Effects of Basicity and Al2O3 Content on Viscosity and Crystallization Behavior of Super-High-Alumina Slag
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Slag Sample Preparation
2.2. Experimental Methods
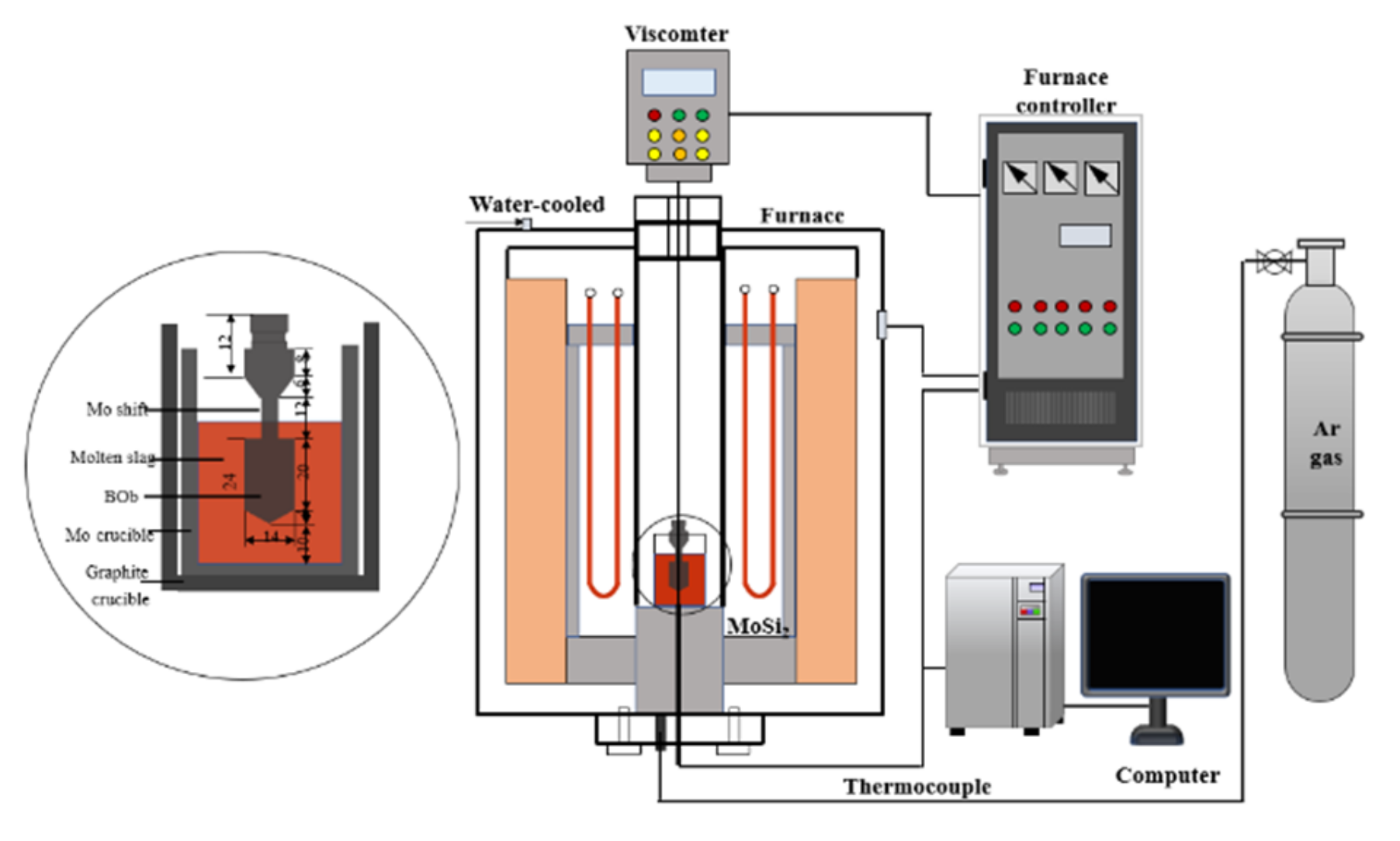
2.2.1. Viscosity Tests
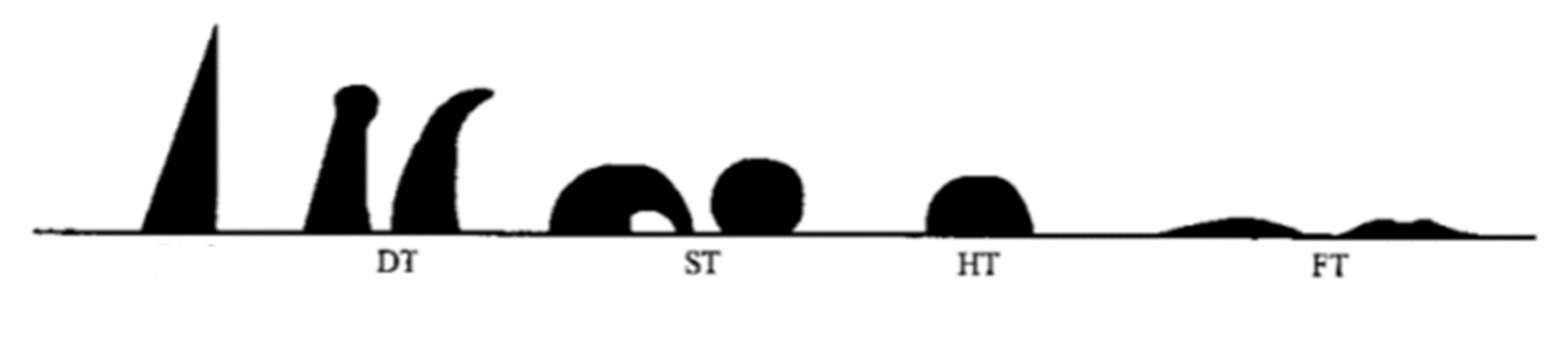
2.2.2. Melting Properties Tests
2.3. Characterization Methods
3. Results
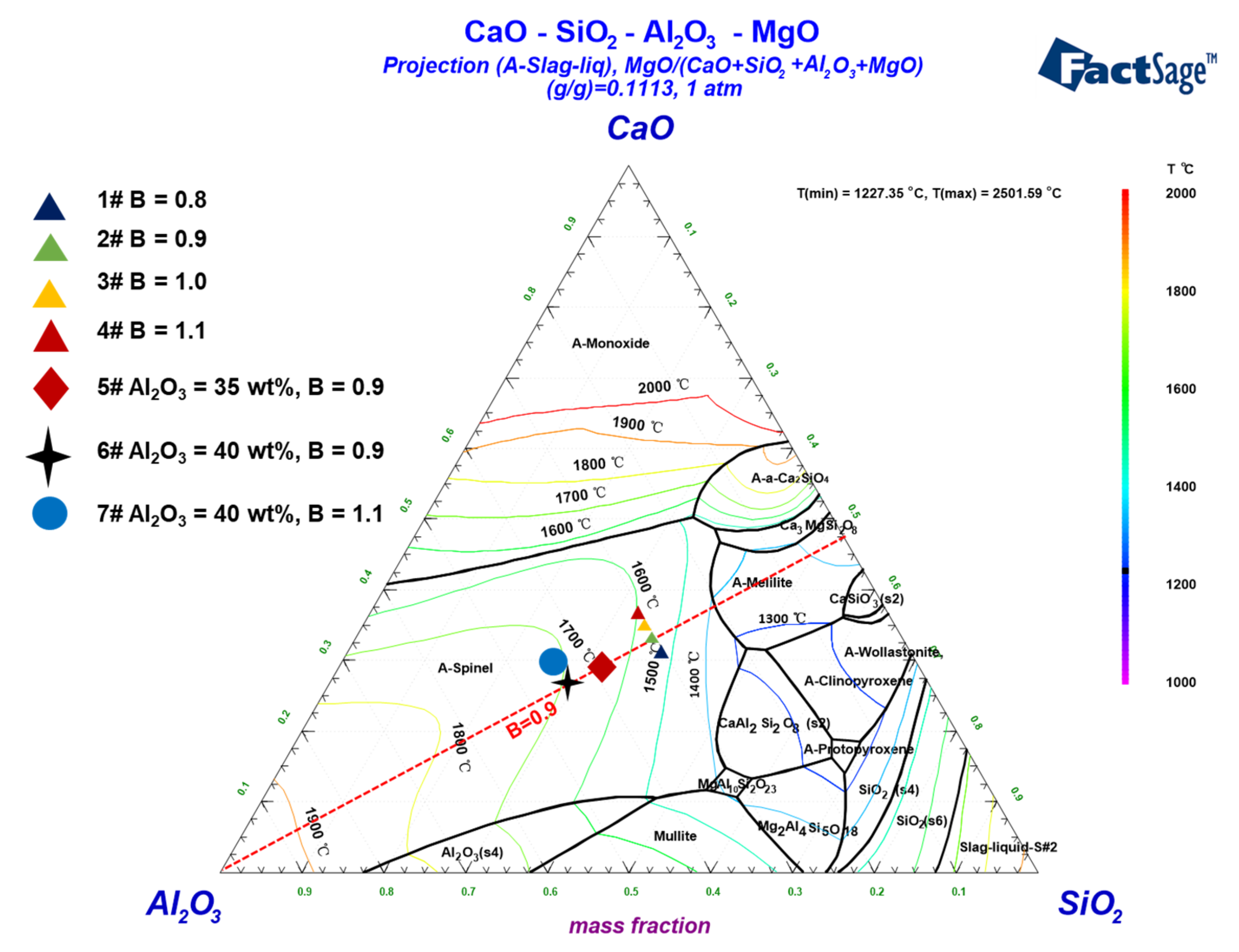
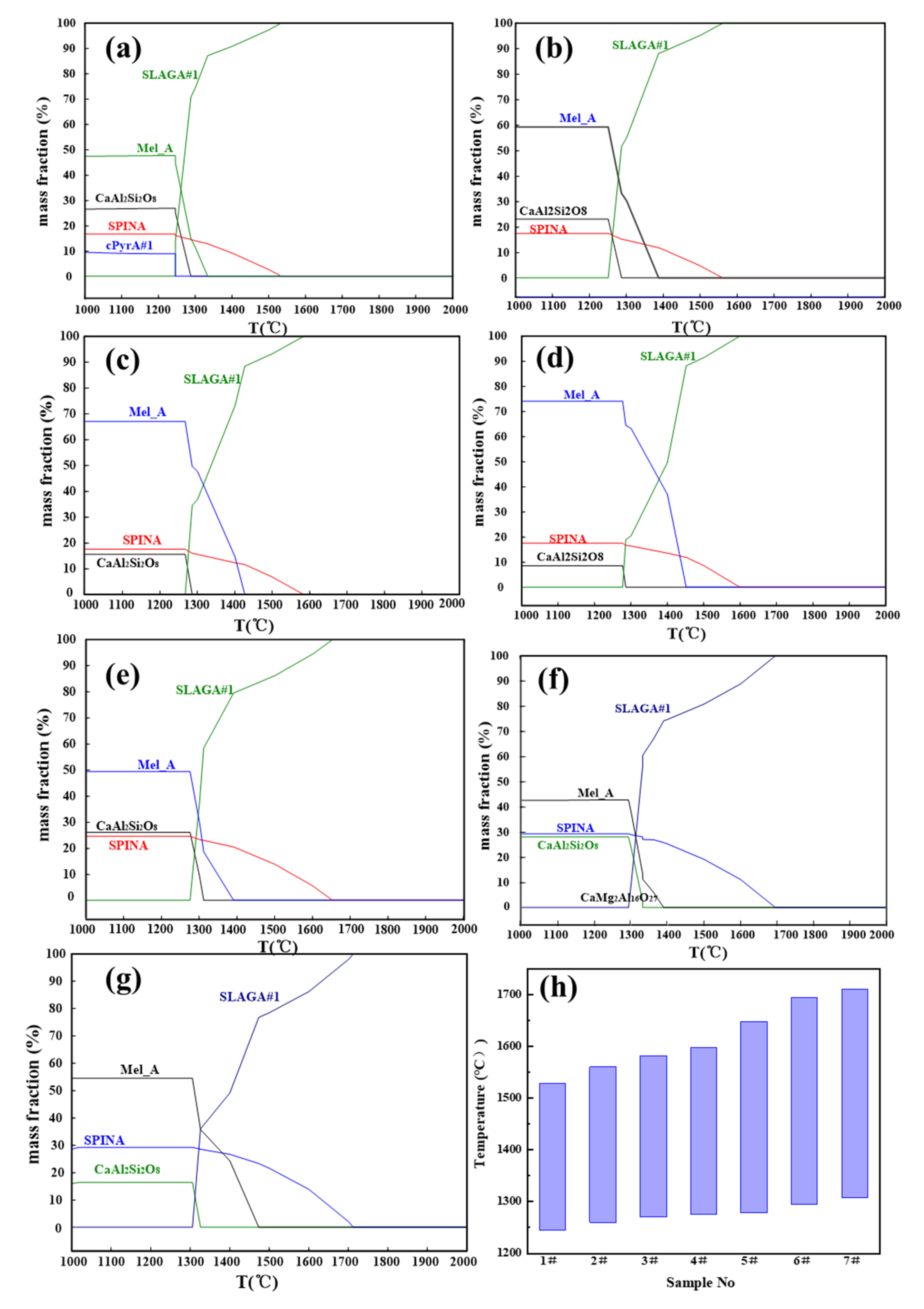
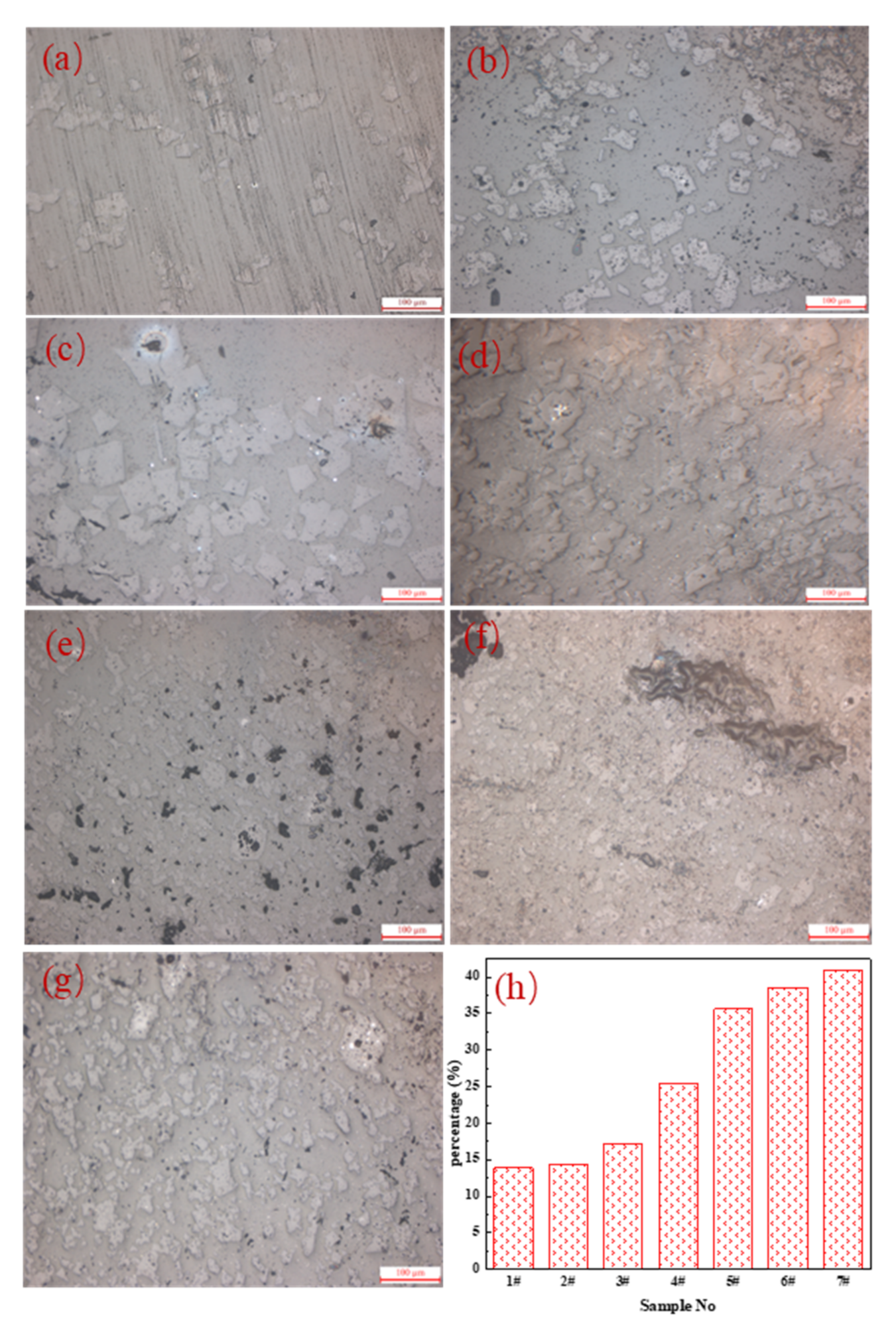
3.1. Crystallization and Phase Transformation of Slags
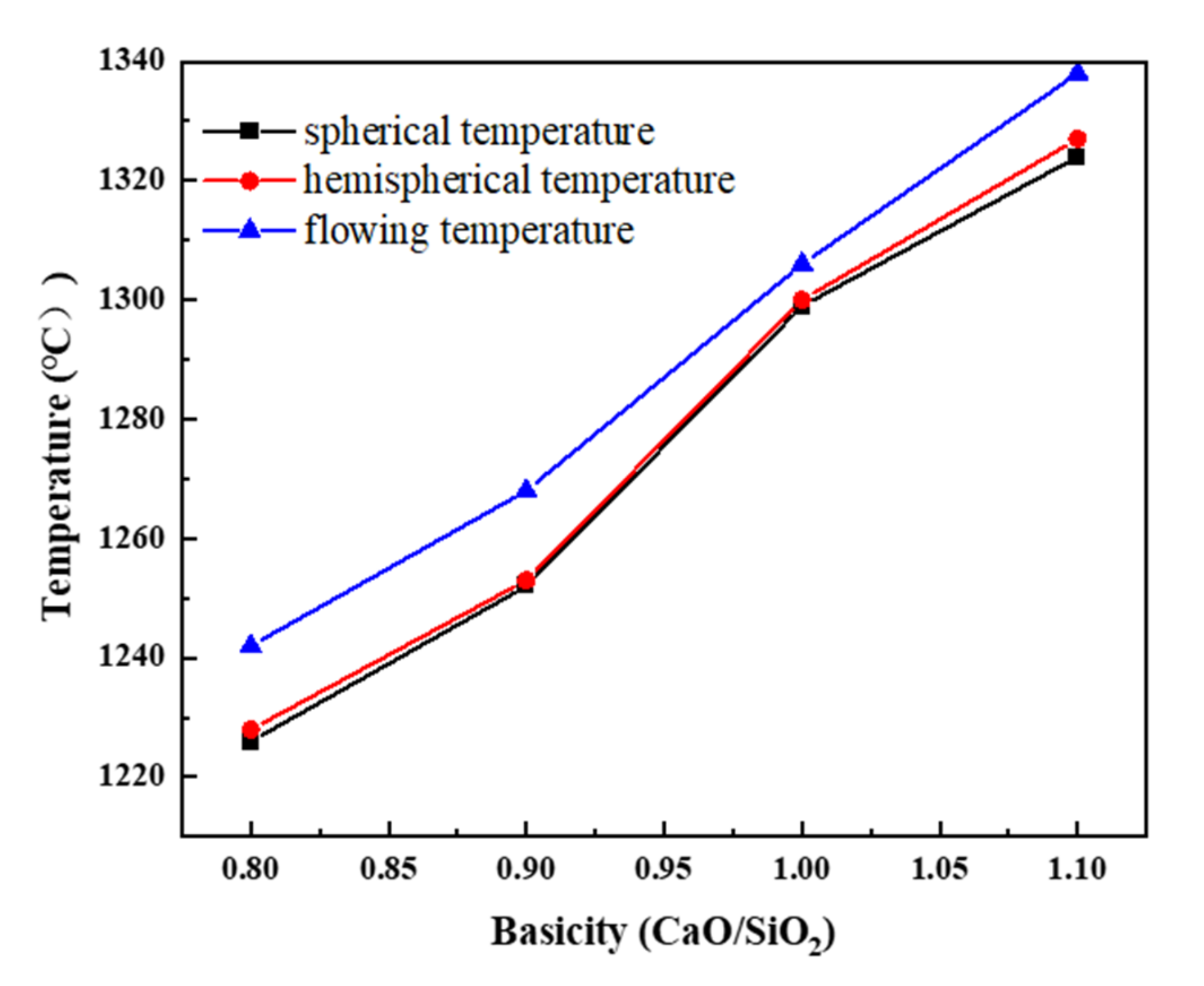
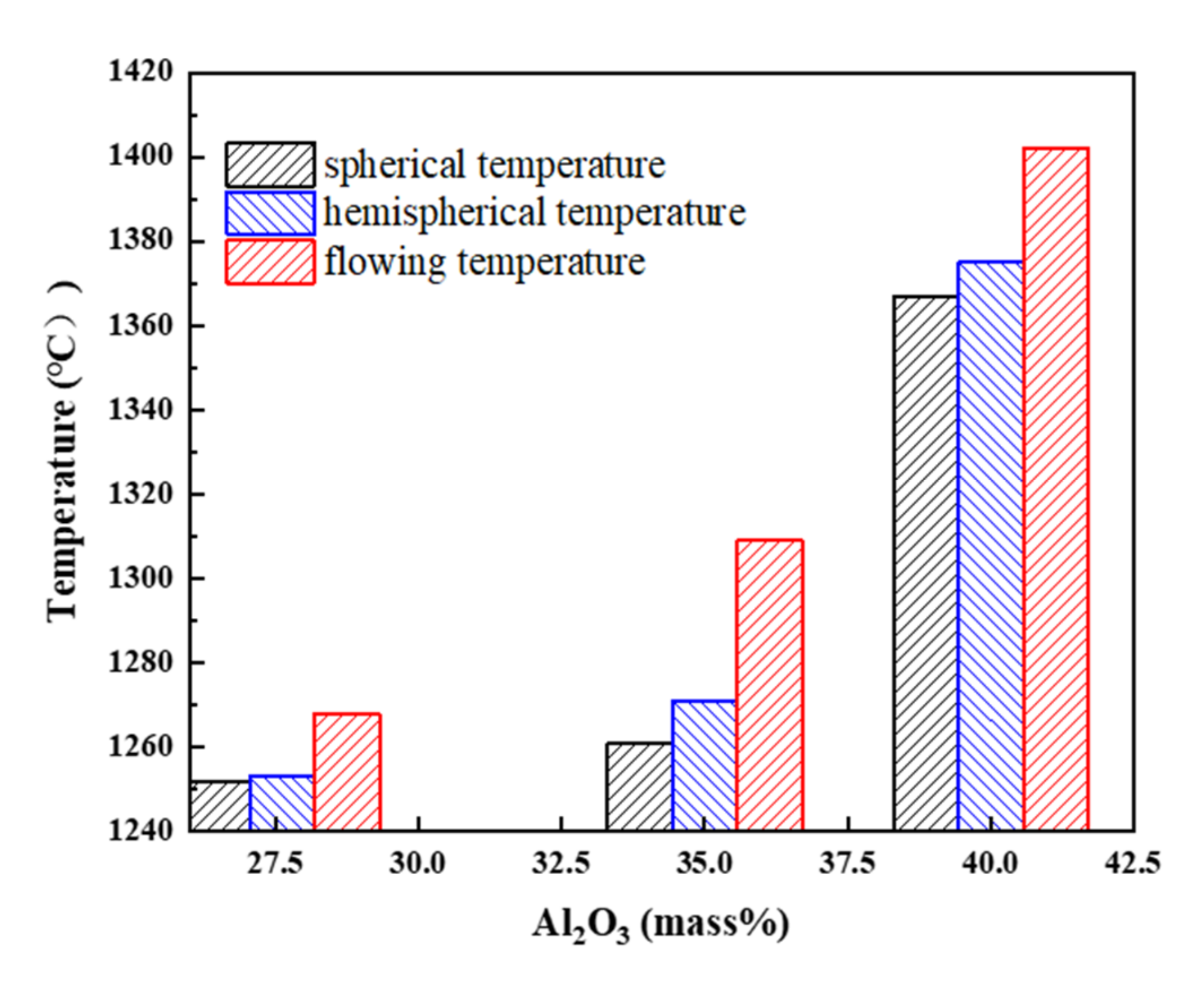
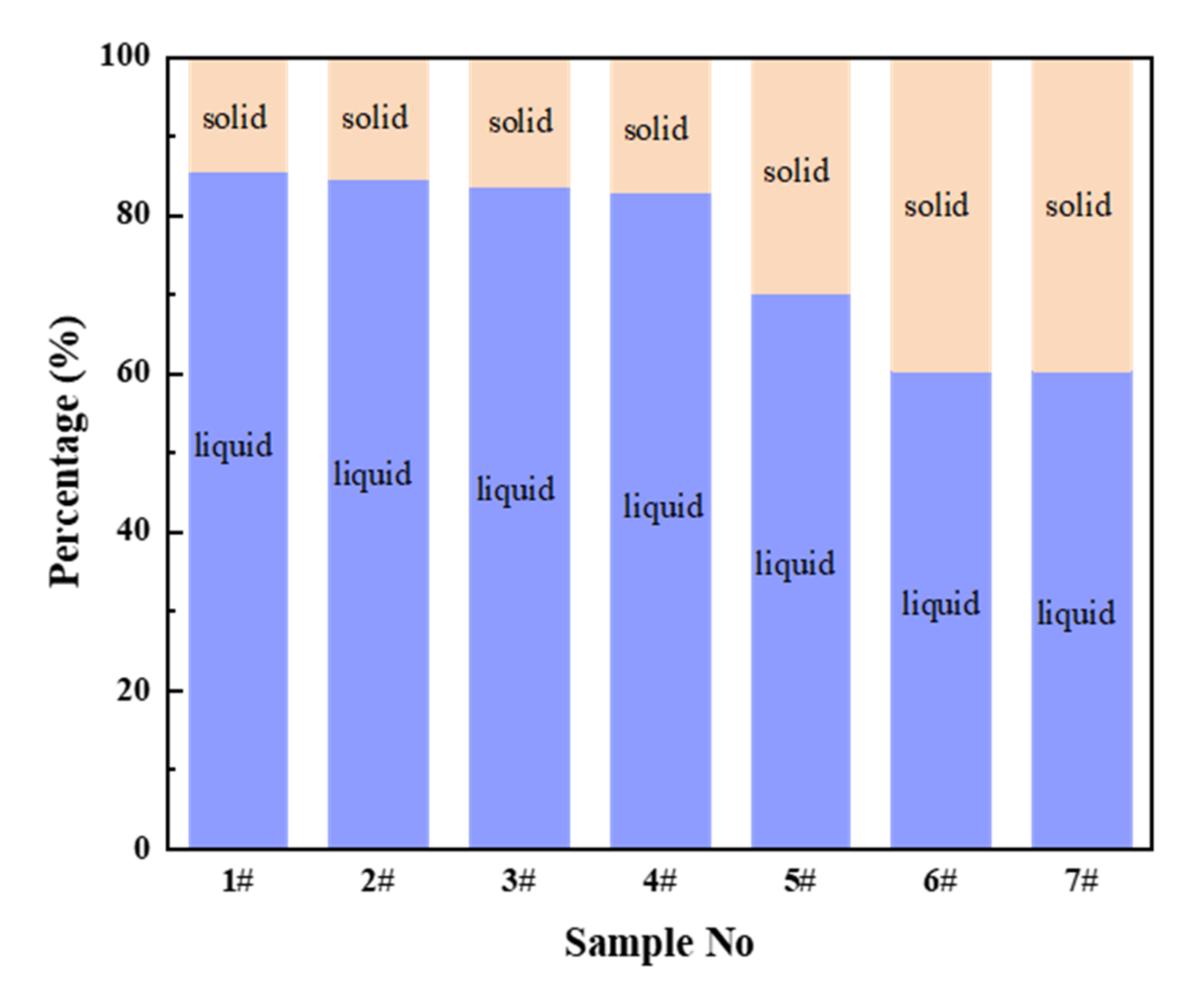
3.2. Melting Properties
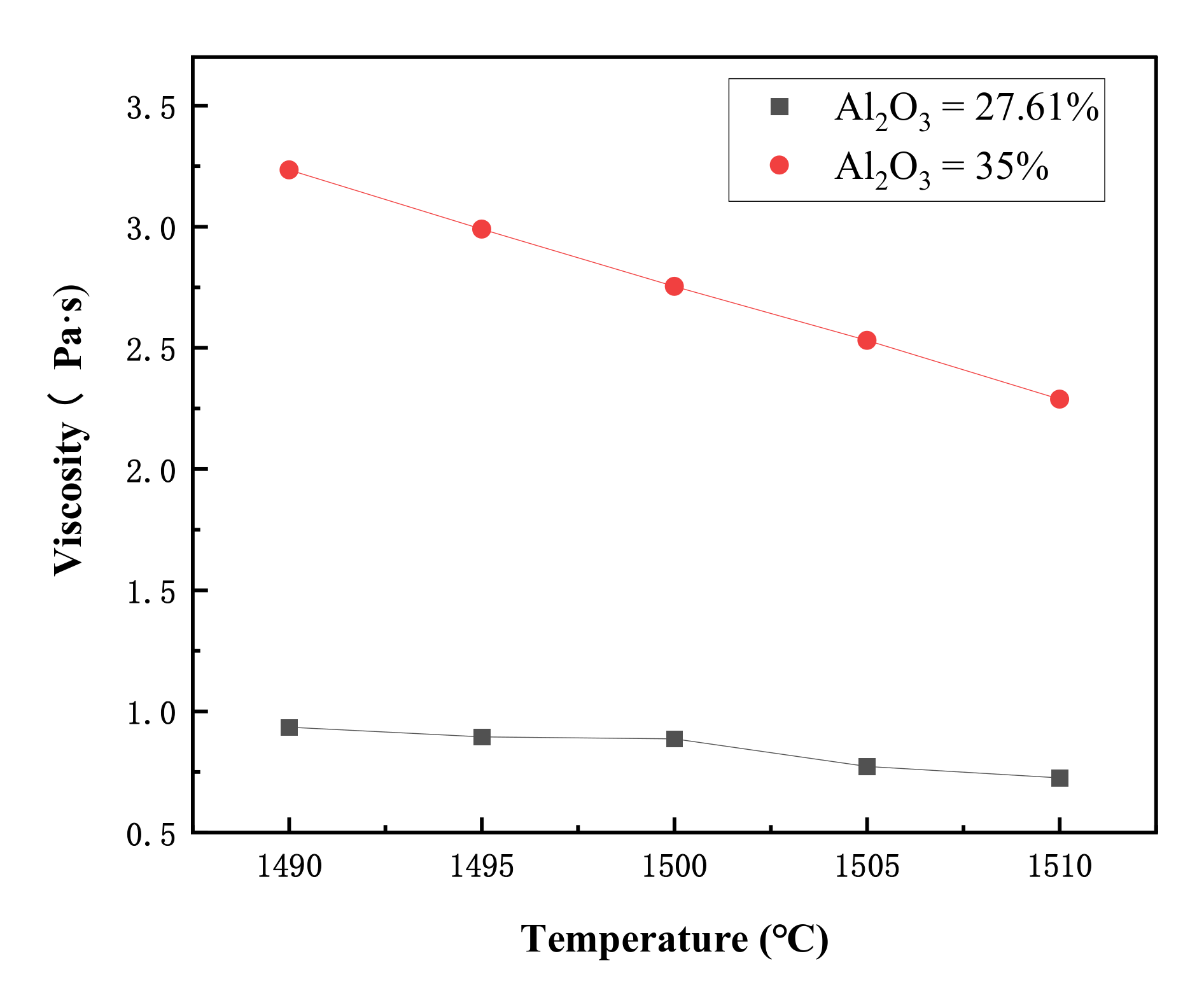
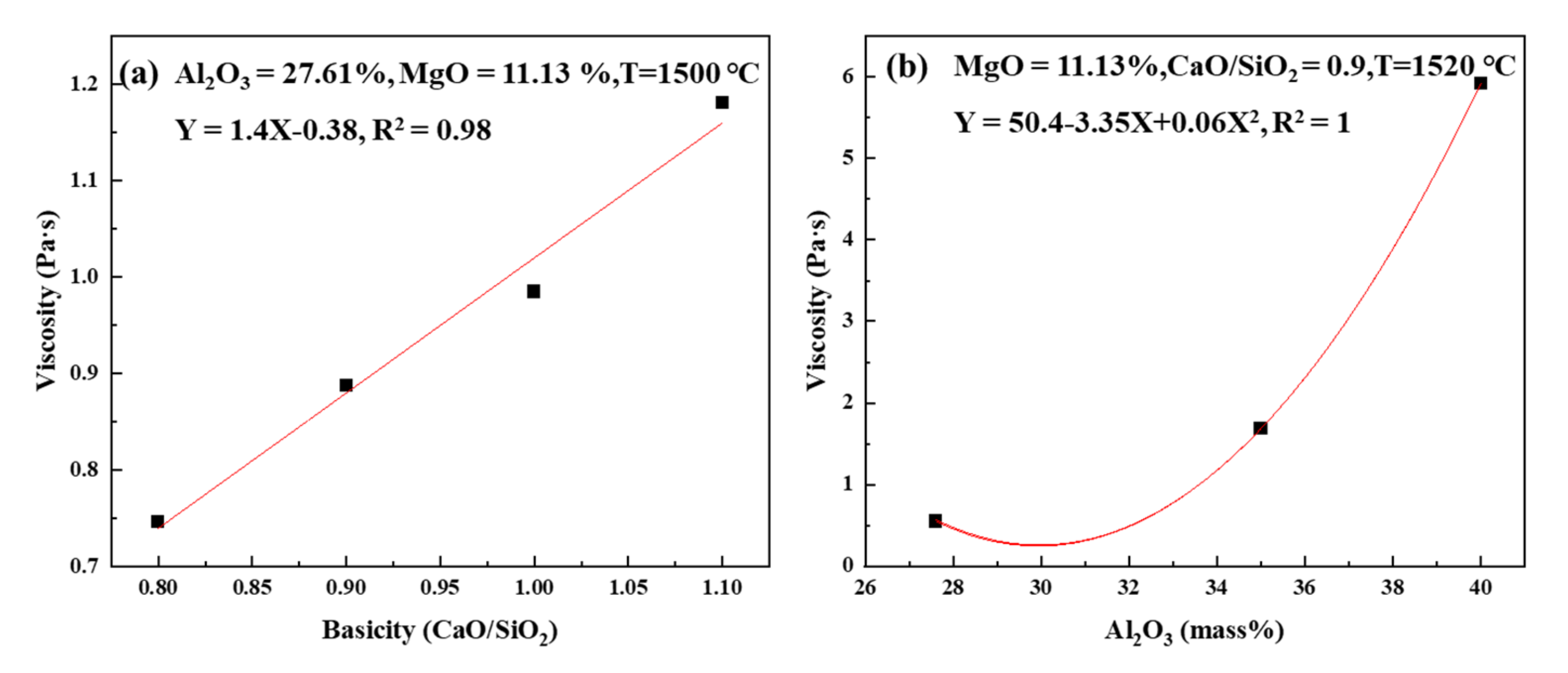
3.3. Slag Viscosity
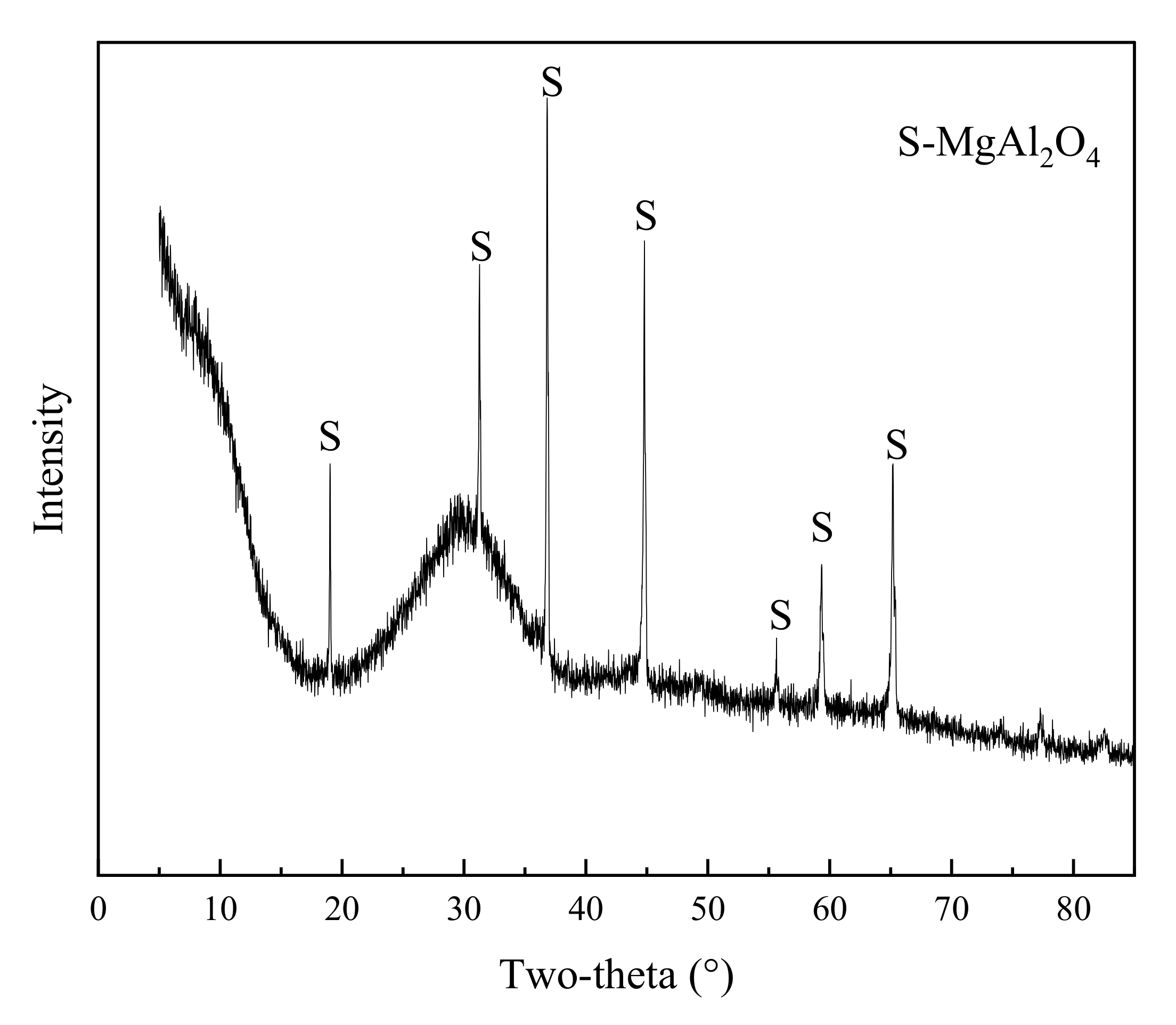
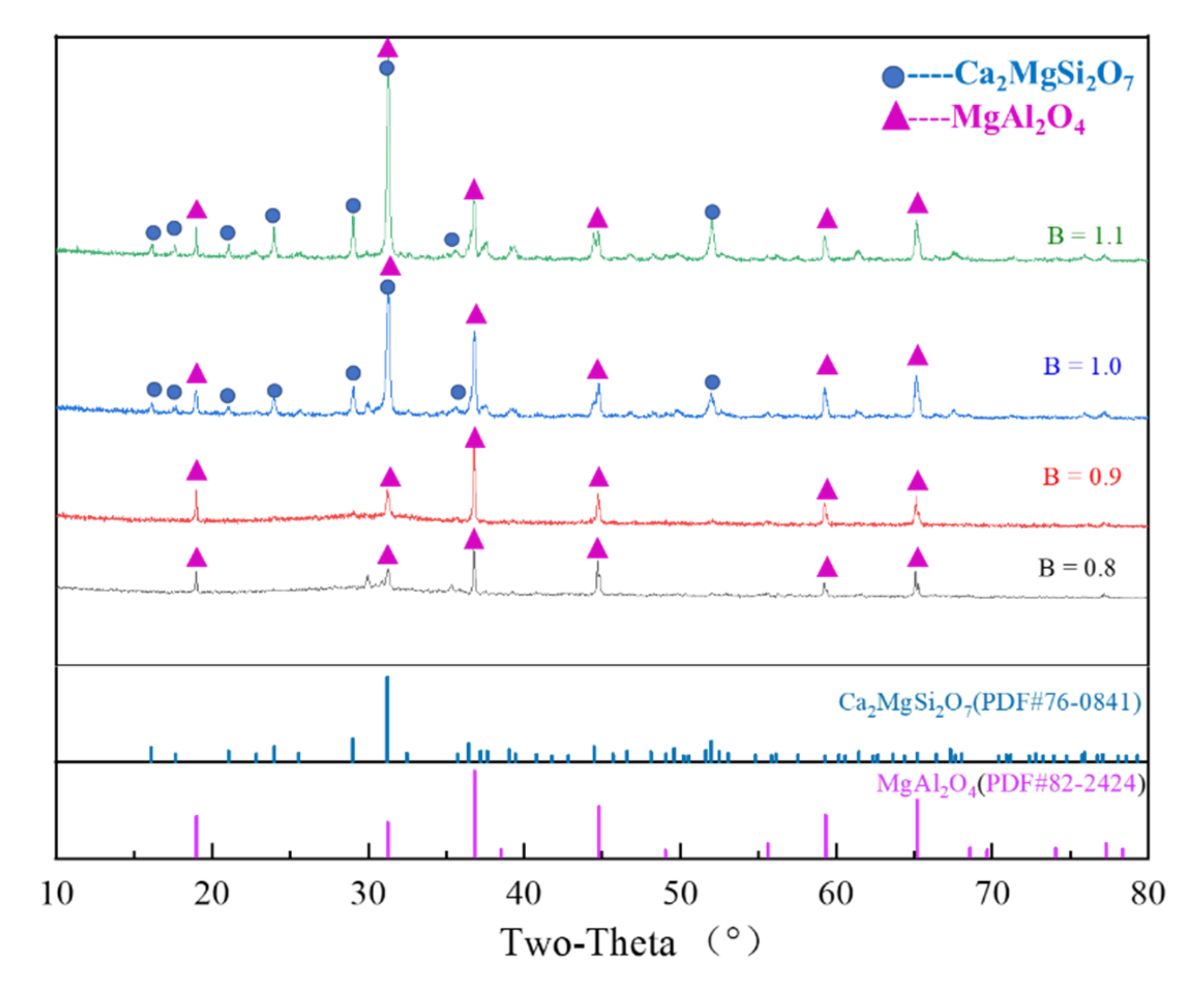
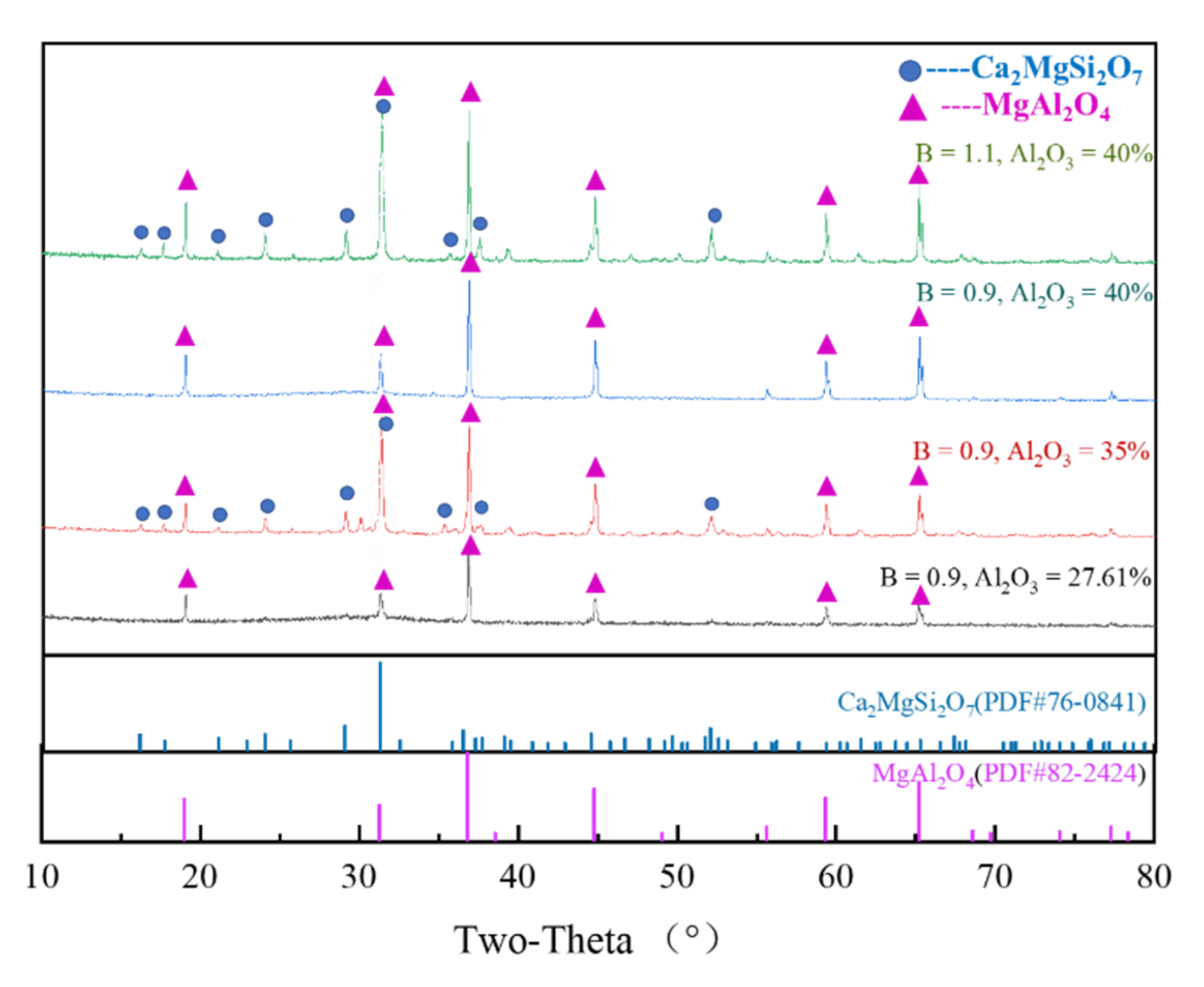
