ZnO under Pressure: From Nanoparticles to Single Crystals
Abstract
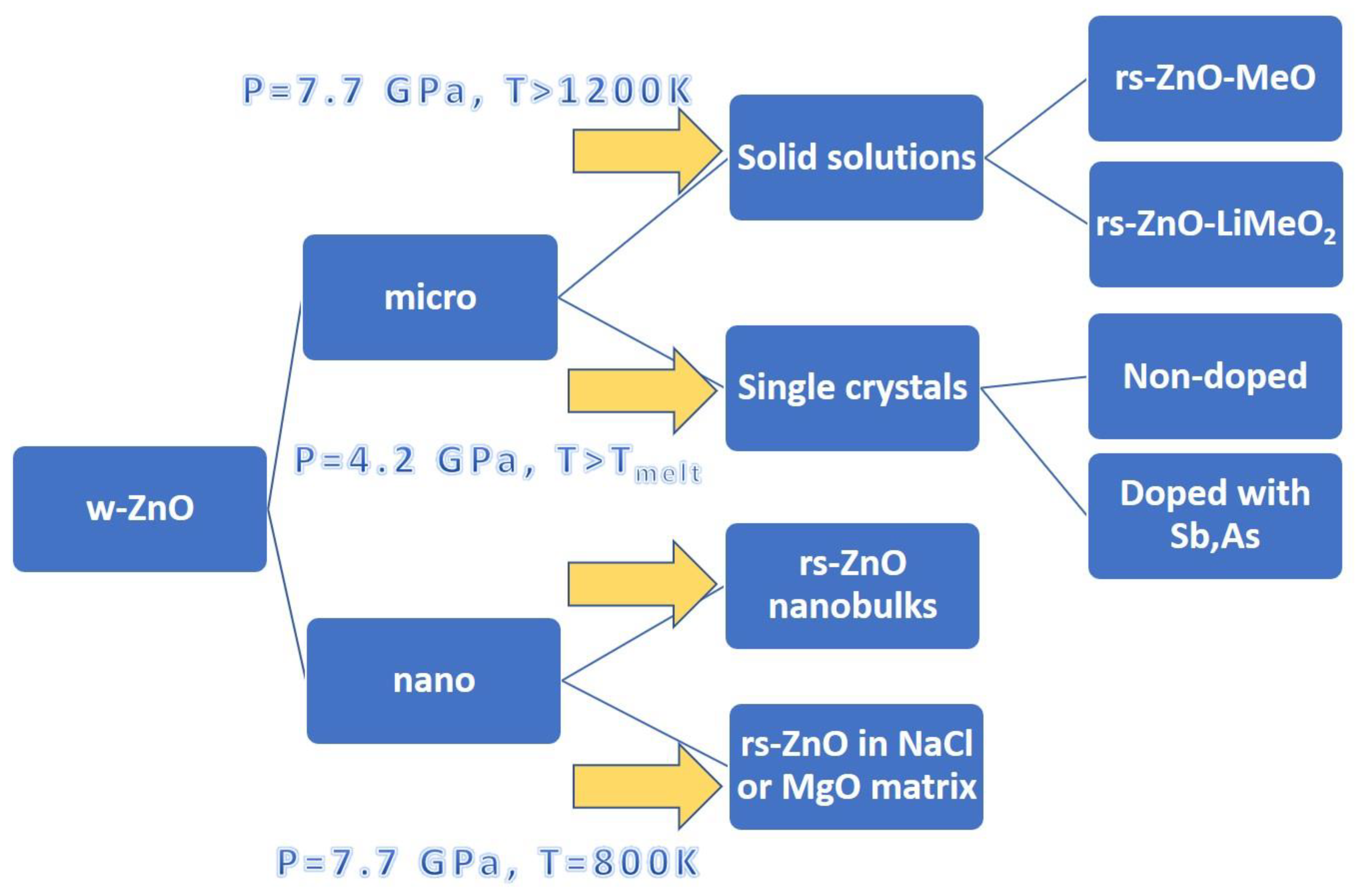
:1. Introduction
- Synthesis of cubic ZnO solid solutions with MeO (where Me = Mg2+, Ni2+, Co2+, Fe2+, Mn2+) and with LiMeO2 (where Me = Sc3+, Ti3+, Fe3+, In3+), and their thermal stability at ambient pressure.
- Synthesis of rs-ZnO using the nanocrystalline state of the initial wurtzite phase, including synthesis in the sodium chloride and magnesium oxide matrixes.
- Growth of zinc oxide single crystals from melts at high pressures and high temperatures (HP and HT).
