WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Wide Angle X-ray Scattering Analysis
3.2. Small Angle X-ray Scattering Analysis
3.3. Differential Scanning Calorimetry Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Gludovatz, B.; Schaible, E.; Dave, N.K.N.; Yang, W.; Meyers, M.A.; Ritchie, R.O. Mechanical adaptability of the Bouligand-type structure in natural dermal armour. Nat. Commun. 2013, 4, 2634. [Google Scholar] [CrossRef] [Green Version]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef]
- Squire, J.M.; Freundlich, A. Direct observation of a transverse periodicity in collagen fibrils. Nature 1980, 288, 410–413. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Li, W. Modelling methods for In Vitro biomechanical properties of the skin: A review. Biomed. Eng. Lett. 2015, 5, 241–250. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular biology of the cell. 4th edn. Ann. Bot. 2003, 91, 401. [Google Scholar]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, L.S.; Park, P.J.; Kim, S.K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef] [PubMed]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Ski. Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; Melo, M.O.; Siqueira César, F.C. Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J. Cosmet. Derm. 2019, 18, 1693–1699. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Luo, Y.; Zhang, S.; Li, B. Effects of collagen peptides intake on skin ageing and platelet release in chronologically aged mice revealed by cytokine array analysis. J. Cell Mol. Med. 2018, 22, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545. [Google Scholar] [CrossRef] [Green Version]
- Altamura, D.; Lassandro, R.; Vittoria, F.; De Caro, L.; Siliqi, D.; Ladisa, M.; Giannini, C. X-ray microimaging laboratory (XMI-LAB). J. Appl. Crystallogr. 2012, 45, 899. [Google Scholar] [CrossRef]
- Siliqi, D.; De Caro, L.; Ladisa, M.; Scattarella FMazzone, A.; Altamura, D.; Sibillano, T.; Giannini, C. SUNBIM: A package for X-ray imaging of nano- and biomaterials using SAXS, WAXS, GISAXS and GIWAXS techniques. J. Appl. Crystallogr. 2016, 49, 1107–1114. [Google Scholar] [CrossRef]
