Ceramic Ti/TiO2/AuNP Film with 1-D Nanostructures for Selfstanding Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
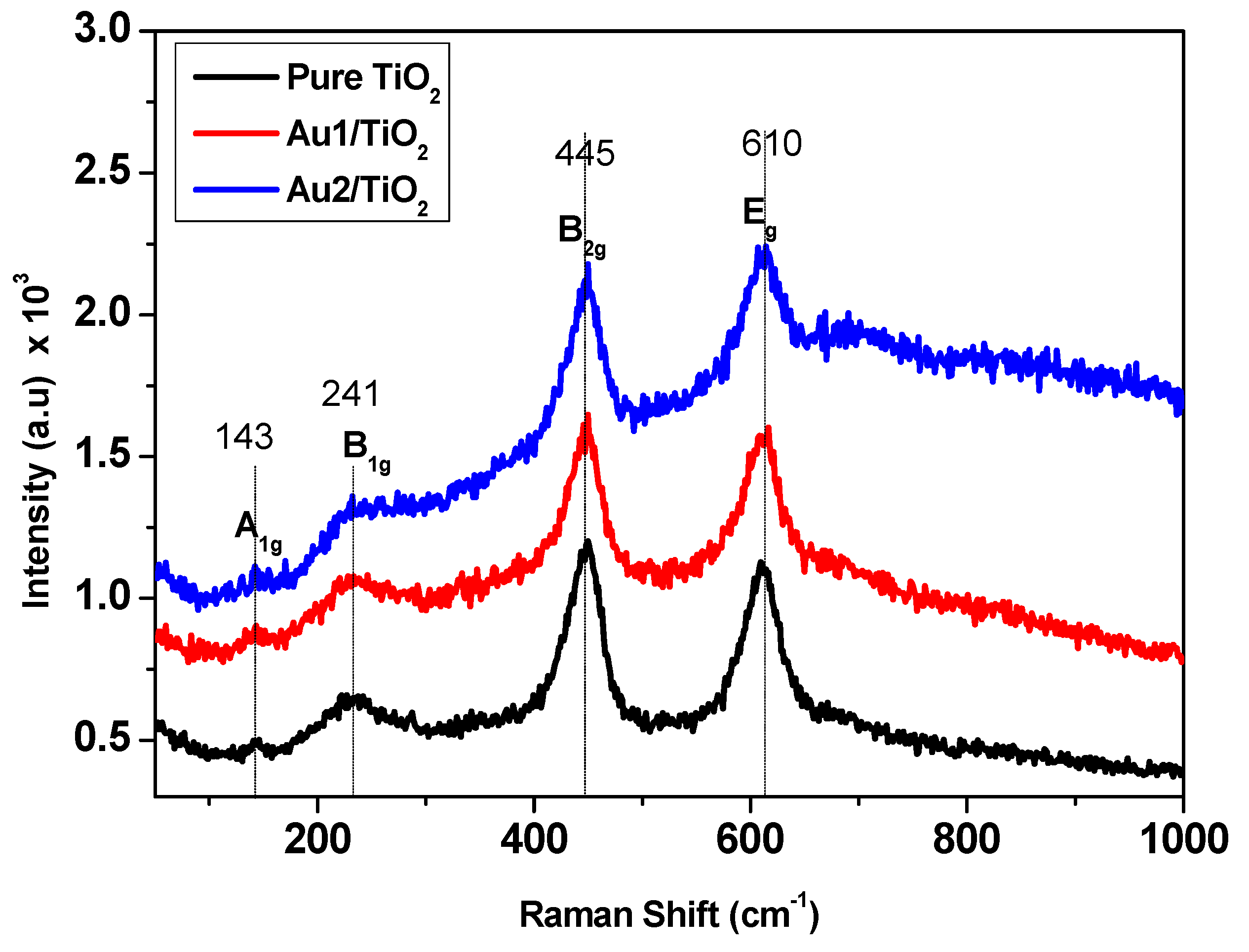
3.1. Au/TiO2 Nanorod Characterizations
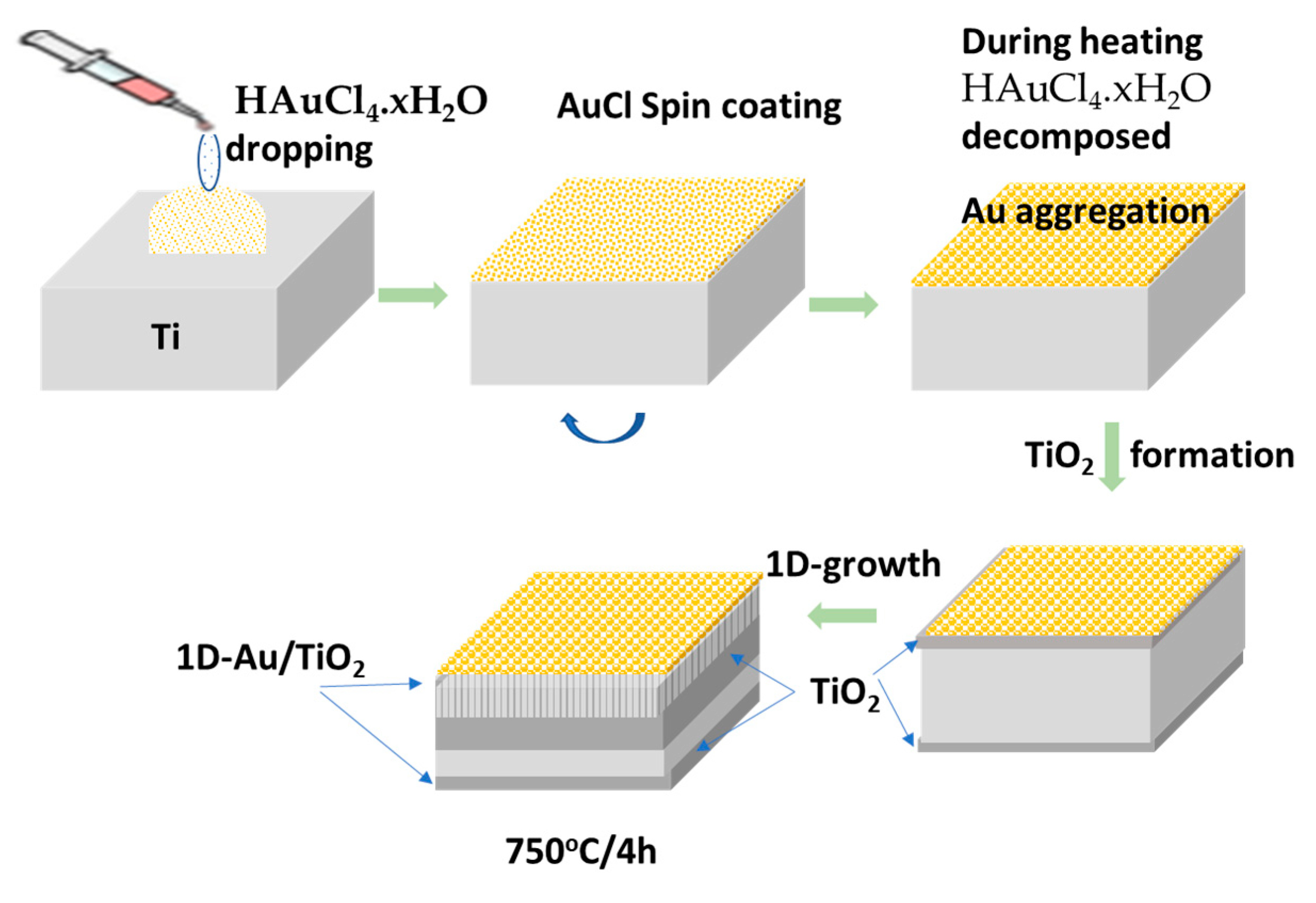
3.2. Au/TiO2 Nanorod Growth Mechanism