2. Results and Discussion
2.1. Wurtzite ZnO
2.2. w-ZnO → rs-ZnO Phase Transition
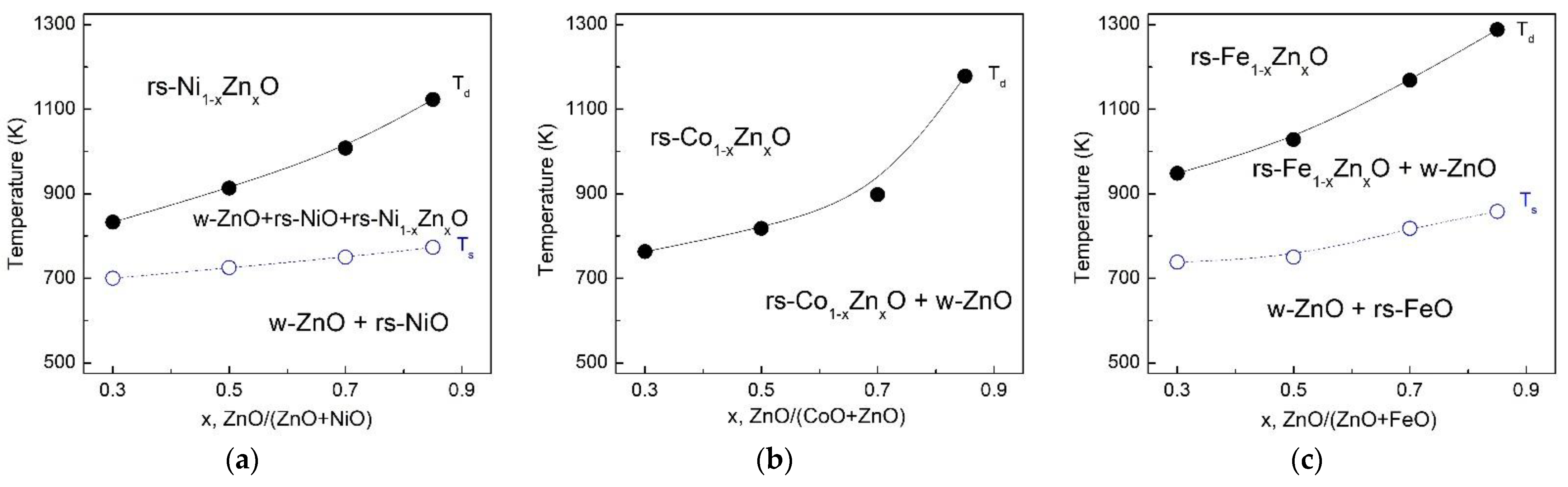
2.3. Stabilization of rs-ZnO in the Form of Solid Solutions
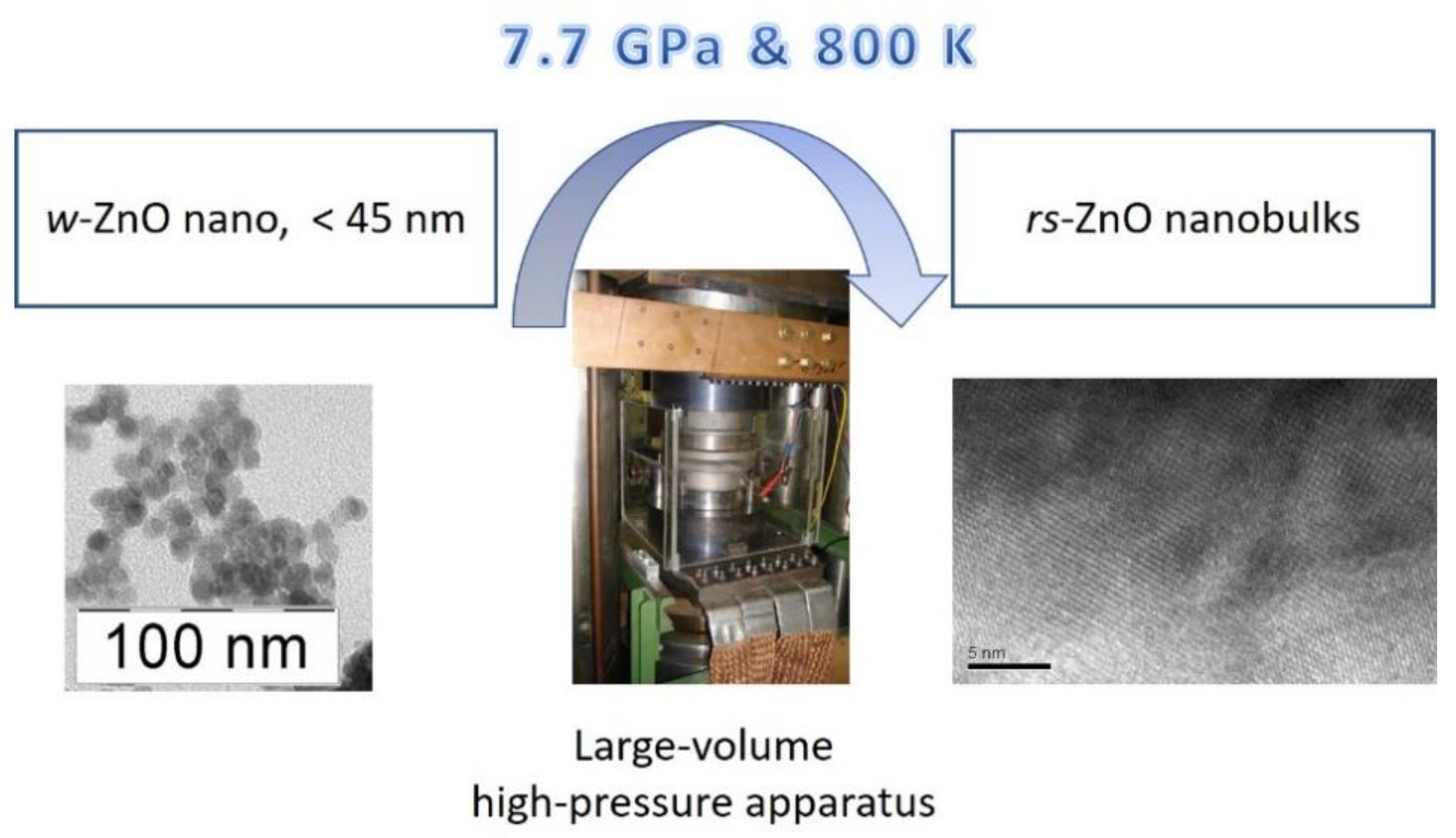
2.4. Synthesis of Single-Phase rs-ZnO Nanobulks
2.5. Melting and Crystallization of Zinc Oxide under Pressure—Growth of Single Crystals
2.6. Growth of Doped ZnO Single Crystals under Pressure
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearton, S.J.; Jagadish, C. Zinc Oxide: Bulk, Thin Films and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yun, F.; Morkoc, H. Ferromagnetism of ZnO and GaN: A Review. J. Mater. Sci. Mater. Electron. 2005, 16, 555–597. [Google Scholar] [CrossRef]
- Bates, C.H.; White, W.B.; Roy, R. New high-pressure polymorph of zinc oxide. Science 1962, 137, 993. [Google Scholar] [CrossRef]
- Bates, C.H.; White, W.B.; Roy, R. The solubility of transition metal oxides in zinc oxide and the reflectance spectra of Mn2+ and Fe2+ in tetrahedral fields. J. Inorg. Nucl. Chem. 1966, 28, 397–405. [Google Scholar] [CrossRef]
- Ellmer, K.; Klein, A.; Rech, D. Transparent Conductive Zinc Oxide; Springer: Berlin, Germany, 2008. [Google Scholar] [CrossRef]
- Kumar, N.; Yadav, S.; Mittal, A.; Kumari, K. Photocatalysis by zinc oxide-based nanomaterials. In Nanostructured Zinc Oxide. Synthesis, Properties and Applications; Awasthi, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Musselman, K.P.; Macmanus-Driscoll, J.L. Research Update: Doping ZnO and TiO2 for solar cells. APL Mater. 2013, 1, 060701. [Google Scholar] [CrossRef]
- Kunisu, M.; Tanaka, I.; Yamamoto, T.; Suga, T.; Mizoguchi, T. The formation of a rock-salt type ZnO thin film by low-level alloying with MgO. J. Phys. Condens. Matter 2004, 16, 3801. [Google Scholar] [CrossRef]
- James Lu, C.-Y.; Tu, Y.-T.; Yan, T.; Trampert, A.; Chang, L.; Ploog, K.H. Growth and stability of rocksalt Zn1−xMgxO epilayers and ZnO/MgO superlattice on MgO (100) substrate by molecular beam epitaxy. J. Chem. Phys. 2016, 144, 214704. [Google Scholar] [CrossRef]
- Egbo, K.O.; Chibueze, T.C.; Raji, A.T.; Ekuma, C.E.; Liu, C.P.; Yu, K.M. Effects of acceptor doping and oxygen stoichiometry on the properties of sputter-deposited p-type rocksalt NixZn1−xO (0.3 ≤ x ≤ 1.0) alloys. J. Alloys Compd. 2022, 905, 164224. [Google Scholar] [CrossRef]
- Liu, C.P.; Egbo, K.O.; Ho, C.Y.; Wang, Y.; Xu, C.K.; Yu, K.M. Wide-Gap Zn1−xNixO alloy: A transparent p-type oxide. Phys. Rev. Appl. 2020, 13, 024049. [Google Scholar] [CrossRef]
- Baranov, A.N.; Solozhenko, V.L.; Chateau, C.; Bocquillon, G.; Petitet, J.P.; Panin, G.N.; Kang, T.W.; Shpanchenko, R.V.; Antipov, E.V.; Oh, Y.J. Cubic MgxZn1–xO wide band gap solid solutions synthesized at high pressures. J. Phys. Cond. Matter 2005, 7, 3377–3384. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Baranov, A.N.; Turkevich, V.Z. High-pressure formation of MgxZn1−xO solid solutions with rock salt structure. Solid State Commun. 2006, 138, 534–537. [Google Scholar] [CrossRef] [Green Version]