3.4. Determination of Crystalline Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, T.; Wang, Q.; Yu, C.; He, S. Structural and viscosity properties of CaO-SiO2-Al2O3-FeO slags based on molecular dynamic simulation. J. Non-Cryst. Solids 2016, 450, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, C.; Lv, X.; Wei, R. Molecular dynamics simulation on the influence of Al2O3 on the slag structure at 1873 K. Mater. Today Proc. 2015, 2, 453–459. [Google Scholar] [CrossRef]
- Sukenaga, S.; Saito, N.; Kawakami, K.; Nakashima, K. Viscosities of CaO-SiO2-Al2O3-(R2O or RO) melts. ISIJ Int. 2006, 46, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Zhan, W.; Liu, Y.; Shao, T.; Han, X.; Pang, Q.; Zhang, J.; He, Z. Evaluating the effect of MgO/Al2O3 ratio on thermal behaviors and structures of blast furnace slag with low carbon consumption. Crystals 2021, 11, 1386. [Google Scholar] [CrossRef]
- Ghosh, D.; Krishnamurthy, V.A.; Sankaranarayanan, S.R. Application of optical basicity to viscosity of high alumina blast furnace slags. J. Min. Metall. 2010, 46, 41–49. [Google Scholar] [CrossRef]
- Liu, W.; Xing, X.D.; Zuo, H.B. Effect of TiO2 on viscosity and sulfide capacity of blast furnace slag containing barium. ISIJ Int. 2020, 60, 1886–1891. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.d.; Matos, P.R.d.; Monteiro, S.N.; Vieira, C.M.F. Rheological and the fresh state properties of alkali-activated mortars by blast furnace slag. Materials 2021, 14, 2069. [Google Scholar] [CrossRef]
- Xiang, D.; Shen, F.; Jiang, X.; Gao, Q.; Zheng, H. Effect of unburned pulverized coal on the melting characteristics and fluidity of blast furnace slag. Crystals 2021, 11, 579. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, S.B.; Hong, C.; Ma, X.J.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt%Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Kong, W.G.; Liu, J.H.; Yu, Y.W.; Hou, X.M.; He, Z.J. Effect of w(MgO)/w(Al2O3) ratio and basicity on microstructure and metallurgical properties of blast furnace slag. Steel Res. Int. 2021, 10, 1223–1232. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; Zhang, J.; Qin, Y.; Bai, C. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5 wt-% TiO2. Can. Metall. Q. 2016, 55, 160215144748001. [Google Scholar] [CrossRef]
- Yao, L.; Ren, S.; Wang, X.; Liu, Q.; Liu, J. Effect of Al2O3, MgO, and CaO/SiO2 on viscosity of high alumina blast furnace slag. Steel Res. Int. 2016, 87, 241–249. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, T.; Xue, X.; Hu, B. Influence of MgO/Al2O3 ratio on viscosity of blast furnace slag with high Al2O3 content. Steel Res. Int. 2016, 87, 87–94. [Google Scholar] [CrossRef]
- Yan, Z.; Pang, Z.; Lv, X.W.; Qiu, G.; Bai, C. Physicochemical Properties of High Alumina Blast Furnace Slag. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2018; Volume 279-286, pp. 279–286. [Google Scholar] [CrossRef]
- Dong, L.; Yan, Z.; Lv, X.; Jie, Z.; Bai, C.B. Transition of blast furnace slag from silicate-based to aluminate-based: Structure evolution by molecular dynamics simulation and raman spectroscopy. Metall. Mater. Trans. B 2016, 48, 1–9. [Google Scholar]
- Zhao, J.; Cui, H.; Bao, S.; Tong, W.; Wu, Y.; Dong, H. Theoretical and experimental study on recovery of Platinum group metals by copper trapping method. Chin. J. Nonferrous Met. 2019, 29, 2820–2825. [Google Scholar]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Mineral. Processing 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total Environ. 2022, 802, 149830. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.; Dai, S.; Huang, D.; Wang, W. Variation of viscosity and crystallization properties of synthetic ferronickel waste slag with Al2O3 content. Ceram. Int. 2021, 47, 22918–22923. [Google Scholar] [CrossRef]
- Biswal, S.S.; Panda, C.; Sahoo, S.; Jena, T.; Panda, K.C. Assessment of factors influencing the elution of chromium from ferrochromium slag using factorial design of experiment. Mater. Today Proc. 2020, 35, 97–101. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, Q.; Wu, X.; Zheng, J.; Hai, O. Recycling of solid wastes ferrochromium slag for preparation of eco-friendly high-strength spinel–corundum ceramics. Mater. Chem. Phys. 2019, 239, 122060. [Google Scholar] [CrossRef]
- Chenglin, Q.; Jianliang, Z.; Jiugang, S.; Weijia, L.; Zhixing, Z.; Xuesong, Z. Study of boronizing mechanism of high-alumina slag. Steel Res. Int. 2011, 82, 1319–1324. [Google Scholar] [CrossRef]
- Shankar, A. Sulphur partition between hot metal and high alumina blast furnace slag. Ironmak. Steelmak. 2006, 33, 413–418. [Google Scholar] [CrossRef]
- Khandelwal, D.; Sanapala, V. Measurement of Viscosity of High Alumina Blast Furnace Slags by Statistical Approach. Ph.D. Thesis, National Institute of Technology Rourkela, Odisha, India, 2015. [Google Scholar]
- Sunahara, K.; Nakano, K.; Hoshi, M.; Inada, T.; Komatsu, S.; Yamamoto, T. Effect of high Al2O3 slag on the blast furnace operations. Isij Int. 2008, 48, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.Y.; Liu, X.H.; Li, J.; Yin, X.T.; Song, S.; Wang, Q. Influence of Al2O3 and MgO on the Viscosity and Stability of CaO-MgO-SiO2-Al2O3 Slags with CaO/SiO2 = 1.0. ISIJ Int. 2017, 57, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Lv, X.M.; Pang, Z.D.; Lv, X.W. Effect of basicity and Al2O3 on viscosity of ferronickel smelting slag. J. Iron Steel Res. Int. 2020, 27, 1400–1406. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhang, Z.T.; Guo, M.; Zhang, M.; Wang, X.D. Viscosities Behavior of CaO-SiO2-MgO-Al2O3 Slag With Low Mass Ratio of CaO to SiO2 and Wide Range of Al2O3 Content. J. Iron Steel Res. Int. 2011, 18, 1–17. [Google Scholar] [CrossRef]
- Zheng, G. Chemical analysis method standards and proficiency testing of laboratory for iron ore. Metall. Anal. 2015, 35, 37–44. [Google Scholar]
- China Standardization Administration. GB/T-219-2008; Determination of Fusibility of Coal ash. China Standardization Administration: Beijing, China, 2008.