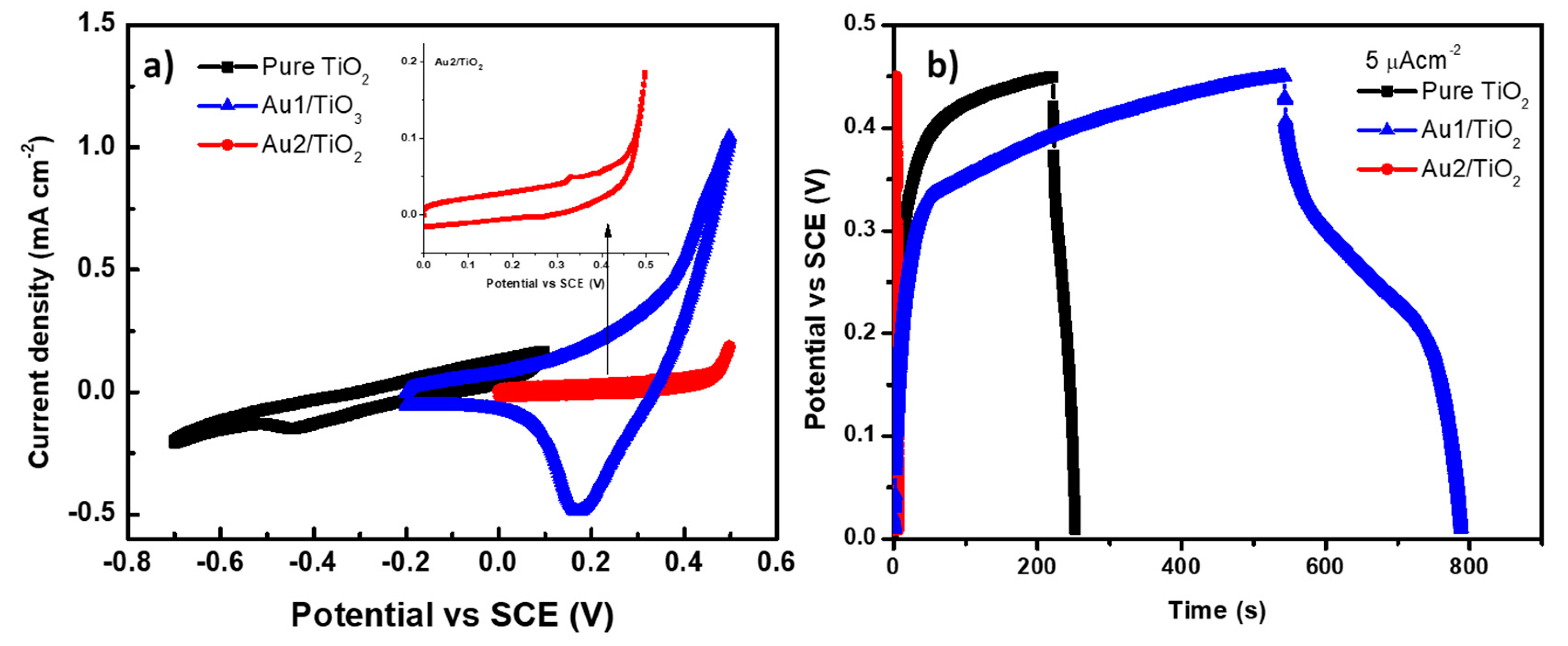
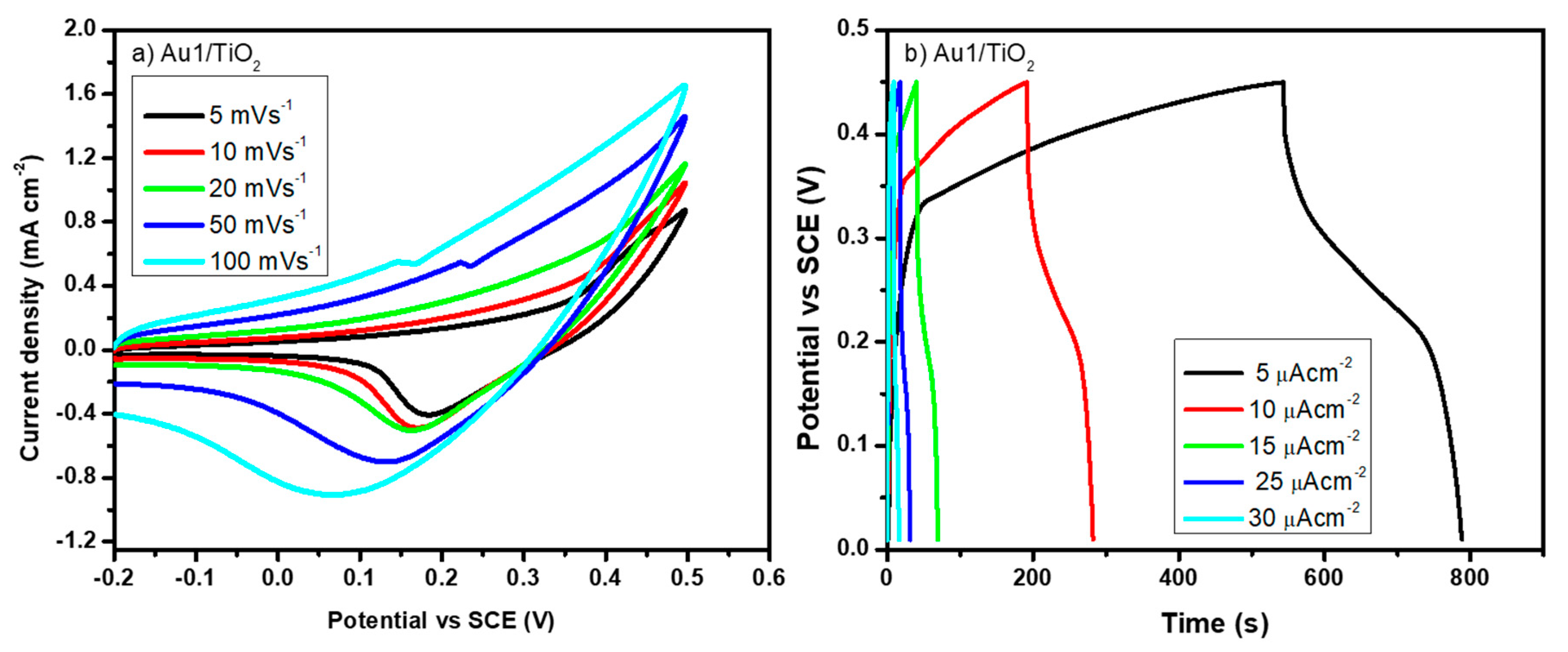
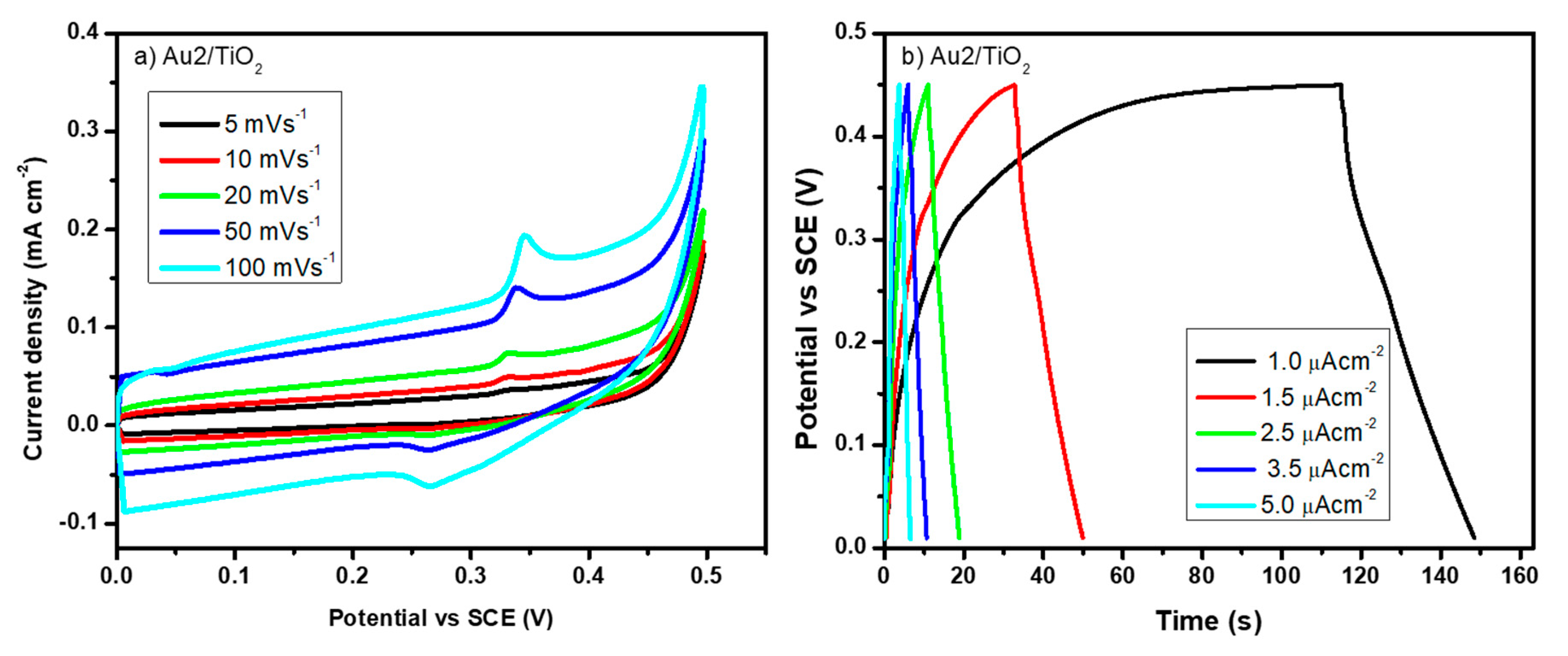