- Baranov, A.N.; Sokolov, P.S.; Tafeenko, V.A.; Lathe, C.; Zubavichus, Y.V.; Veligzhanin, A.A.; Chukichev, M.V.; Solozhenko, V.L. Nanocrystallinity as a route to metastable phases: Rock salt ZnO. Chem. Mater. 2010, 25, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Klimm, D.; Ganschow, S.; Schulz, D.; Fornari, R. The growth of ZnO crystals from the melt. J. Cryst. Growth 2008, 310, 3009–3013. [Google Scholar] [CrossRef] [Green Version]
- Kusaba, K.; Syonom, Y.; Kikegawam, T. Phase transition of ZnO under high pressure and temperature. Proc. Jpn. Acad. Ser. B 1999, 75, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, doping and processing. Mater. Today 2004, 7, 34–40. [Google Scholar] [CrossRef]
- Norton, D.P.; Pearton, S.J.; Hebard, A.F.; Theodoropoulou, N.; Boatner, L.A.; Wilson, R.G. Ferromagnetism in Mn-implanted ZnO:Sn single crystal. Appl. Phys. Lett. 2003, 82, 239–241. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, T.; Toyosaki, H.; Yamada, Y. Magnetic oxide semiconductors. Semicond. Sci. Technol. 2005, 20, S103–S111. [Google Scholar] [CrossRef] [Green Version]
- Makino, T.; Segawa, Y.; Kawasaki, M.; Ohtomo, A.; Shiroki, R.; Tamura, K.; Yasuda, T.; Koinuma, H. Band gap engineering based on MgxZn1–xO and CdyZn1–yO ternary alloy films. Appl. Phys. Lett. 2001, 78, 1237–1239. [Google Scholar] [CrossRef] [Green Version]
- Decremps, F.; Zhang, J.; Liebermann, R.C. New phase boundary and high-pressure thermoelasticity of ZnO. Europhys. Lett. 2000, 51, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.K.; Navrotsky, A. Thermodynamics of solid solution formation in NiO-MgO and NiO-ZnO. J. Solid State Chem. 1981, 38, 264–276. [Google Scholar] [CrossRef]
- Seko, A.; Obam, F.; Kuwabara, A.; Tanaka, I. Pressure-induced phase transition in ZnO and ZnO-MgO pseudobinary system: A first-principles lattice dynamics study. Phys. Rev. B 2005, 72, 024107. [Google Scholar] [CrossRef]
- Sowa, H.; Ahsbahs, H. High-pressure X-ray investigation of zincite ZnO single crystal using diamond anvils with an improved shape. J. Appl. Crystallogr. 2006, 39, 169–175. [Google Scholar] [CrossRef]
- Decremps, F.; Zhang, J.; Li, B.; Liebermann, R.C. Pressure-induced softening of shear modes in ZnO. Phys. Rev. B 2002, 63, 224105. [Google Scholar] [CrossRef]
- Segura, A.; Sans, J.A.; Manjon, F.J.; Munoz, A.; Herrera-Cabrera, M.J. Optical properties and electronic structure of rock-salt ZnO under pressure. Appl. Phys. Lett. 2003, 83, 278–280. [Google Scholar] [CrossRef]
- Sans, J.A.; Segura, A.; Manjon, F.J.; Mari, B.; Munoz, A.; Herrera-Cabrera, M.J. Optical properties of wurtzite and rock-salt ZnO under pressure. Microelectr. J. 2005, 36, 928–932. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Olsen, J.S.; Gerward, L.; Frost, D.; Rubie, D.; Peyronneau, J. Structural stability in nanocrystalline ZnO. Europhys. Lett. 2000, 50, 48–53. [Google Scholar] [CrossRef]
