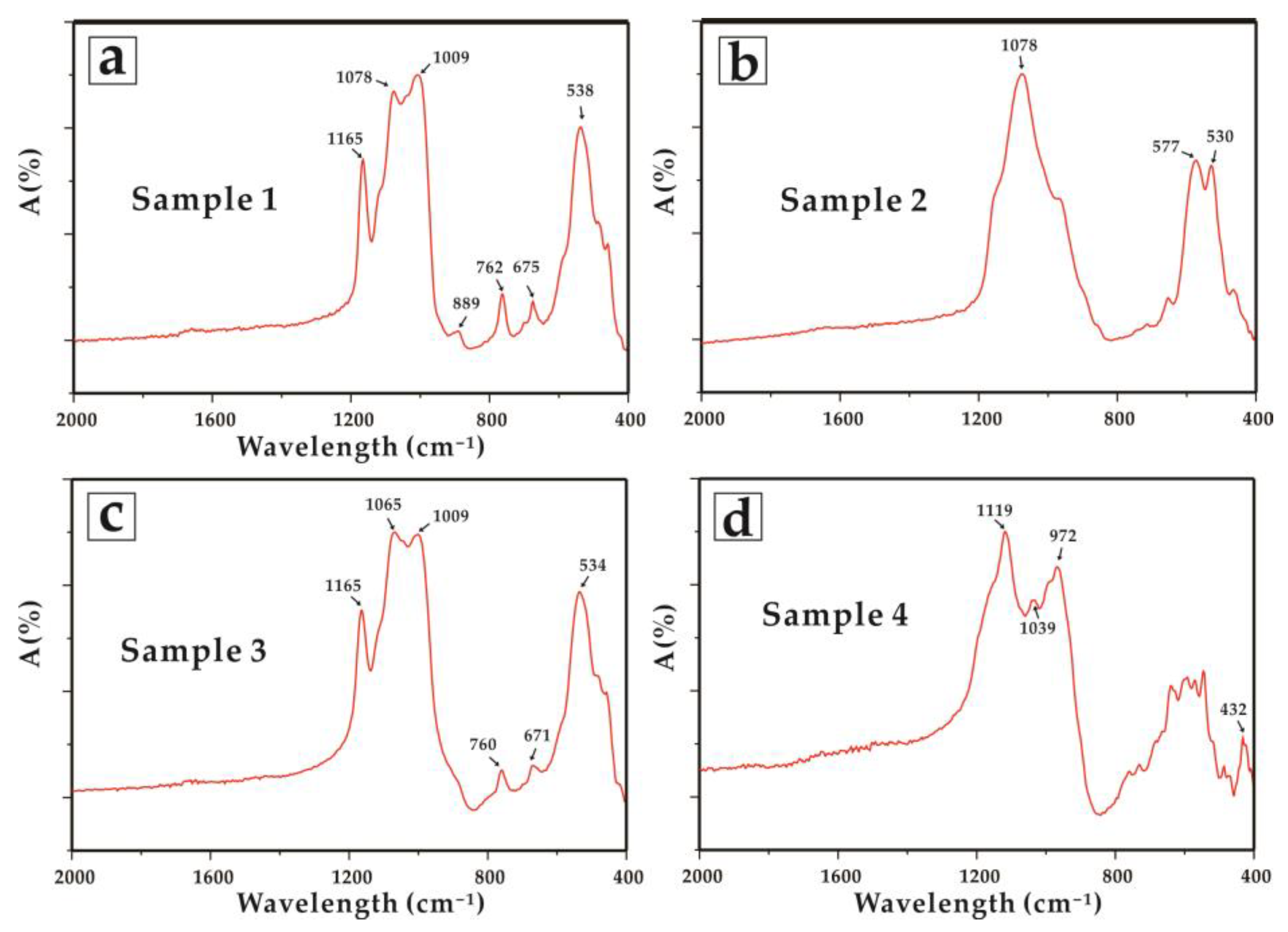


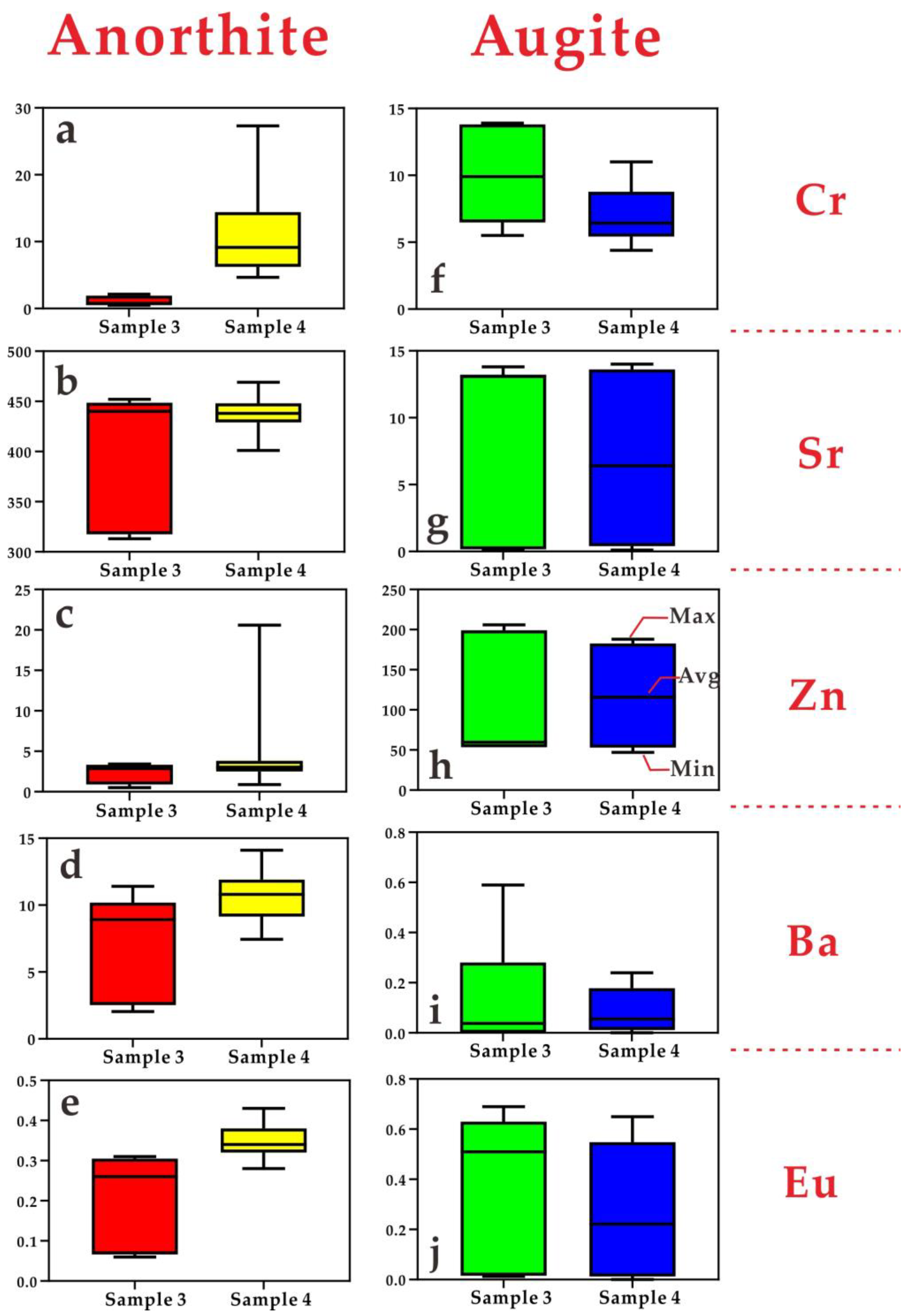
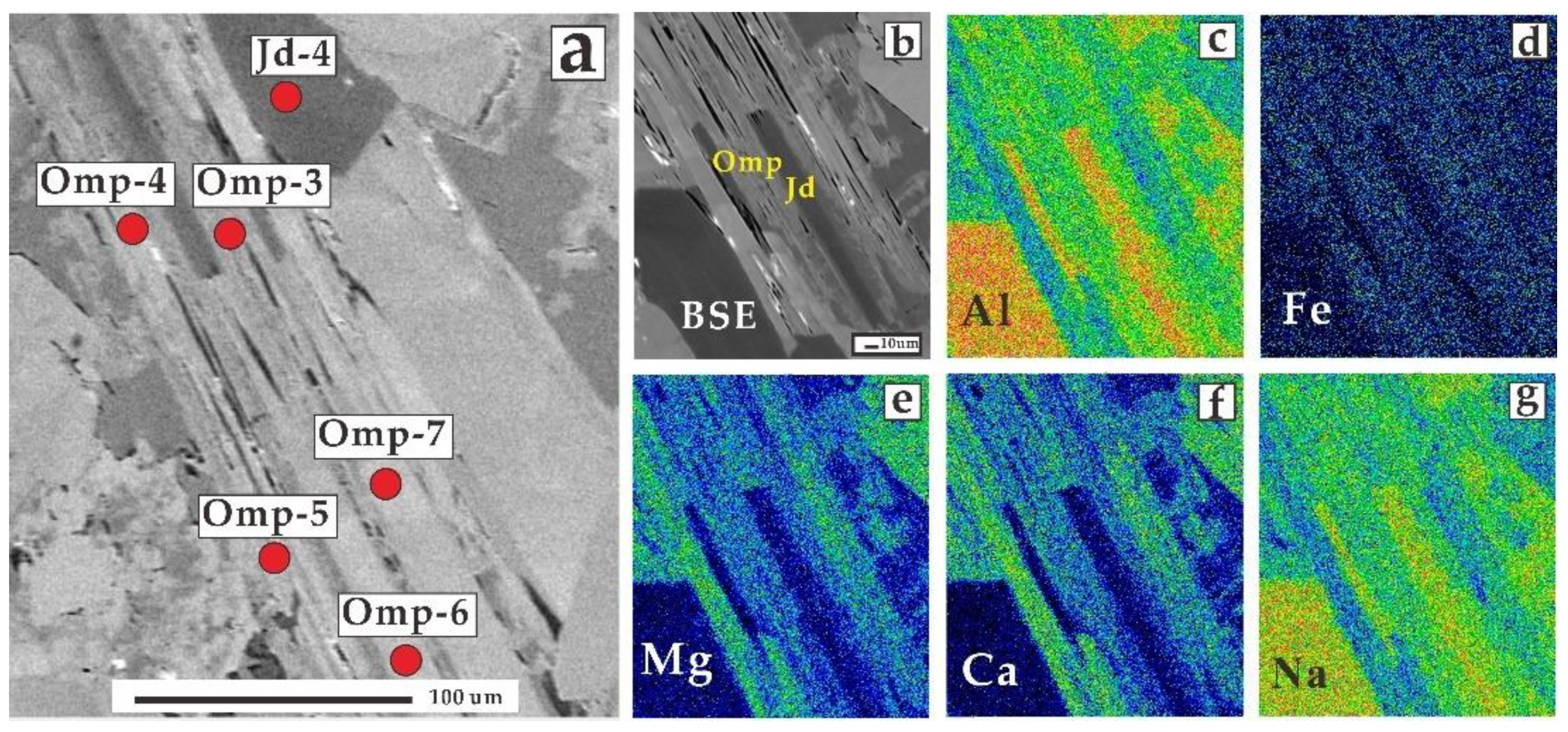
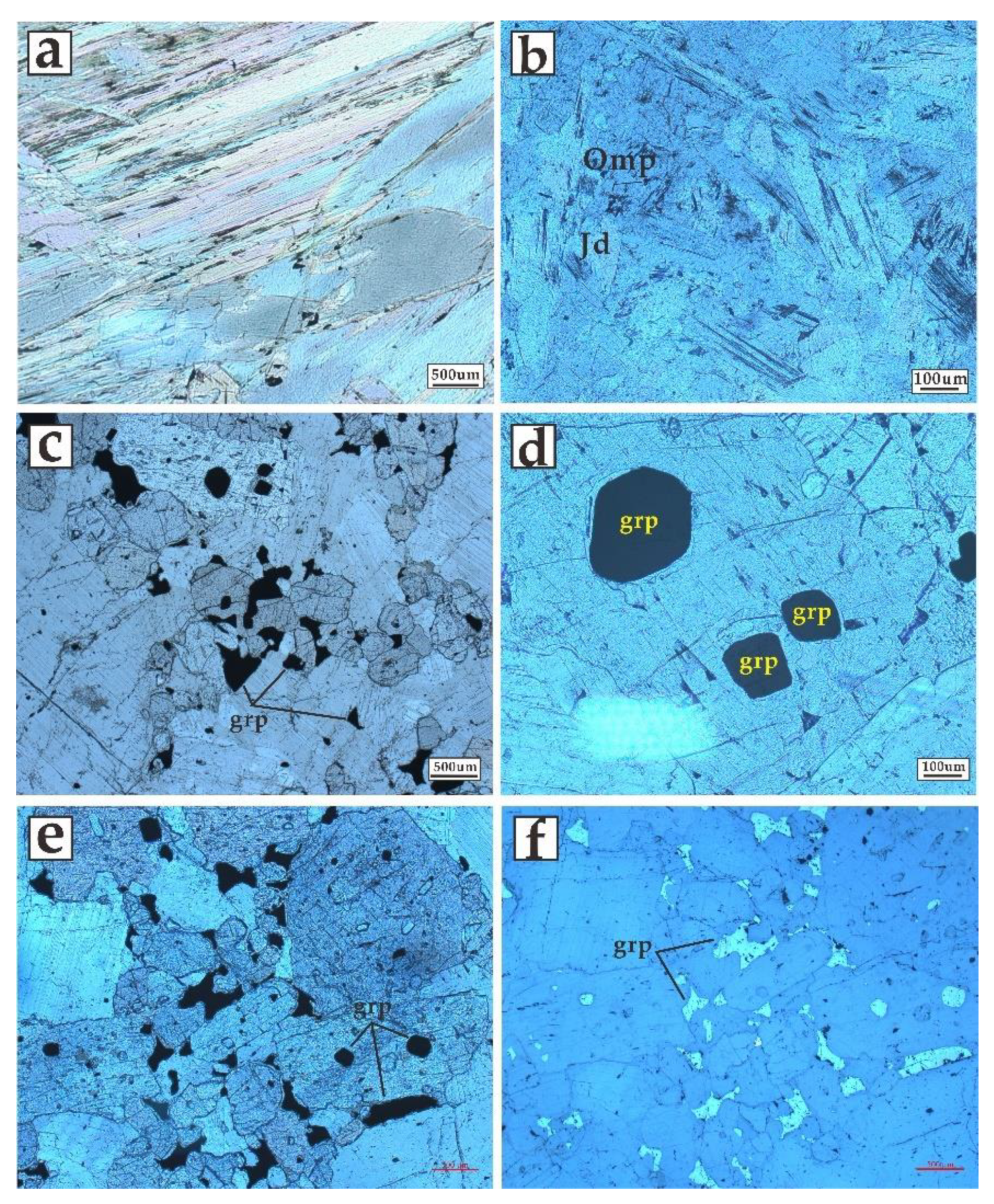
| Sample 1 | Sample 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 |
| Type | Katophorite | Omphacite | Jadeite | |||||||||||||
| SiO2 | 54.54 | 55.08 | 57.30 | 56.96 | 59.40 | 57.36 | 56.49 | 57.35 | 55.86 | 56.34 | 55.78 | 56.23 | 59.17 | 59.94 | 59.22 | 59.26 |
| TiO2 | 0.08 | 0.06 | 0.02 | 0.04 | 0.03 | 0.07 | 0.09 | 0.14 | 0.22 | 0.20 | 0.08 | 0.08 | 0.29 | 0.27 | 0.05 | 0.11 |
| Al2O3 | 9.08 | 6.78 | 5.97 | 7.21 | 5.04 | 10.67 | 10.71 | 14.91 | 10.38 | 14.25 | 11.85 | 11.32 | 22.13 | 22.73 | 21.64 | 22.41 |
