The Potential Effect of Annealing Mesostructured Titanium Dioxide Electrode in a Closed Box Furnace on the Concentration of Lead (II) Iodide Solution Required for Optimal Performance of Mesoscopic Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
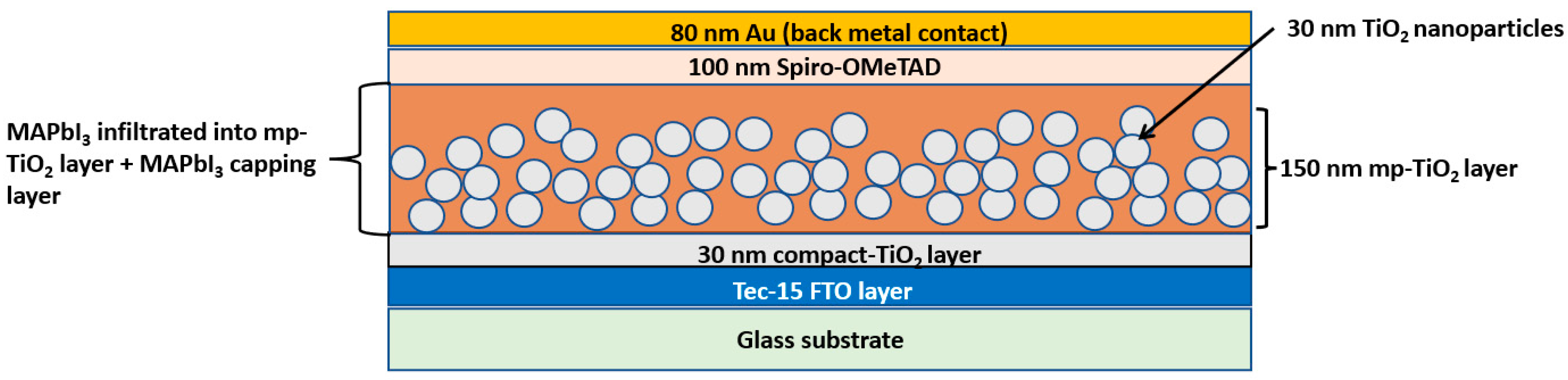
2.2. Preparation of Compact and Mesoporous TiO2 Films
2.3. Deposition of the Perovskite Active Layer
2.4. Characterization
2.5. Device Fabrication
2.6. Device Characterization
3. Results
3.1. Determining the Perovskite Content Using GI-XRD
3.2. Influence of PbI2 on the Absorbance before and after Conversion to MAPbI3
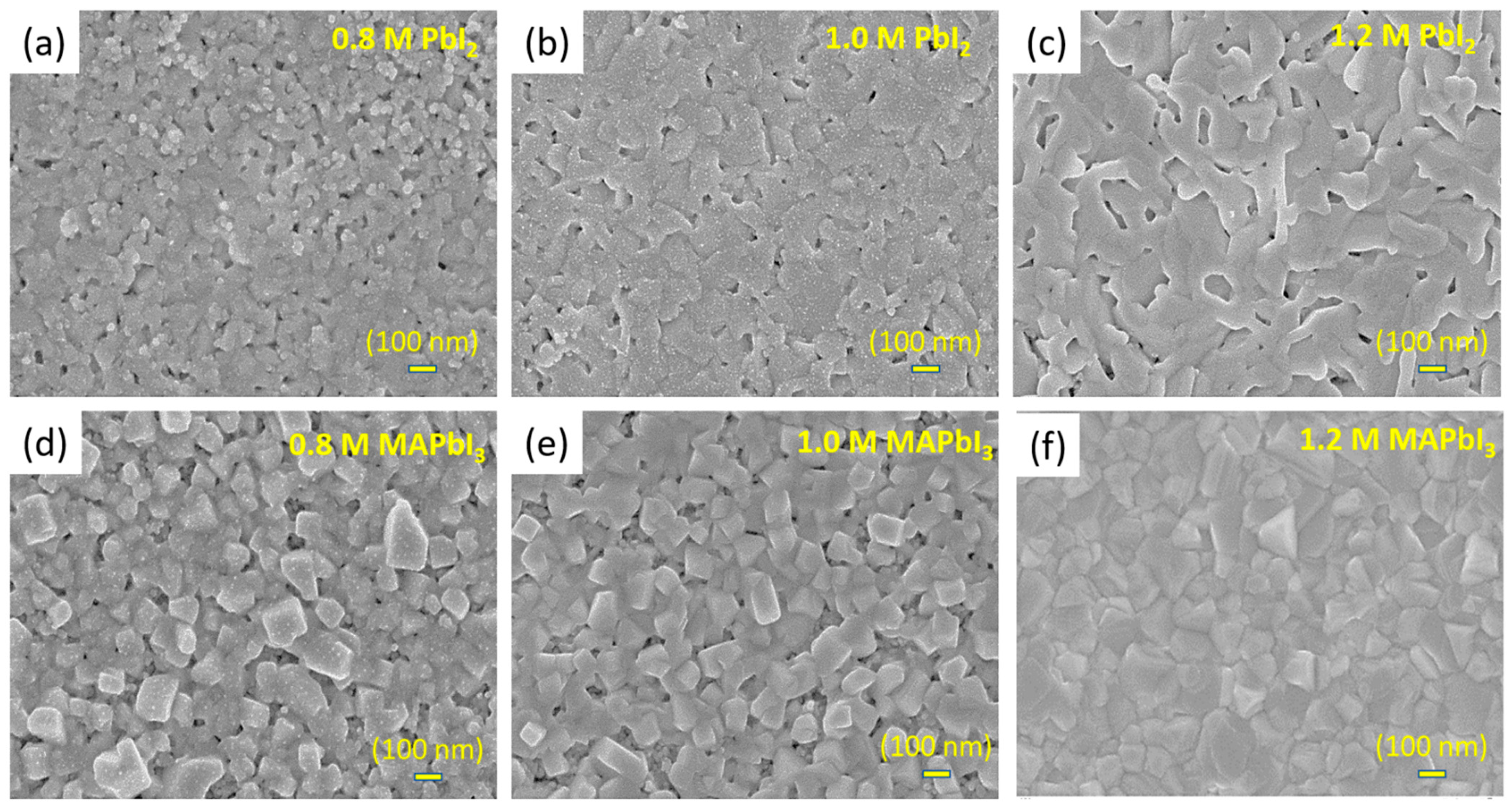
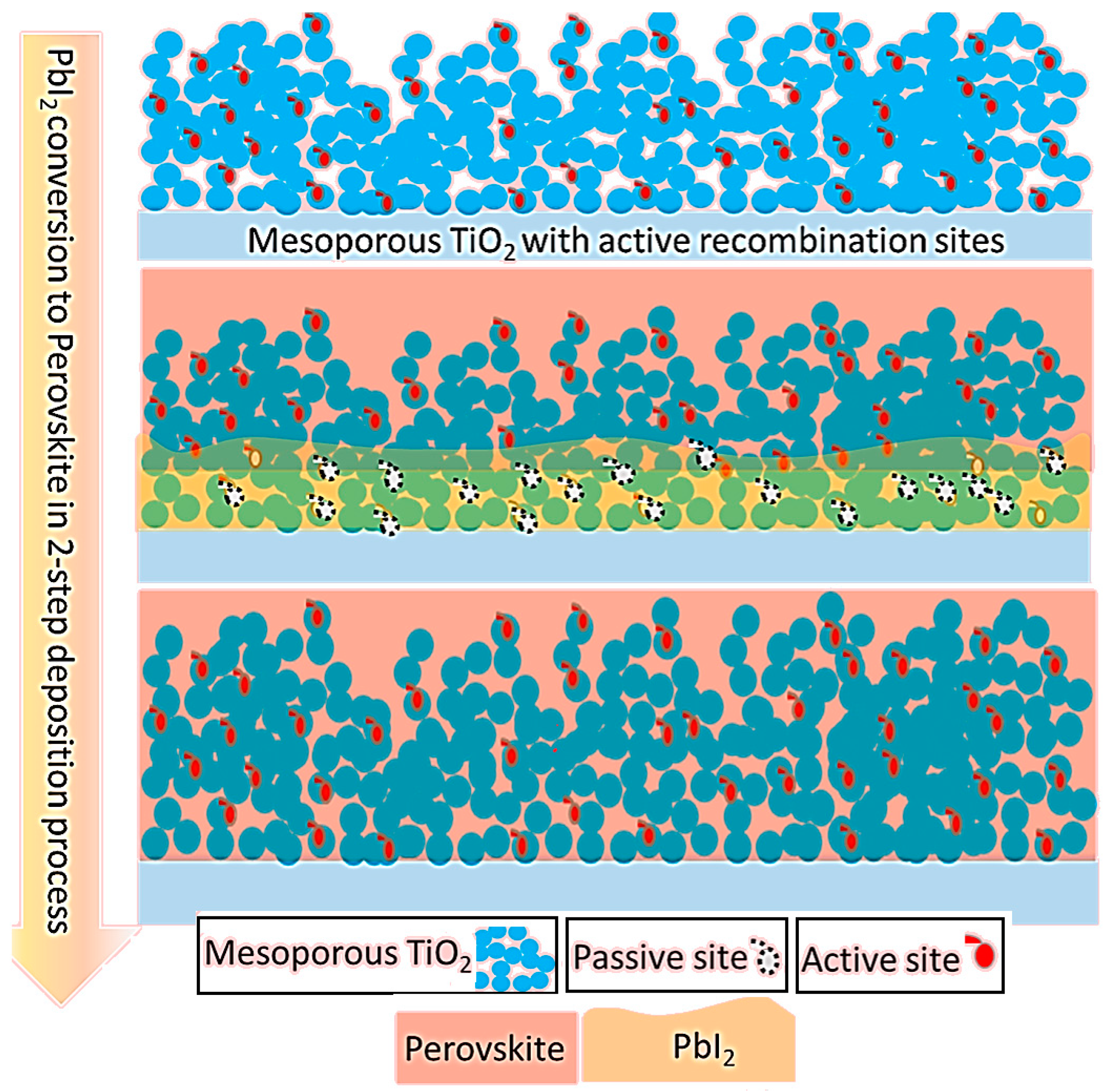
3.3. Morphology of the Capping Layer before and after Conversion to MAPbI3
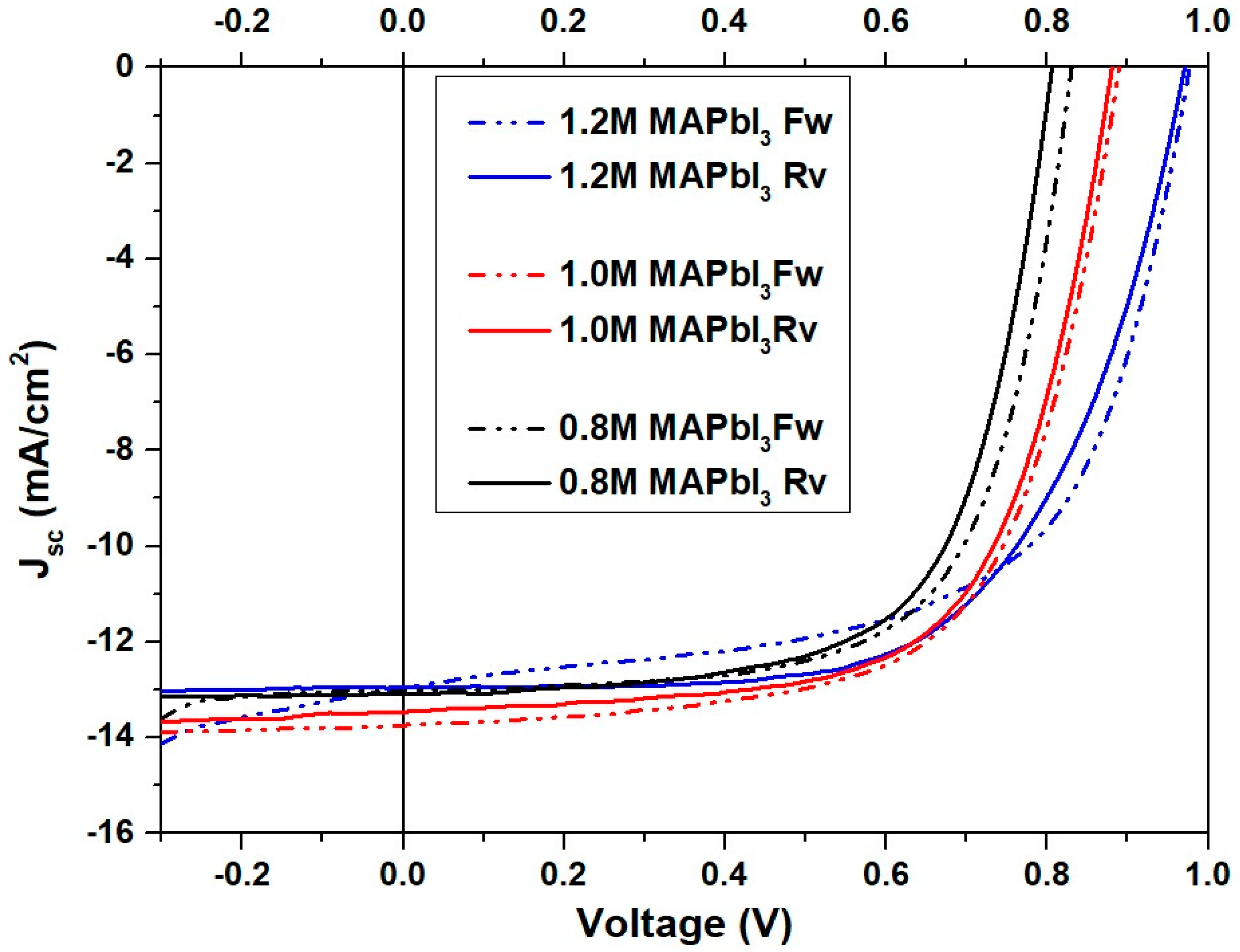
3.4. The Influence of PbI2 Concentration on the Device Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burlakov, V.M.; Docampo, P.; Goriely, A. Morphological Control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Kim, H.-S.; Park, N.-G. Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3. APL Mater. 2014, 2, 081510. [Google Scholar] [CrossRef]
- Im, J.-H.; Jang, I.-H.; Pellet, N.; Grätzel, M.; Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef]