- Yu, C.; Yu, Q.; Gao, C.; Yang, H.; Li, B.; Peng, G.; Han, Y.; Zhang, D.; Cui, X.; Liu, C.; et al. Phase transformation and resistivity of dumbbell-like ZnO microcrystals under high pressure. J. Appl. Phys. 2008, 103, 114901. [Google Scholar] [CrossRef]
- Decremps, F.; Datchi, F.; Saitta, A.M.; Polian, A.; Pascarelli, S.; Di Cicco, A.; Itie, J.P.; Baudelet, F. Local structure of condensed zinc oxide. Phys. Rev. B 2003, 68, 104101. [Google Scholar] [CrossRef] [Green Version]
- Karzel, H.; Potzel, W.; Köfferlein, M.; Schiessl, W.; Steiner, M.; Hiller, U.; Kalvius, G.M.; Mitchell, D.W.; Das, T.P.; Blaha, P.; et al. Lattice dynamics and hyperfine interactions in ZnO and ZnSe at high external pressures. Phys. Rev. B 1996, 53, 11425–11438. [Google Scholar] [CrossRef] [PubMed]
- Decremps, F.; Pellicer-Porres, J.; Marco Saitta, A.; Chervin, J.-C.; Polian, A. High-pressure Raman spectroscopy study of wurtzite ZnO. Phys. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef] [Green Version]
- Manjon, F.J.; Syassen, K.; Lauck, R. Effect of pressure on phonon modes in wurtzite zinc oxide. High Press. Res. 2002, 22, 299–304. [Google Scholar] [CrossRef]
- Bayarjargal, L.; Winkler, B.; Haussuhl, E.; Boehler, R. Influence of deviatoric stress on the pressure-induced structural phase transition of ZnO studied by optical second harmonic generation measurements. Appl. Phys. Lett. 2009, 95, 061907. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Gerward, L.; Frost, D.; Secco, R.; Peyronneau, J.; Olsen, J.S. Grain-size effect on pressure-induced semiconductor-to-metal transition in ZnS. J. Appl. Phys. 1999, 86, 6608–6610. [Google Scholar] [CrossRef] [Green Version]
- Baranov, A.N.; Sokolov, P.S.; Kurakevych, O.O.; Tafeenko, V.A.; Trots, D.; Solozhenko, V.L. Synthesis of rock-salt MeO–ZnO solid solutions (Me = Ni2+, Co2+, Fe2+, Mn2+) at high pressure and high temperature. High Press. Res. 2008, 4, 515. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S. The synthesis of cubic ZnO and its solid solutions at high pressures and high temperatures. Ph.D. Thesis, Université Paris Nord, Villetaneuse, France, 2010. [Google Scholar]
- Bhadram, V.S.; Cheng, Q.; Chan, C.K.; Liu, Y.; Lany, S.; Landskron, K.; Strobel, T.A. ZnxMn1–xO solid solutions in the rocksalt structure: Optical, charge transport, and photoelectrochemical properties. ACS Appl. Energy Mater. 2018, 1, 260. [Google Scholar] [CrossRef]
- Sokolov, P.S.; Baranov, A.N.; Lathe, C.; Solozhenko, V.L. High-pressure synthesis of FeO–ZnO solid solutions with rock salt structure: In situ X-ray diffraction studies. High Press. Res. 2010, 30, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Lathe, C.; Turkevich, V.Z.; Solozhenko, V.L. High-pressure synthesis of MnO–ZnO solid solutions with rock salt structure: In situ X-ray diffraction studies. High Press. Res. 2011, 31, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Tafeenko, V.A.; Solozhenko, V.L. High pressure synthesis of LiMeO2–ZnO (Me = Fe3+, Ti3+) solid solutions with a rock salt structure. High Press. Res. 2011, 31, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Raghavan, S.; Hajra, J.P.; Iyengar, G.N.K.; Abraham, K.P. Terminal solid solubilities at 900–1000 °C in the magnesium oxide-zinc oxide system measured using a magnesium fluoride solid-electrolyte galvanic cell. Thermochim. Acta 1991, 189, 151–158. [Google Scholar] [CrossRef]