- Ma, J.; Li, W.; Fu, G.; Zhu, M. Effect of Al2O3 on the viscosity and crystallization behavior of CaO-SiO2-MgO-Al2O3-TiO2-Cr2O3 slag. JOM 2021, 74, 159–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bao, S.; Chen, T.; Han, J. Calculation of mineral phase and liquid phase formation temperature during roasting of vanadium-bearing stone coal using FactSage software. Int. J. Mineral. Process. 2013, 124, 150–153. [Google Scholar] [CrossRef]
- Bale, C.W.; Belisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melancon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, M.; Guo, Y.F.; Jiang, T.; Zhao, B.J. Comparison of phase equilibria between FactSage predictions and experimental results in titanium oxide-containing system. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2018, 63, 77–81. [Google Scholar] [CrossRef]
- Rocha, V.C.d.; Silva, M.L.d.; Bielefeldt, W.V.; Vilela, A.C.F. Assessment of viscosity calculation for calcium-silicate based slags using computational thermodynamics. REM Int. Eng. J. 2018, 71, 243–252. [Google Scholar] [CrossRef]
- Eriksson, G.; Konigsberger, E. FactSage and ChemApp: Two tools for the prediction of multiphase chemical equilibria in solutions. Pure Appl. Chem. 2008, 80, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.-H.; Van Ende, M.-A. Computational thermodynamic calculations: FactSage from CALPHAD thermodynamic database to virtual process simulation. Metall. Mater. Trans. B-Process. Metall. Mater. Process. Sci. 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Kim, H.; Matsuura, H. Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate–based slags containing 10 mass pct MgO. Metall. Mater. Trans. B 2013, 44, 5–12. [Google Scholar] [CrossRef]
- Kim, Y.; Min, D.-J. Viscosity and structural investigation of high-concentration Al2O3 and MgO slag system for FeO reduction in electric arc furnace processing. Metals 2021, 11, 1169. [Google Scholar] [CrossRef]
- Shen, X.; Chen, M.; Wang, N.; Wang, D. Viscosity property and melt structure of CaO-MgO-SiO2-Al2O3-FeO slag system. ISIJ Int. 2019, 59, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-J.; Zhao, Y.-H.; Yu, S.; Zhang, L.-X.; Ye, Z.-C.; Wang, N.; Chen, M. Viscosity property and structure analysis of FeO-SiO2-V2O3-TiO2-Cr2O3 Slags. ISIJ Int. 2016, 56, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zhang, X.; Zhang, M.; Jia, X. Effect of CaF2 on viscosity, structure and properties of CaO-Al2O3-MgO-SiO2 slag glass ceramics. J. Non-Cryst. Solids 2018, 500, 310–316. [Google Scholar] [CrossRef]
- Li, T.; Sun, C.; Song, S.; Wang, Q. Influences of Al2O3 and TiO2 content on viscosity and structure of CaO–8%MgO–Al2O3–SiO2–TiO2–5%FeO blast furnace primary slag. Metals 2019, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.-Y.; Wang, C.; Xu, R.-Z.; Zhang, J.-L.; Jiao, K.-X. Effect of Al2O3 on the viscosity of CaO-SiO2-Al2O3-MgO-Cr2O3 slags. Int. J. Miner. Metall. Mater. 2021, 28, 797–803. [Google Scholar] [CrossRef]
- Roscoe, R. The viscosity of suspensions of rigid spheres. Br. J. Appl. Phys 1952, 3, 267. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zheng, Y.L.; Chou, K.C. Influence of TiC on the Viscosity of CaO-MgO-Al2O3-SiO2-TiC Suspension System. ISIJ Int. 2015, 55, 922–927. [Google Scholar] [CrossRef] [Green Version]



| SiO2 | CaO | Al2O3 | MgO | FeO | TFe | MnO | S | TiO2 | Cr2O3 | MFe |
| 27.9 | 24.90 | 23.8 | 9.59 | 1.63 | 1.40 | 1.36 | 0.97 | 0.58 | 0.57 | 0.13 |
| No. | Slag Composition | Binary Basicity (B = CaO/SiO2) | |||
|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | ||
| 1# | 27.23 | 34.03 | 11.13 | 27.61 | 0.8 |
| 2# | 29.02 | 32.24 | 11.13 | 27.61 | 0.9 |
| 3# | 30.63 | 30.63 | 11.13 | 27.61 | 1.0 |
| 4# | 32.09 | 29.17 | 11.13 | 27.61 | 1.1 |
| 5# | 25.52 | 28.35 | 11.13 | 35.00 | 0.9 |
| 6# | 23.15 | 25.72 | 11.13 | 40.00 | 0.9 |
| 7# | 25.60 | 23.27 | 11.13 | 40.00 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiang, Y.; Guo, Y.; Yang, Z.; Chen, F.; Yang, L.; Li, G. Effects of Basicity and Al2O3 Content on Viscosity and Crystallization Behavior of Super-High-Alumina Slag. Crystals 2022, 12, 851. https://doi.org/10.3390/cryst12060851
Wang S, Jiang Y, Guo Y, Yang Z, Chen F, Yang L, Li G. Effects of Basicity and Al2O3 Content on Viscosity and Crystallization Behavior of Super-High-Alumina Slag. Crystals. 2022; 12(6):851. https://doi.org/10.3390/cryst12060851
Chicago/Turabian StyleWang, Shuai, Ying Jiang, Yufeng Guo, Zhuang Yang, Feng Chen, Lingzhi Yang, and Guang Li. 2022. "Effects of Basicity and Al2O3 Content on Viscosity and Crystallization Behavior of Super-High-Alumina Slag" Crystals 12, no. 6: 851. https://doi.org/10.3390/cryst12060851