- Chen, H. Two-step sequential deposition of organometal halide perovskite for photovoltaic application. Adv. Funct. Mater. 2017, 27, 1605654. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.-B.; Spiccia, L. A Fast Deposition-Crystallization Procedure for Highly Efficient Lead Iodide Perovskite Thin-Film Solar Cells; Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Sakai, N.; Pathak, S.; Chen, H.-W.; Haghighirad, A.; Stranks, S.D.; Miyasaka, T.; Snaith, H.J. The mechanism of toluene-assisted crystallization of organic-inorganic perovskites for highly efficienct solar cells. J. Mater. Chem. A 2016, 4, 4464–4471. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.-P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n−i−p) and Inverted (p−i−n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Somsongkul, V.; Lang, F.; Jeong, A.R.; Rusu, M.; Arunchaiya, M.; Dittrich, T. Hole blocking PbI2/CH3NH3PbI3 interface. Phys. Status Solidi RRL 2014, 8, 763–766. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, M.; Chai, L. Effects of the concentration of PbI2 and CH3NH3I on the perovskite films and the performance of perovskite solar cells based on ZnO/TiO2 nanorod arrays. Superlattices Microstruct. 2018, 123, 189–200. [Google Scholar] [CrossRef]
- Bi, D.; El-Zohry, A.M.; Hagfeldt, A.; Boschloo, G. Unraveling the Effect of PbI2 Concentration on Charge Recombination Kinetics in Perovskite Solar Cells. ACS Photonics 2015, 2, 589–594. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Hoque, M.N.F.; Stoneham, E.; Warzywoda, J.; Dallas, T.; Fan, Z. Reduction of Oxygen Vacancy Related Traps in TiO2 and the Impacts on Hybrid Perovskite Solar Cells. J. Phys. Chem. C 2017, 121, 23939–23946. [Google Scholar] [CrossRef]
- Huang, H.; Yan, H.; Duan, M.; Ji, J.; Liu, X.; Jiang, H.; Liu, B.; Sajid, S.; Cui, P.; Li, Y.; et al. TiO2 surface oxygen vacancy passivation towards mitigated interfacial lattice distortion and efficient perovskite solar cell. Appl. Surf. Sci. 2021, 544, 148583. [Google Scholar] [CrossRef]
- Klasen, A.; Baumli, P.; Sheng, Q.; Johannes, E.; Bretschneider, S.A.; Hermes, I.M.; Bergmann, V.W.; Gort, G.; Axt, A.; Weber, S.A.L.; et al. Removal of Surface Oxygen Vacancies Increases Conductance Through TiO2 Thin Films for Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 13458–13466. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.T.; Weinberger, C.; Sarfraz, J.; Rosqvist, E.; Sandén, S.; Sandberg, O.J.; Vivo, P.; Hashmi, G.; Lund, P.D.; Österbacka, R.; et al. Impact of Film Thickness of Ultrathin Dip-Coated Compact TiO2 Layers on the Performance of Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 17906–17913. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.T.; Weinbergera, C.; Qudsia, S.; Rosqvista, E.; Sandberg, O.J.; Nyman, M.; Sandén, S.; Vivo, P.; Aitola, K.; Lund, P.D.; et al. Influence of titanium dioxide surface activation on the performance of mesoscopic perovskite solar cells. Thin Solid Film. 2019, 686, 137418. [Google Scholar] [CrossRef]
- Hu, H. Defect-states Passivation Strategy in Perovskite Solar Cells. E3S Web Conf. 2021, 245, 01031. [Google Scholar] [CrossRef]
- Wang, S.; Dong, W.; Fang, X.; Zhang, Q.; Zhou, S.; Deng, Z.; Tao, R.; Shao, J.; Xia, R.; Song, C.; et al. Credible evidence for the passivation effect of remnant PbI2 in CH3NH3PbI3 films in improving the performance of perovskite solar cells. Nanoscale 2016, 8, 6600–6608. [Google Scholar] [CrossRef]
- Hidayat, R.; Nurunnizar, A.A.; Fariz, A.; Rosa, E.S.; Oizumi, T.; Fujii, A.; Ozaki, M. Revealing the charge carrier kinetics in perovskite solar cells affected by mesoscopic structures and defect states from simple transient photovoltage measurements. Sci. Rep. 2020, 10, 19197. [Google Scholar] [CrossRef]