- Sokolov, P.S.; Baranov, A.N.; Dobrokhotova, Z.V.; Solozhenko, V.L. Synthesis and thermal stability of cubic ZnO in the salt nanocomposites. Russ. Chem. Bull. 2010, 59, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Tafeenko, V.A.; Solozhenko, V.L. Synthèse des solutions solides cubiques ZnO-LiMeO2 (Me = Sc3+, Ti3+, Fe3+, In3+) sous hautes pressions et hautes températures. In Proceedings of the 7e Forum de Technologie des Hautes Pressions, Biarritz, France, 11–15 October 2010. [Google Scholar]
- Fernandes, A.A.R.; Santamaria, J.; Bud’ko, S.L.; Nakamura, O.; Guimpel, J.; Schuller, I.K. Effect of physical and chemical pressure on the superconductivity of high-temperature oxide superconductors. Phys. Rev. B 1991, 44, 7601. [Google Scholar] [CrossRef] [PubMed]
- Urusov, V.S. Interaction of Cations on Octahedral and Tetrahedral Sites in Simple Spinels. Phys. Chem. Miner. 1983, 10, 194–195. [Google Scholar] [CrossRef]
- Maksimov, V.I.; Dubinin, S.F.; Baranov, A.N.; Sokolov, V.I.; Sokolov, P.S.; Parkhomenko, V.D. Structural state of metastable cubic compounds Ni1−xZnxO (0.6≤x≤0.99). Phys. Met. Metallogr. 2013, 114, 734–740. [Google Scholar] [CrossRef]
- Dubinin, S.F.; Maksimov, V.I.; Parkhomenko, V.D.; Sokolov, V.I.; Baranov, A.N.; Sokolov, P.S.; Dorofeev, Y.A. Fine structure and magnetism of the cubic oxide compound Ni0.3Zn0.7O. Phys. Solid State 2011, 53, 1362–1366. [Google Scholar] [CrossRef]
- Gurskiy, S.I.; Tafeenko, V.A.; Baranov, A.N. Use of integrated intensities of X-ray powder diffraction patterns of Mg1−xZnxO solid solutions for the quantitative determination of their composition. Russ. J. Inorg. Chem. 2008, 53, 111–116. [Google Scholar] [CrossRef]
- Babanov, Y.A.; Ponomarev, D.A.; Ustinov, V.V. Visualization of the atomic structure of solid solutions with the NaCl structure. Phys. Solid State. 2015, 57, 717–721. [Google Scholar] [CrossRef]
- Babanov, Y.A.; Ponomarev, D.A.; Ustinov, V.V.; Baranov, A.N.; Zubavichus, Y.V. Local atomic structure of solid solutions with overlapping shells by EXAFS: The regularization method. J. Electron Spectrosc. 2016, 211, 1–11. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Pustovarov, V.A.; Churmanov, V.N.; Ivanov, V.Y.; Gruzdev, N.B.; Sokolov, P.S.; Baranov, A.N.; Moskvin, A.S. Unusual x-ray excited luminescence spectra of NiO suggest self-trapping of the d-d charge-transfer exciton. Phys. Rev. B 2012, 86, 115128. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, V.I.; Pustovarov, V.A.; Churmanov, V.N.; Ivanov, V.Y.; Gruzdev, N.B.; Sokolov, P.S.; Baranov, A.N.; Moskvin, A.S. Self-trapping of the d-d charge transfer exciton in bulk NiO evidenced by X-ray excited luminescence. JETP Lett. 2012, 95, 528–533. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Pustovarov, V.A.; Churmanov, V.N.; Ivanov, V.Y.; Gruzdev, N.B.; Sokolov, P.S.; Baranov, A.N.; Moskvin, A.S. Self-trapping of the d-d charge transfer exciton in rock-salt structured Zn1–xNixO evidenced by soft X-ray excited luminescence. Phys. Status Solidi C 2013, 10, 1329–1335. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Pustovarov, V.A.; Ivanov, V.Y.; Gruzdev, N.B.; Sokolov, P.S.; Baranov, A.N. The influence of temperature on narrow I1 and I2 lines in the luminescence spectrum of Ni0.6Zn0.4O. Opt. Spectrosc. 2014, 116, 798–801. [Google Scholar] [CrossRef]
- Churmanov, V.N.; Sokolov, V.I.; Gruzdev, N.B.; Ivanov, V.Y.; Pustovarov, V.A. Exciton lines in luminescence spectra of NixZn1−xO under inner shell excitation. Phys. Procedia 2015, 76, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, V.I.; Pustovarov, V.A.; Churmanov, V.N.; Gruzdev, N.B.; Uimin, M.A.; Byzov, I.V.; Druzhinin, A.V.; Mironova-Ulmane, N.A. Luminescence and optical spectroscopy of charge transfer processes in solid solutions NicMg1−cO and NixZn1−xO. J. Lumin. 2016, 169, 641–644. [Google Scholar] [CrossRef]
- Churmanov, V.N.; Sokolov, V.I.; Gruzdev, N.B.; Ivanov, V.Y.; Pustovarov, V.A. p-d charge transfer excitons in Zn1−xNixO under inner shell excitation. Phys. Status Solidi C 2016, 13, 610–613. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellani, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Li, B.; Sougrati, M.T.; Rousse, G.; Morozov, A.V.; Dedryvere, R.; Iadecola, A.; Senyshyn, A.; Zhang, L.; Abakumov, A.A.; Doublet, M.; et al. Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox. Nat. Chem. 2021, 13, 1070–1080. [Google Scholar] [CrossRef]
- Cambaz, M.A.; Vinayan, B.P.; Euchner, H.; Guda, A.A.; Mazilkin, A.; Rusalev, Y.V.; Trigub, A.L.; Gross, A.; Fichtner, M. Design of nickel-based cation-disordered rock-salt oxides: The effect of transition metal (M = V, Ti, Zr) substitution in LiNi0.5M0.5O2 binary systems. ACS Appl. Mater. Interfaces 2018, 10, 21957–21964. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, Y.; Hua, Y.; Han, Y.; Li, L. Low-temperature sintering and microwave dielectric properties of Li2ZnTi3O8 ceramics. Mater. Lett. 2013, 107, 351–353. [Google Scholar] [CrossRef]
- Yuan, L.L.; Bian, J.J. Microwave dielectric properties of the lithium containing compounds with rock salt structure. Ferroelectrics 2009, 387, 123–129. [Google Scholar] [CrossRef]
- Viegas, J.I.; Moreira, R.L.; Dias, A. Optical-vibration properties of Li2ZnGeO4 dielectric ceramics. Vib. Spectrosc. 2020, 110, 103130. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P. Microstructure and microwave dielectric properties of low loss materials Li3(Mg0.95A0.05)2NbO6 (A = Ca2+, Ni2+, Zn2+, Mn2+) with rock-salt structure. J. Alloys Compds. 2016, 658, 744–748. [Google Scholar] [CrossRef]