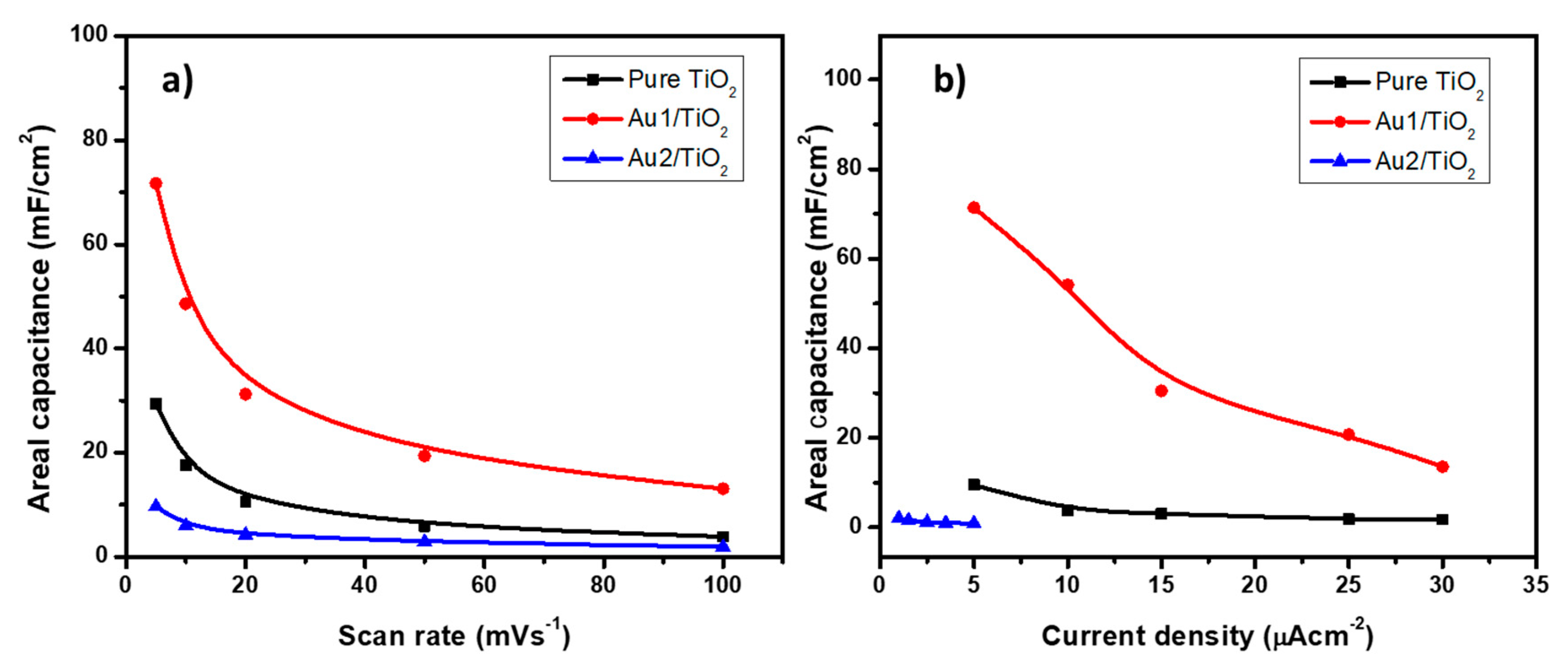
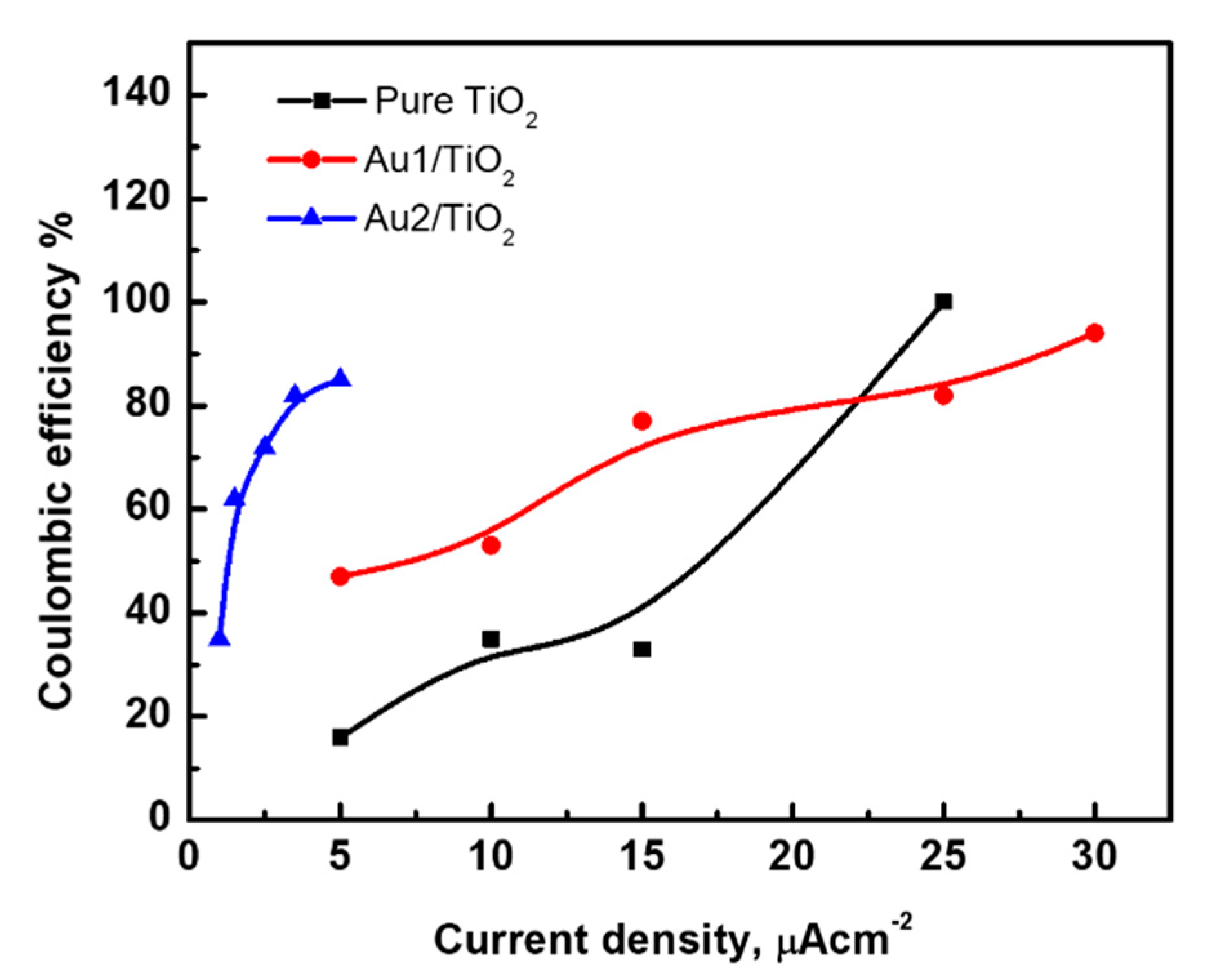
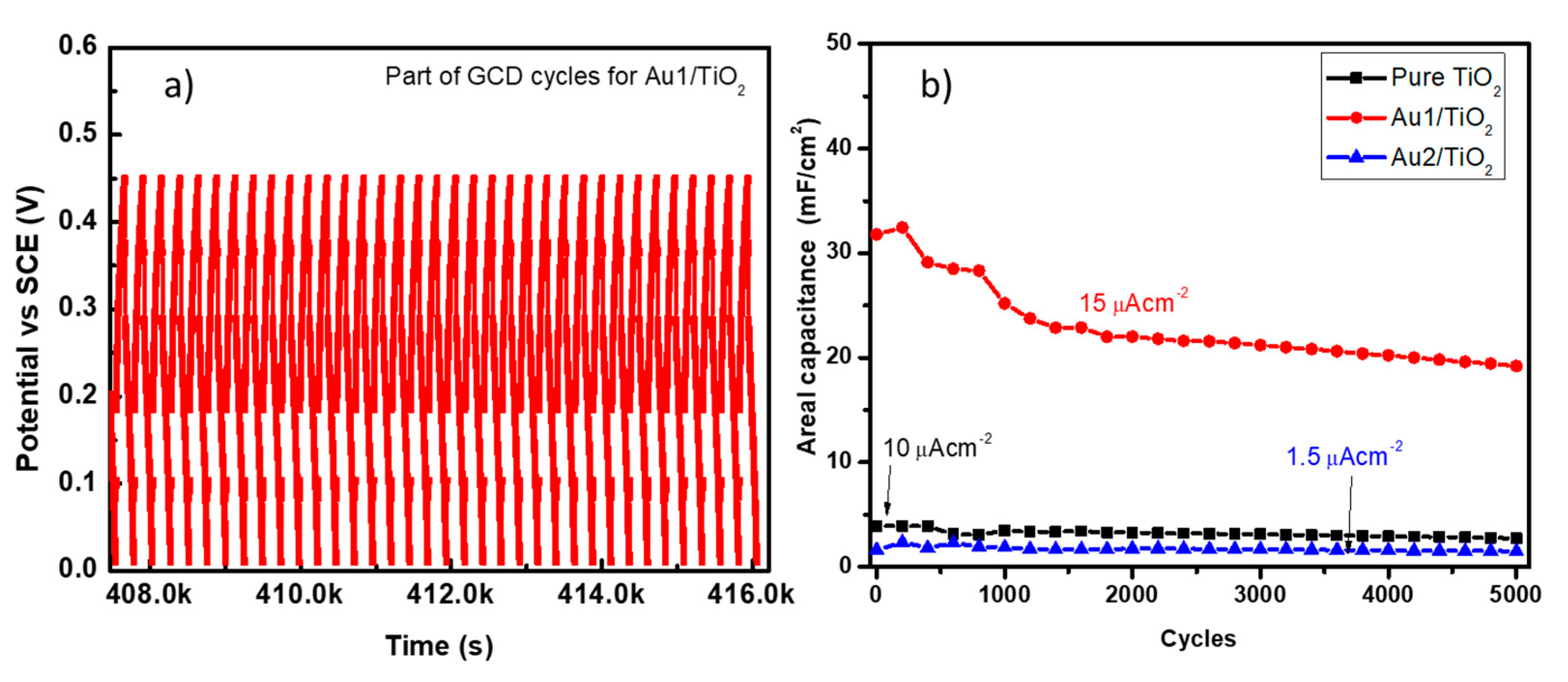
3.3. Au/TiO2 Electrochemical Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funabashi, T. Energy Storage System—An overview|ScienceDirect Topics. 2016. Available online: https://www.sciencedirect.com/topics/materials-science/supercapacitor-energy-storage-system (accessed on 13 March 2022).