- Idígoras, J.; Contreras-Bernal, L.; Cave, J.M.; Courtier, N.E.; Barranco, Á.; Borras, A.; Sánchez-Valencia, J.R.; Anta, J.A.; Walker, A.B. The role of surface recombination on the performance of perovskite solar cells: Effect of morphology and crystalline phase of TiO2 contact. Adv. Mater. Interfaces 2018, 5, 1801076. [Google Scholar] [CrossRef]
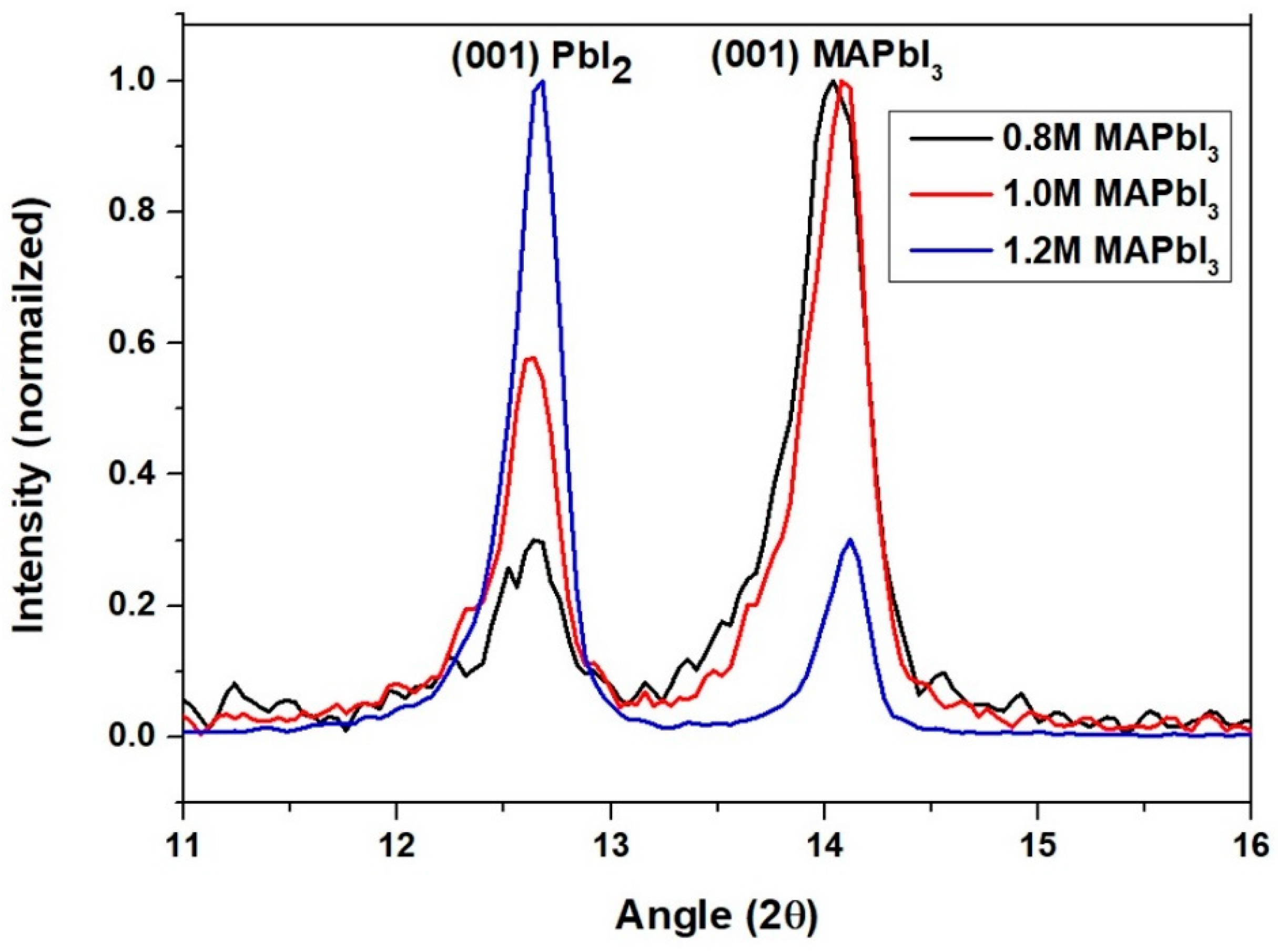

| PbI2 Concentration | % Perovskite |
|---|---|
| 0.8 M | 74.48 |
| 1.0 M | 62.89 |
| 1.2 M | 23.62 |
| (a) Forwards Sweep | ||||
| PbI2 Concentration | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
| 0.8 M | 13.115 ± 0.374 | 0.839 ± 0.013 | 0.673 ± 0.025 | 7.425 ± 0.485 |
| 1.0 M | 13.850 ± 0.365 | 0.891 ± 0.017 | 0.620 ± 0.024 | 7.655 ± 0.342 |
| 1.2 M | 13.507 ± 0.939 | 0.975 ± 0.004 | 0.617 ± 0.006 | 8.120 ± 0.607 |
| (b) Reverse Sweep | ||||
| PbI2 Concentration | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
| 0.8 M | 13.155 ± 0.392 | 0.829 ± 0.021 | 0.693 ± 0.022 | 7.579 ± 0.532 |
| 1.0 M | 13.395 ± 0.556 | 0.880 ± 0.022 | 0.635 ± 0.017 | 7.470 ± 0.321 |
| 1.2 M | 13.407 ± 0.800 | 0.972 ± 0.008 | 0.620 ± 0.010 | 8.130 ± 0.638 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, M.T.; Safdar, A.; Akram, M.A.; Javed, S.; Qudsia, S. The Potential Effect of Annealing Mesostructured Titanium Dioxide Electrode in a Closed Box Furnace on the Concentration of Lead (II) Iodide Solution Required for Optimal Performance of Mesoscopic Perovskite Solar Cells. Crystals 2022, 12, 833. https://doi.org/10.3390/cryst12060833