- Salvatore, L.; Calò, E.; Bonfrate, V.; Pedone, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Madaghiele, M. Exploring the effects of the crosslink density on the physicochemical properties of collagen-based scaffolds. Polym. Test. 2021, 93, 106966. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Curci, C.; Picerno, A.; Gallone, A.; Vulpi, M.; Vitarelli, A.; Ditonno, P.; Cascione, M.; Sallustio, F.; et al. Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration. Materials 2021, 14, 7648. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.G. THE SKIN|Functional morphology of the integumentary system in fishes. In Encyclopedia of Fish Physiology; Academic Press: Cambridge, MA, USA, 2011; pp. 476–488. [Google Scholar]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydratation structure of collagen peptide. Structure 1995, 3, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Bella, J. A new method for describing the helical conformation of collagen: Dependence of the tripe helical twist on amino acid sequence. J. Struct. Biol. 2010, 170, 377–391. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [Green Version]
- Sibillano, T.; Terzi, A.; De Caro, L.; Ladisa, M.; Altamura, D.; Moliterni, A.; Lassandro, R.; Scattarella, F.; Siliqi, D.; Giannini, C. Wide Angle X-ray Scattering to Study the Atomic Structure of Polymeric Fibers. Crystals 2020, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; De Caro, L.; et al. Investigation of processing-induced structural changes in horse type-I collagen at sub and supramolecular levels. Front. Bioeng. Biotechnol. 2019, 7, 203. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H.C. The Molecular Structure of Collagen. J. Mol. Biol. 1971, 3, 483–506. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Rault, I.; Frei, V.; Herbage, D.; Abdul-Malak, N.; Huc, A. Evaluation of different chemical methods for cros-linking collagen gel, films and sponges. J. Mater. Sci. Mater. Med. 1996, 7, 215–221. [Google Scholar] [CrossRef]
- Jafari, H.; Alberto Lista, A.; Manuela Mafosso Siekapen, M.M.; Pejman Ghaffari-Bohlouli, P.G.; Lei Nie, L.; Alimoradi, H.; Shavandi, A. Composition of Collagen Extracted from the Skin of Three Different Varieties of Fish. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.F.; Von Hippel, P.H. The Structure Of Collagen And Gelatin. Adv. Protein Chem. 1962, 16, 1–138. [Google Scholar]