- Niu, H.; Zhou, H.; Lin, T. Electrospun carbon nanofibers as electrode materials for supercapacitor applications. In Electrospun Polymers and Composites; Woodhead Publishing: Sawston, UK, 2021; pp. 641–688. [Google Scholar] [CrossRef]
- Adil, M.; Abdelkareem, M.A.; Sayed, E.T.; Rodriguez, C.; Ramadan, M.; Olabi, A.-G. Progress of Metal Chalcogenides in Supercapacitors. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 424–433. [Google Scholar] [CrossRef]
- Maitra, A.; Bera, S.; Halder, L.; Khatua, B.B. Advanced functional materials and devices for energy conversion and storage applications. In Sustainable Materials and Green Processing for Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 43–96. [Google Scholar] [CrossRef]
- De, S.; Acharya, S.; Sahoo, S.; Nayak, G.C. Present status of biomass-derived carbon-based composites for supercapacitor application. In Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–415. [Google Scholar] [CrossRef]
- El-Shafei, M.H.; Hassanin, A.H.; Shaalan, N.M.; Sharshar, T.; El-Moneim, A.A. Free-standing interconnected carbon nanofiber electrodes: New structural designs for supercapacitor application. Nanotechnology 2020, 31, 185403. [Google Scholar] [CrossRef]
- El-Deen, A.G.; El-Shafei, M.H.; Hessein, A.; Hassanin, A.H.; Shaalan, N.M.; El-Moneim, A.A. High-performance asymmetric supercapacitor based hierarchical NiCo2 O4 @ carbon nanofibers//Activated multichannel carbon nanofibers. Nanotechnology 2020, 31, 365404. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Pal, N. Nanoporous metal oxide composite materials: A journey from the past, present to future. Adv. Colloid Interface Sci. 2020, 280, 102156. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chhetri, K.; Kim, H.; Ji, S.; Chae, S.-H.; Kim, T.; Kim, H.Y. Self-assembled polypyrrole hierarchical porous networks as the cathode and porous three dimensional carbonaceous networks as the anode materials for asymmetric supercapacitor. J. Energy Storage 2021, 33, 102080. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.-H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hanafy, T.A.; Rashad, M. Dual optical properties of NiO-doped PVA nanocomposite films. Opt. Mater. 2021, 119, 111325. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hamad, D.; Aljaafari, A.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation and Characterization of Developed CuxSn1−xO2 Nanocomposite and Its Promising Methane Gas Sensing Properties. Sensors 2019, 19, 2257. [Google Scholar] [CrossRef] [Green Version]
- Shaalan, N.M.; Morsy, A.E.A.; Abdel-Rahim, M.A.; Rashad, M. Simple preparation of Ni/CuO nanocomposites with superior sensing activity toward the detection of methane gas. Appl. Phys. A Mater. Sci. Process 2021, 127, 455. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Lokhande, C.D. SILAR deposited TiO2 thin film for supercapacitor application. AIP Conf. Proc. 2013, 1536, 787–788. [Google Scholar] [CrossRef]
- Naeem, F.; Naeem, S.; Zhao, Y.; Wang, D.; Zhang, J.; Mei, Y.F.; Huang, G. TiO2 Nanomembranes Fabricated by Atomic Layer Deposition for Supercapacitor Electrode with Enhanced Capacitance. Nanoscale Res. Lett. 2019, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, X.; Mu, H.; Tian, Q.; Li, Q.; Liu, Y.; Zhang, X.; Zhao, Z.; Su, X. Construction of hierarchical sea urchin-like manganese substituted nickel cobaltite@tricobalt tetraoxide core-shell microspheres on nickel foam as binder-free electrodes for high performance supercapacitors. J. Colloid Interface Sci. 2021, 596, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Su, X.; Zhao, Z.; Han, C.; Wang, Z.; Zhao, P. Facile synthesis of Ni0.5Mn0.5Co2O4 nanoflowers as high-performance electrode material for supercapacitors. J. Am. Ceram. Soc. 2019, 102, 6893–6903. [Google Scholar] [CrossRef]
- Iqbal, M.; Saykar, N.G.; Arya, A.; Banerjee, I.; Alegaonkar, P.S.; Mahapatra, S.K. High-performance supercapacitor based on MoS2@TiO2 composite for wide range temperature application. J. Alloys Compd. 2021, 883, 160705. [Google Scholar] [CrossRef]
- Hodaei, A.; Dezfuli, A.S.; Naderi, H.R. A high-performance supercapacitor based on N-doped TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 14596–14604. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Castelo-Quibén, J.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Carbon-TiO2 composites as high-performance supercapacitor electrodes: Synergistic effect between carbon and metal oxide phases. J. Mater. Chem. A 2018, 6, 633–644. [Google Scholar] [CrossRef]
- Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple preparation of ceramic-like materials based on 1d-agx(X=0, 5, 10, 20, 40 mm)/tio2 nanostructures and their photocatalysis performance. Crystals 2020, 10, 1024. [Google Scholar] [CrossRef]
- Qu, B.; Hu, L.; Chen, Y.; Li, C.; Li, Q.; Wang, Y.; Wei, W.; Chen, L.; Wang, T. Rational design of Au–NiO hierarchical structures with enhanced rate performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7023–7026. [Google Scholar] [CrossRef]
- Kim, S.-I.; Thiyagarajan, P.; Jang, J.-H. Great improvement in pseudocapacitor properties of nickel hydroxide via simple gold deposition. Nanoscale 2014, 6, 11646–11652. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Supercapacitor electrode of nano-Co3O4 decorated with gold nanoparticles via in-situ reduction method. J. Power Sources 2017, 363, 1–8. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Kong, L.; Kang, L.; Ran, F. Synthesis of ultra-small gold nanoparticles decorated onto NiO nanobelts and their high electrochemical performance. Dalt. Trans. 2018, 47, 8078–8086. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bhunia, K.; Terashima, C.; Fujishima, A.; Pradhan, D. Citrate-Capped Hybrid Au-TiO2 Nanomaterial for Facile and Enhanced Electrochemical Hydrazine Oxidation. ACS Omega 2017, 2, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Tuschel, D. The Correlation Method for the Determination of Spectroscopically Active Vibrational Modes in Crystals. Spectroscopy 2015, 30, 17–22. Available online: https://www.spectroscopyonline.com/view/correlation-method-determination-spectroscopically-active-vibrational-modes-crystals (accessed on 6 April 2022).