Masood MT, Safdar A, Akram MA, Javed S, Qudsia S. The Potential Effect of Annealing Mesostructured Titanium Dioxide Electrode in a Closed Box Furnace on the Concentration of Lead (II) Iodide Solution Required for Optimal Performance of Mesoscopic Perovskite Solar Cells. Crystals. 2022; 12(6):833. https://doi.org/10.3390/cryst12060833
Chicago/Turabian StyleMasood, Muhammad Talha, Amna Safdar, Muhammad Aftab Akram, Sofia Javed, and Syeda Qudsia. 2022. "The Potential Effect of Annealing Mesostructured Titanium Dioxide Electrode in a Closed Box Furnace on the Concentration of Lead (II) Iodide Solution Required for Optimal Performance of Mesoscopic Perovskite Solar Cells" Crystals 12, no. 6: 833. https://doi.org/10.3390/cryst12060833
APA StyleMasood, M. T., Safdar, A., Akram, M. A., Javed, S., & Qudsia, S. (2022). The Potential Effect of Annealing Mesostructured Titanium Dioxide Electrode in a Closed Box Furnace on the Concentration of Lead (II) Iodide Solution Required for Optimal Performance of Mesoscopic Perovskite Solar Cells. Crystals, 12(6), 833. https://doi.org/10.3390/cryst12060833