- Kumar, R.S.; Cornelius, A.L.; Nicol, A.F. Structure of nanocrystalline ZnO up to 85 GPa. Curr. Appl. Phys. 2007, 7, 135–138. [Google Scholar] [CrossRef]
- Grzanka, E.; Gierlotkam, S.; Stelmakh, S.; Palosz, B.; Strachowski, T.; Swiderska-Srode, A.; Kalisz, G.; Lojkowski, W.; Porsch, F. Phase transition in nanocrystalline ZnO. Z. Kristallogr. 2006, 23, 337–342. [Google Scholar] [CrossRef]
- Bayarjargal, L.; Wiehl, L.; Winkler, B. Influence of grain size, surface energy, and deviatoric stress on the pressure-induced phase transition of ZnO and AlN. High Press. Res. 2013, 33, 642–651. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S. The high-pressure phase of zincite. J. Synchrotron Radiat. 1995, 2, 233–235. [Google Scholar] [CrossRef]
- Baranov, A.N.; Kurakevych, O.O.; Tafeenko, V.A.; Sokolov, P.S.; Panin, G.N.; Solozhenko, V.L. High-pressure synthesis and luminescent properties of cubic ZnO/MgO nanocomposites. J. Appl. Phys. 2010, 107, 073519. [Google Scholar] [CrossRef] [Green Version]
- Kamenev, K.V.; Courac, A.; Sokolov, P.S.; Baranov, A.N.; Sharikov, F.Y.; Solozhenko, V.L. Heat capacities of nanostructured wurtzite and rock salt ZnO: Challenges of ZnO nano-phase diagram. Solids 2021, 2, 121–128. [Google Scholar] [CrossRef]
- Sokolov, P.S.; Baranov, A.N.; Bell, A.M.T.; Solozhenko, V.L. Low-temperature thermal expansion of rock-salt ZnO. Solid State Commun. 2014, 177, 65–67. [Google Scholar] [CrossRef] [Green Version]
- Sharikov, F.Y.; Sokolov, P.S.; Baranov, A.N.; Solozhenko, V.L. On the thermodynamic aspect of zinc oxide polymorphism: Calorimetric study of metastable rock salt ZnO. Mendeleev Commun. 2017, 27, 613–614. [Google Scholar] [CrossRef] [Green Version]
- Leitner, J.; Kamrádek, M.; Sedmidubský, D. Thermodynamic properties of rock-salt ZnO. Thermochim. Acta 2013, 572, 1–5. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O.; Sokolov, P.S.; Baranov, A.N. Kinetics of the wurtzite to rock-salt phase transformation in ZnO at high pressure. J. Phys. Chem. A 2011, 115, 4354–4358. [Google Scholar] [CrossRef] [PubMed]
- Potuzak, M.; Dingwell, D.B.; Ledda, B.; Courtial, P. A partial molar volume for ZnO in silicate melts. Am. Mineral. 2006, 91, 366. [Google Scholar] [CrossRef]
- Iwanaga, H.; Kunishige, A.; Takeuchi, S. Anisotropic thermal expansion in wurtzite-type crystals. J. Mater. Sci. 2000, 35, 2451. [Google Scholar] [CrossRef]
- Belov, G.V.; Iorish, V.S.; Yungman, V.S. IVTANTHERMO for Windows—Database on Thermodynamic Properties and Related Software. Calphad 1999, 23, 173, Thermodynamic Data for w-ZnO and Liquid-ZnO. Available online: http://www.chem.msu.su/rus/handbook/ivtan/263.html (accessed on 20 May 2022). [CrossRef]
- Batsanov, S.S. Structural Chemistry. Book of Facts; Dialog-MSU: Moscow, Russia, 2000; p. 37, Table 1.27 (Data of National Metrological Institute of Russia, VNIIFRTI); Available online: http://www.chem.msu.su/rus/elibrary/bazanov/welcome.html (accessed on 20 May 2022).