- Fateley, W.G.; McDevitt, N.T.; Bentley, F.F. Infrared and Raman Selection Rules for Lattice Vibrations: The Correlation Method. Appl. Spectrosc. 1971, 25, 155–173. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Lang, J.W.; Kong, L.B.; Wu, W.J.; Luo, Y.C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 35, 4213–4215. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Zhao, W.; Ma, J.; Mhaisalkar, S.; Maria, T.L.; Yang, Y.; Zhang, H.; Hng, H.H.; et al. Synthesis of porous NiO nanocrystals with controllable surface area and their application as supercapacitor electrodes. Nano Res. 2010, 3, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, K.N.; Chaudhari, S.; Yu, J.-S. Synthesis and supercapacitor performance of Au-nanoparticle decorated MWCNT. J. Electroanal. Chem. 2016, 761, 98–105. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Gao, X.; Liu, W.; Zhu, H. 3D hierarchically gold-nanoparticle-decorated porous carbon for high-performance supercapacitors. Sci. Rep. 2019, 9, 17065. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Kong, L.; Kang, L.; Ran, F. Nano-Au@PANI core-shell nanoparticles via in-situ polymerization as electrode for supercapacitor. J. Alloys Compd. 2017, 722, 1–7. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Buremoh, W.; Owoeye, V.A.; Ajayeoba, Y.A.; Salau, A.O.; Busari, H.K.; Tijani, M.A.; Taleatu, B.A. Preparation and characterization of TiO2 thin film electrode for optoelectronic and energy storage Potentials: Effects of Co incorporation. Chem. Phys. Lett. 2021, 779, 138854. [Google Scholar] [CrossRef]
- Achour, A.; Porto, R.L.; Soussou, M.A.; Islam, M.; Boujtita, M.; Aissa, K.A.; le Brizoual, L.; Djouadi, A.; Brousse, T. Titanium nitride films for micro-supercapacitors: Effect of surface chemistry and film morphology on the capacitance. J. Power Sources 2015, 300, 525–532. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [Green Version]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, F.; Wang, F.; Wang, J.; Dong, R.; Zhuang, X.; Schmidt, O.G.; Feng, X. Stimulus-Responsive Micro-Supercapacitors with Ultrahigh Energy Density and Reversible Electrochromic Window. Adv. Mater. 2017, 29, 1604491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Peng, J.; Yu, Z.; Zhou, Y.; Guo, Y.; Li, Z.; Lin, Y.; Ruan, K.; Wu, C.; Xie, Y. Acid-Assisted Exfoliation toward Metallic Sub-nanopore TaS2 Monolayer with High Volumetric Capacitance. J. Am. Chem. Soc. 2018, 140, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Carminati, M.; Ferrari, G.; Bianchi, D. Impedance Spectroscopy for Biosensing: Circuits and Applications. In Handbook of Biochips; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–24. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (Eis): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]




| Sample Type | Selfstanding | Areal Capacitance | Ref. |
|---|---|---|---|
| Codoped TiO2 Thin film | yes | 15.86 mFcm−2 | [36] |
| TiN thin film | yes | 12.00 mFcm−2 | [37] |
| Laser-GO | yes | 3.67 mFcm−2 | [38] |
| MoS2 film | yes | 700 Fcm−3 (thickness 5 µm) | [39] |
| V2O5 nanoribbons film | exfoliated graphene-based hybrid and viologen as electrode | 3.92 mFcm−2 | [40] |
| TaS2 monolayer | yes | 508 Fcm−3 (thickness 330 nm | [41] |
| Au/TiO2 | yes | 32 mFcm−2 (thickness 5 µm) | Present result |
| Sample | Rs (Ω) | Rct (Ω) | Wo1-R (Ω) |
|---|---|---|---|
| Pure TiO2 | 10 | 3900 | 1200 |
| Au1/TiO2 | 14 | 2000 | 2000 |
| Au2/TiO2 | 16 | / | 28,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.M.; Ahmed, F.; Rashad, M.; Kumar, S.; Saber, O.; Al-Naim, A.F.; Kotb, H.M.; Ezzeldien, M.; Mahmoud, A.Z. Ceramic Ti/TiO2/AuNP Film with 1-D Nanostructures for Selfstanding Supercapacitor Electrodes. Crystals 2022, 12, 791. https://doi.org/10.3390/cryst12060791
Shaalan NM, Ahmed F, Rashad M, Kumar S, Saber O, Al-Naim AF, Kotb HM, Ezzeldien M, Mahmoud AZ. Ceramic Ti/TiO2/AuNP Film with 1-D Nanostructures for Selfstanding Supercapacitor Electrodes. Crystals. 2022; 12(6):791. https://doi.org/10.3390/cryst12060791
Chicago/Turabian StyleShaalan, Nagih M., Faheem Ahmed, Mohamed Rashad, Shalendra Kumar, Osama Saber, Abdullah F. Al-Naim, Hicham M. Kotb, Mohammed Ezzeldien, and Amera Z. Mahmoud. 2022. "Ceramic Ti/TiO2/AuNP Film with 1-D Nanostructures for Selfstanding Supercapacitor Electrodes" Crystals 12, no. 6: 791. https://doi.org/10.3390/cryst12060791