| FeO | 5.67 | 4.77 | 4.01 | 4.21 | 4.04 | 2.91 | 3.89 | 3.96 | 5.69 | 4.67 | 4.87 | 4.73 | 1.45 | 1.32 | 1.82 | 1.79 |
| MnO | 0.10 | 0.08 | 0.10 | 0.14 | 0.13 | 0.10 | 0.11 | 0.13 | 0.11 | 0.13 | 0.20 | 0.16 | 0.01 | 0.00 | 0.08 | 0.02 |
| MgO | 15.08 | 17.13 | 17.13 | 16.11 | 17.67 | 8.17 | 7.88 | 5.66 | 7.67 | 6.39 | 7.51 | 7.63 | 1.45 | 1.31 | 1.72 | 1.53 |
| CaO | 1.67 | 2.55 | 1.26 | 1.01 | 1.46 | 12.85 | 12.26 | 8.57 | 11.11 | 8.74 | 12.62 | 12.82 | 2.10 | 1.76 | 2.71 | 2.20 |
| Na2O | 8.85 | 8.61 | 9.32 | 9.43 | 8.01 | 6.66 | 6.87 | 9.75 | 7.24 | 8.93 | 7.12 | 7.21 | 12.62 | 12.07 | 12.24 | 13.56 |
| K2O | 0.24 | 0.25 | 0.32 | 0.17 | 0.35 | 0.00 | 0.01 | 0.00 | 0.04 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | 0.00 |