- Thermodynamic Properties of Individual Substances (TSIV) Database (in Russian). Available online: http://www.chem.msu.su/rus/tsiv/Zn/ZnO_c.html (accessed on 20 May 2022).
- Kerridge, D.H.; Sturton, I.A. Fused zinc chloride part II: Some solubility measurements. Inorg. Chim. Acta 1974, 8, 27–30. [Google Scholar] [CrossRef]
- Mukhanov, V.A.; Sokolov, P.S.; Baranov, A.N.; Timoshenko, V.Y.; Zhigunov, D.M.; Solozhenko, V.L. Congruent melting and rapid single-crystal growth of ZnO at 4 GPa. CrystEngComm 2013, 32, 6318–6322. [Google Scholar] [CrossRef]
- Baranov, A.N.; Shestakov, M.V.; Chukichev, M.V.; Tafeenko, V.A.; Mukhanov, V.A.; Solozhenko, V.L. Cathodoluminescence of zinc and ytterbium oxide poly- and single crystals grown from melt under high pressure. Trends Phys. Chem. 2021, 21, 81–86. [Google Scholar]
- Fan, J.C.; Sreekanth, K.M.; Xie, Z.; Chang, S.L.; Rao, K.V. p-Type ZnO materials: Theory, growth, properties and devices. Prog. Mater. Sci. 2013, 58, 874–985. [Google Scholar] [CrossRef]
- Taibarei, N.O.; Kytin, V.G.; Konstantinova, E.A.; Kulbachinskii, V.A.; Shalygina, O.A.; Pavlikov, A.V.; Savilov, S.V.; Tafeenko, V.A.; Mukhanov, V.A.; Solozhenko, V.L.; et al. Doping nature of group V elements in ZnO single crystals grown from melts at high pressure. Cryst. Growth Des. 2022, 22, 2452–2461. [Google Scholar] [CrossRef]



| ZnO Mole Fraction, x | NiO-ZnO | MgO-ZnO | CoO-ZnO | FeO-ZnO | MnO-ZnO |
|---|---|---|---|---|---|
| 0.95 | w | w | w | w | w |
| 0.90 | w + rs | w | w | w | w |
| 0.80 | rs | w + rs | w | w | w + rs |
| 0.70 | rs | rs | w | w | w + rs |
| 0.60 | rs | rs | w + rs | rs + w | rs + w |
| 0.50 | rs | rs | rs | rs | rs + w |
| 0.40 | rs | rs | rs | rs | rs |
| 0.30 | rs | rs | rs | rs | rs |
| 0.20 | rs | rs | rs | rs | rs |
| 0.10 | rs | rs | rs | rs | rs |
| 0.00 | rs | rs | rs | rs | rs |
| Solute | Cation Radius (Å) [45] | Range and Structure of Solid Solutions |
|---|---|---|
| LiTiO2 | Ti3+ (0.67) | x > 0.8 (w + rs); x ≤ 0.8 (rs) |
| LiFeO2 | Fe3+ (0.74) | x > 0.8 (w + rs); x ≤ 0.8 (rs) |
| LiScO2 | Sc3+ (0.74) | x > 0.8 (w + rs); 0.5 ≤ x ≤ 0.8 (rs) |
| LiInO2 | In3+ (0.80) | x > 0.8 (w + rs); 0.5 ≤ x ≤ 0.8 (rs) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranov, A.N.; Sokolov, P.S.; Solozhenko, V.L. ZnO under Pressure: From Nanoparticles to Single Crystals. Crystals 2022, 12, 744. https://doi.org/10.3390/cryst12050744
Baranov AN, Sokolov PS, Solozhenko VL. ZnO under Pressure: From Nanoparticles to Single Crystals. Crystals. 2022; 12(5):744. https://doi.org/10.3390/cryst12050744
Chicago/Turabian StyleBaranov, Andrei N., Petr S. Sokolov, and Vladimir L. Solozhenko. 2022. "ZnO under Pressure: From Nanoparticles to Single Crystals" Crystals 12, no. 5: 744. https://doi.org/10.3390/cryst12050744