- Flandin, F.; Buffevant, C.; Herbage, D. A differential scanning calorimetry analysis of the age-related changes in the thermal stability of rat skin collagen. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1984, 791, 205–211. [Google Scholar] [CrossRef]
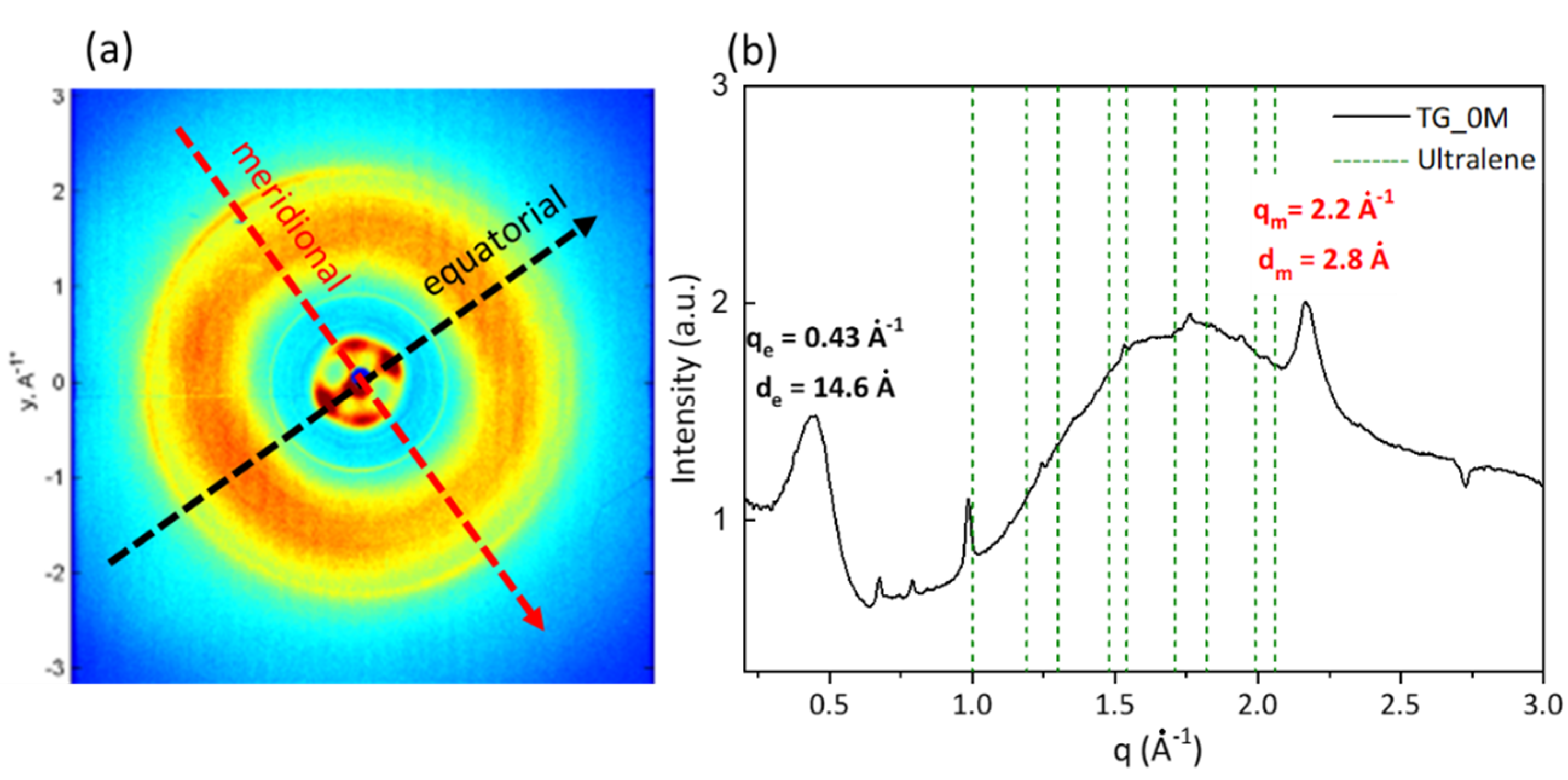
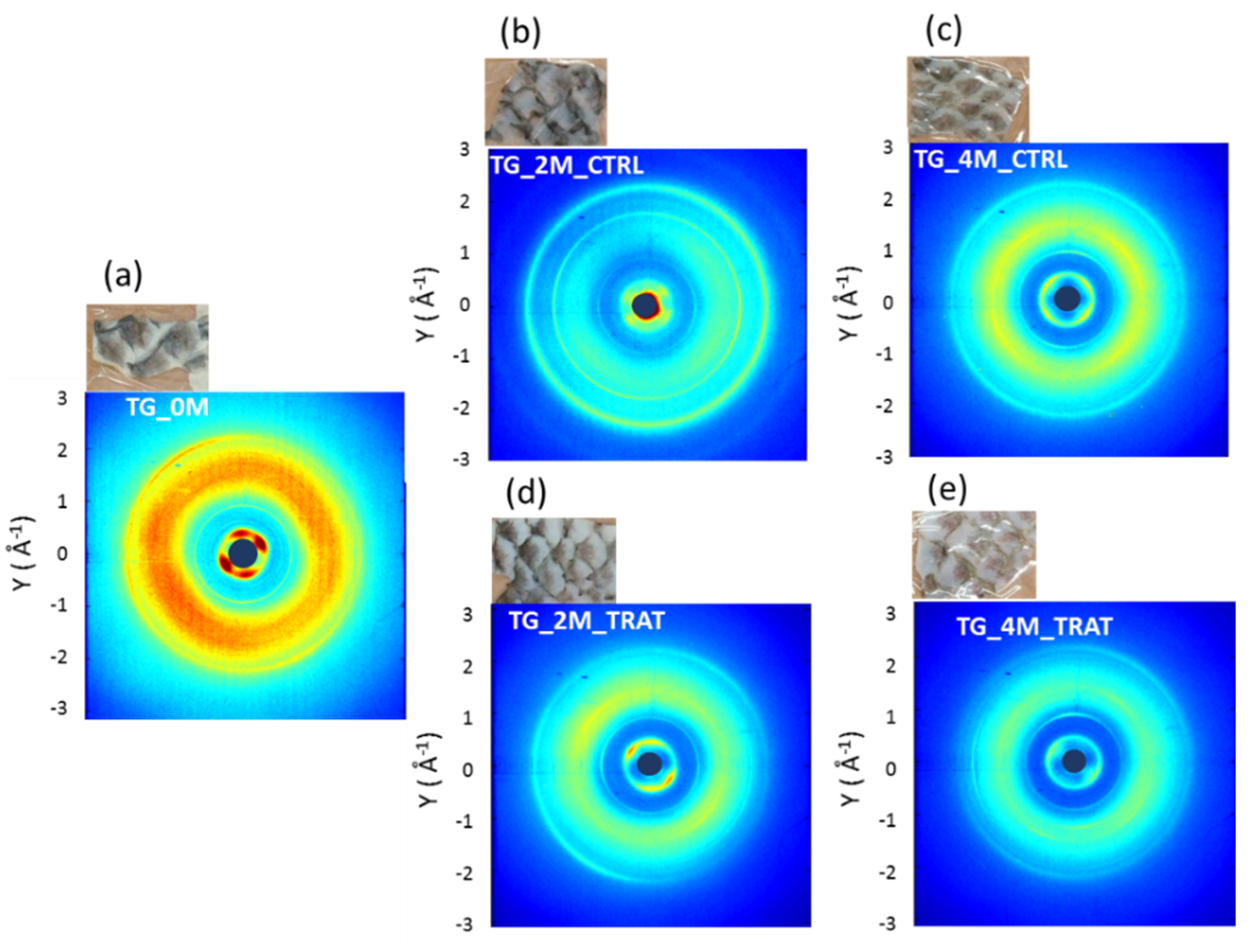




| 0 Month | 2 Months | 4 Months | |
|---|---|---|---|
| no collagen-rich diet | TG_0M | TG_2M_CTRL | TG_4M_CTRL |
| collagen-rich diet | / | TG_2M_TRAT | TG_4M_TRAT |
| Sample | Td (°C) | ΔH (J/g) |
|---|---|---|
| TG_0M | 66.2 ± 0.4 | 19.7 ± 1.5 |
| TG_2M_CTRL | 66.6 ± 0.3 | 21.6 ± 1.5 |
| TG_2M_TRAT | 65.8 ± 0.3 | 34.5 ± 3.0 |
| TG_4M_CTRL | 66.5 ± 0.3 | 27.9 ± 1.9 |
| TG_4M_TRAT | 67.3 ± 0.1 | 32.3 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzi, A.; Sibillano, T.; De Caro, L.; Altamura, D.; Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L.; Blasi, F.S.; Corallo, A.; et al. WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant. Crystals 2022, 12, 700. https://doi.org/10.3390/cryst12050700
Terzi A, Sibillano T, De Caro L, Altamura D, Gallo N, Natali ML, Sannino A, Salvatore L, Blasi FS, Corallo A, et al. WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant. Crystals. 2022; 12(5):700. https://doi.org/10.3390/cryst12050700
Chicago/Turabian StyleTerzi, Alberta, Teresa Sibillano, Liberato De Caro, Davide Altamura, Nunzia Gallo, Maria Lucia Natali, Alessandro Sannino, Luca Salvatore, Federica Stella Blasi, Angelo Corallo, and et al. 2022. "WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant" Crystals 12, no. 5: 700. https://doi.org/10.3390/cryst12050700
APA StyleTerzi, A., Sibillano, T., De Caro, L., Altamura, D., Gallo, N., Natali, M. L., Sannino, A., Salvatore, L., Blasi, F. S., Corallo, A., & Giannini, C. (2022). WAXS and SAXS Investigation of Collagen-Rich Diet Effect on Multiscale Arrangement of Type I Collagen in Tilapia Skin Fed in Aquaponics Plant. Crystals, 12(5), 700. https://doi.org/10.3390/cryst12050700








