Controlled Synthesis and Photoresponsive Properties of Spiropyran End-Functionalized Poly(vinyl ether)s
Abstract
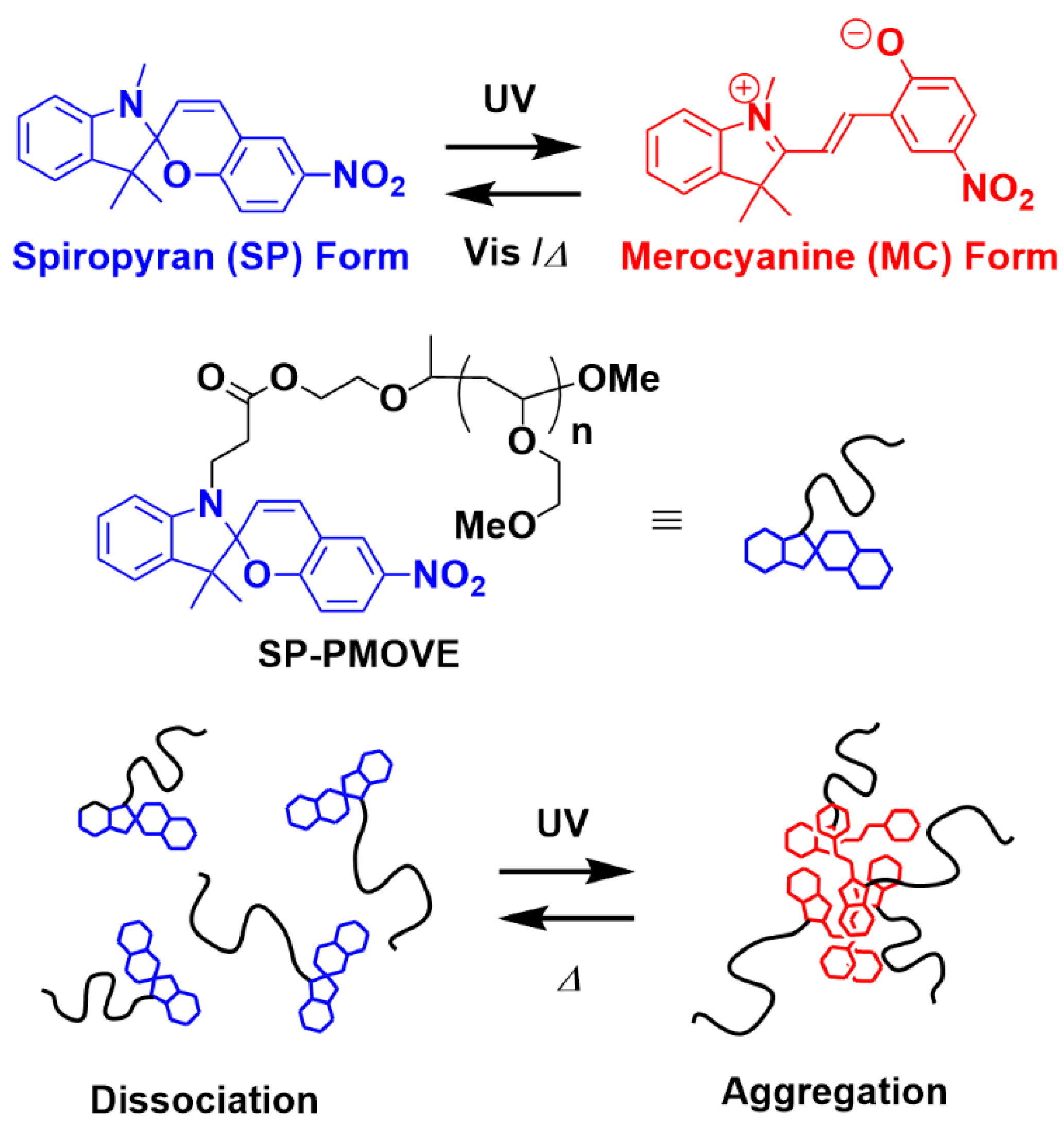
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Methods
2.3. Synthesis of SPVE
2.4. Cationic Polymerization of Vinyl Ether Monomers with SPVE-TFA
3. Results and Discussion
3.1. Synthesis of SPVE-TFA
3.2. Cationic Polymerization of IBVE Monomer with SPVE-TFA

3.3. Synthesis of SP-PMOVE
3.4. Photoresponsive Properties of SP-PMOVE
3.5. Self-Assembly of SP-PMOVE via Photoirradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vriezem, D.M.; Aragonès, M.C.; Elemans, J.A.A.W.; Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M. Self-assembled nanoreactors. Chem. Rev. 2005, 105, 1445–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, D.D.; Kühbeck, D.; Koopmans, R.J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems based on magnetic nanoparticles and thermo- or pH-responsive polymers: An update and future perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-based smart drug delivery systems for skin application and demonstration of stimuli-responsiveness. Polymers 2021, 13, 1285. [Google Scholar] [CrossRef]
- Scott, P.J.; Kasprzak, C.R.; Feller, K.D.; Meenakshisundaram, V.; Williams, C.B.; Long, T.E. Light and latex: Advances in the photochemistry of polymer colloids. Polym. Chem. 2020, 11, 3498–3524. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef]
- Weis, P.; Wu, S. Light-switchable azobenzene-containing macromolecules: From UV to near infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef] [Green Version]
- Minkin, V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Chen, S.; Cao, Z.; Wang, G. A photo, temperature, and pH responsive spiropyran-functionalized polymer: Synthesis, self-assembly and controlled release. Polymer 2016, 83, 85–91. [Google Scholar] [CrossRef]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.M.; Tovar, J.D. Pendant photochromic conjugated polymers incorporating a highly functionalizable thieno[3,4-b]thiophene switching motif. J. Am. Chem. Soc. 2019, 141, 3146–3152. [Google Scholar] [CrossRef]
- Ipe, B.I.; Mahima, S.; Thomas, K.G. Light-induced modulation of self-assembly on spiropyran-capped gold nanoparticles: A potential system for the controlled release of amino acid derivatives. J. Am. Chem. Soc. 2003, 125, 7174–7175. [Google Scholar] [CrossRef]
- Zhu, M.-Q.; Zhu, L.; Han, J.J.; Wu, W.; Hurst, J.K.; Li, A.D.Q. Spiropyran-based photochromic polymer nanoparticles with optically switchable luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. [Google Scholar] [CrossRef] [Green Version]
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran-Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087. [Google Scholar] [CrossRef]