| Total | 95.31 | 95.30 | 95.43 | 95.27 | 96.12 | 98.79 | 98.31 | 100.46 | 98.31 | 99.64 | 100.02 | 100.18 | 99.22 | 99.43 | 99.48 | 100.88 |
| Number | Sample 3 | Sample 4 | Sample 3 | Sample 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Type | Augite | Anorthite | ||||||||||
| SiO2 | 52.88 | 51.98 | 52.98 | 52.21 | 52.23 | 51.96 | 46.50 | 46.54 | 43.77 | 45.90 | 44.49 | 44.13 |
| TiO2 | 0.18 | 0.20 | 0.20 | 0.20 | 0.22 | 0.18 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.00 |
| Al2O3 | 1.06 | 1.30 | 1.14 | 1.06 | 1.06 | 1.24 | 33.56 | 33.38 | 35.62 | 33.64 | 34.60 | 34.95 |
| FeO | 20.01 | 20.13 | 20.08 | 20.51 | 19.57 | 19.76 | 0.45 | 0.40 | 0.44 | 0.60 | 0.30 | 0.28 |
| MnO | 0.68 | 0.64 | 0.70 | 0.70 | 0.73 | 0.69 | 0.02 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 |
| MgO | 23.21 | 23.19 | 23.62 | 23.14 | 23.39 | 23.04 | 0.05 | 0.06 | 0.02 | 0.18 | 0.01 | 0.00 |
| CaO | 1.49 | 1.61 | 1.05 | 1.17 | 1.76 | 1.35 | 17.02 | 16.70 | 19.14 | 17.35 | 18.38 | 18.66 |
| Na2O | 0.00 | 0.00 | 0.02 | 0.01 | 0.02 | 0.01 | 1.38 | 1.65 | 0.49 | 1.22 | 0.75 | 0.60 |
| K2O | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| Total | 99.51 | 99.06 | 99.80 | 99.00 | 98.98 | 98.22 | 99.02 | 98.77 | 99.50 | 98.91 | 98.54 | 98.63 |
| An | 87.21 | 84.81 | 95.61 | 88.69 | 93.11 | 94.47 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Li, K.; Zhang, Y. Gemological Characteristics and Chemical Composition of a New Type of Black Jadeite and Three Imitations. Crystals 2022, 12, 658. https://doi.org/10.3390/cryst12050658
Zheng B, Li K, Zhang Y. Gemological Characteristics and Chemical Composition of a New Type of Black Jadeite and Three Imitations. Crystals. 2022; 12(5):658. https://doi.org/10.3390/cryst12050658
Chicago/Turabian StyleZheng, Beiqi, Ke Li, and Yuyang Zhang. 2022. "Gemological Characteristics and Chemical Composition of a New Type of Black Jadeite and Three Imitations" Crystals 12, no. 5: 658. https://doi.org/10.3390/cryst12050658
APA StyleZheng, B., Li, K., & Zhang, Y. (2022). Gemological Characteristics and Chemical Composition of a New Type of Black Jadeite and Three Imitations. Crystals, 12(5), 658. https://doi.org/10.3390/cryst12050658