- Choi, W.O.; Sawamoto, M.; Higashimura, T. Living cationic polymerization of 2-phenoxyethyl vinyl ether and its ring-substituted derivatives: Effects of para-substituents. Polym. J. 1987, 19, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Motoyanagi, J.; Miyabara, R.; Suzuki, M.; Miki, S.; Minoda, M. A novel [60]fullerene-appended initiator for living cationic polymerization and its application to the synthesis of [60]fullerene-end-capped poly(vinyl ether)s. Polym. Chem. 2012, 3, 329–331. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Kurata, A.; Minoda, M. Self-assembly behavior of amphiphilic C60-end-capped poly(vinyl ether)s in water and dissociation of the aggregates by the complexing of the C60 moieties with externally added γ-cyclodextrins. Langmuir 2015, 31, 2256–2261. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Higashi, K.; Minoda, M. Synthesis of brush-shaped polymers consisting of a poly(phenylacetylene) backbone and pendant poly(vinyl ether)s via selective reaction of 2-vinyloxyethyl 4-ethynylbenzoat. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2800–2805. [Google Scholar] [CrossRef]
- Ishikawa, T.; Motoyanagi, J.; Minoda, M. Synthesis of brush-shaped π-conjugated polymers based on well-defined thiophene-end-capped poly(vinyl ether)s. Chem. Lett. 2016, 45, 415–417. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Ishikawa, T.; Minoda, M. Stimuli-responsive brush-shaped conjugated polymers with pendant well-defined poly(vinyl ether)s. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3318–3325. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Kawamura, S.; Minoda, M. Controlled synthesis of poly(vinyl ether)-grafted poly(phenylacetylene)s by a combination of living coordination polymerization and living cationic polymerization. ACS Omega 2020, 5, 5854–5861. [Google Scholar] [CrossRef]
- Wu, S.; Lu, J.; Zeng, F.; Chen, Y.; Tong, Z. Photoinduced formation of microscopic ordering and macroscopic pattern in spiropyran-containing polyacrylate-tetraoctylammonium bromide films. Macromolecules 2007, 40, 5060–5066. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1753. [Google Scholar] [CrossRef]
- Suzuki, T.; Kato, T.; Shinozaki, H. Photo-reversible Pb2+-complexation of thermosensitive poly(N-isopropyl acrylamide-co-spiropyran acrylate) in water. Chem. Commun. 2004, 2036–2037. [Google Scholar] [CrossRef]
- Wojtyk, J.T.C.; Wasey, A.; Kazmaier, P.M.; Hoz, S.; Buncel, E. Thermal reversion mechanism of N-functionalized merocyanines to spiropyrans: A solvatochromic, solvatokinetic, and semiempirical study. J. Phys. Chem. A 2000, 104, 9046–9055. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoyanagi, J.; Fujishima, A.; Minoda, M. Controlled Synthesis and Photoresponsive Properties of Spiropyran End-Functionalized Poly(vinyl ether)s. Crystals 2022, 12, 742. https://doi.org/10.3390/cryst12050742
Motoyanagi J, Fujishima A, Minoda M. Controlled Synthesis and Photoresponsive Properties of Spiropyran End-Functionalized Poly(vinyl ether)s. Crystals. 2022; 12(5):742. https://doi.org/10.3390/cryst12050742
Chicago/Turabian StyleMotoyanagi, Jin, Ayane Fujishima, and Masahiko Minoda. 2022. "Controlled Synthesis and Photoresponsive Properties of Spiropyran End-Functionalized Poly(vinyl ether)s" Crystals 12, no. 5: 742. https://doi.org/10.3390/cryst12050742
APA StyleMotoyanagi, J., Fujishima, A., & Minoda, M. (2022). Controlled Synthesis and Photoresponsive Properties of Spiropyran End-Functionalized Poly(vinyl ether)s. Crystals, 12(5), 742. https://doi.org/10.3390/cryst12050742





