Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Specimen Characterization
3. Results and Discussion
3.1. Determination of Aggregate Activity
3.1.1. Chemical Compositions of Four Types of Aggregates
3.1.2. Mineral Compositions of the Four Types of Aggregates
3.1.3. Petrographic Analysis of Aggregates
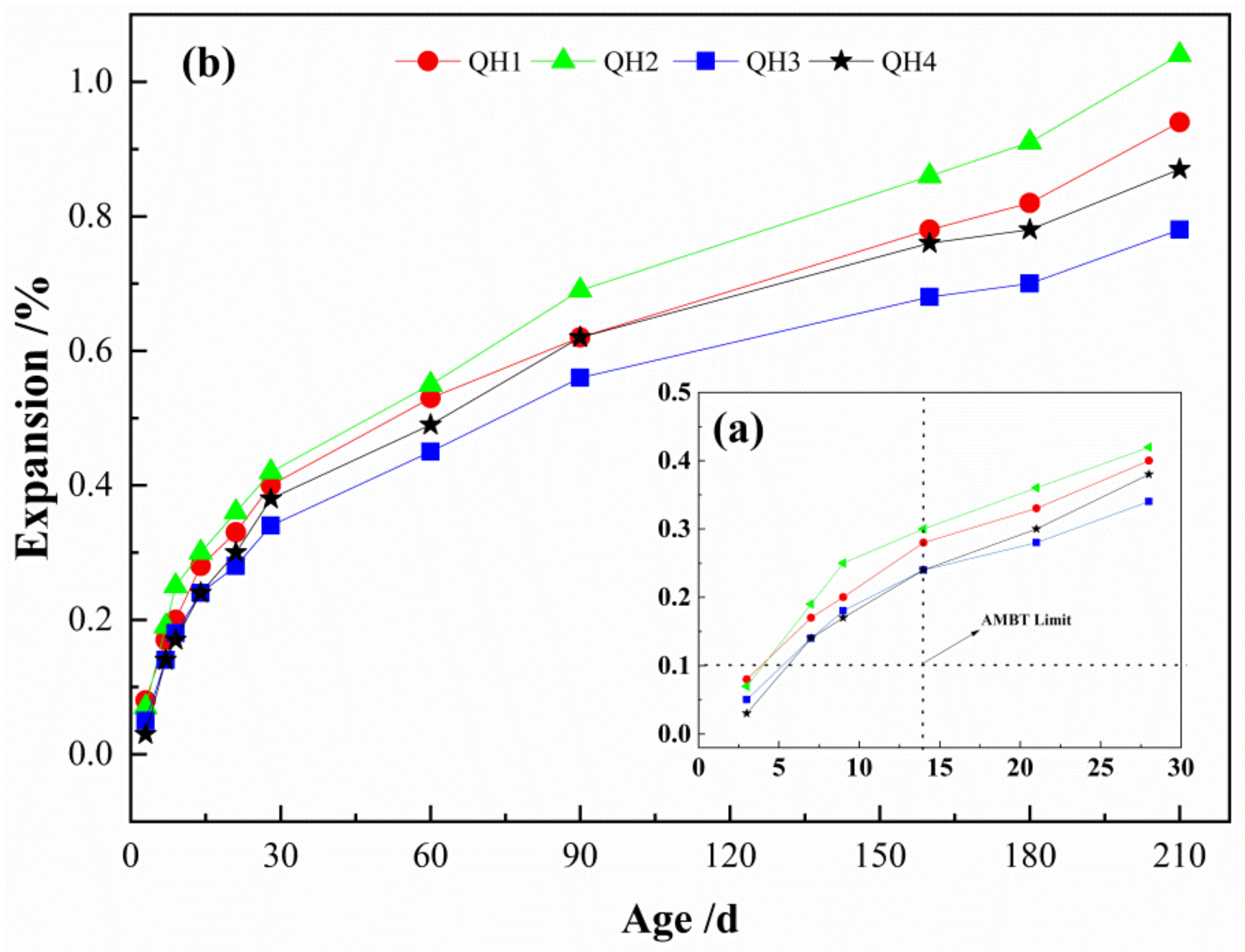
3.1.4. Expansion Rate of Four Types of Aggregates
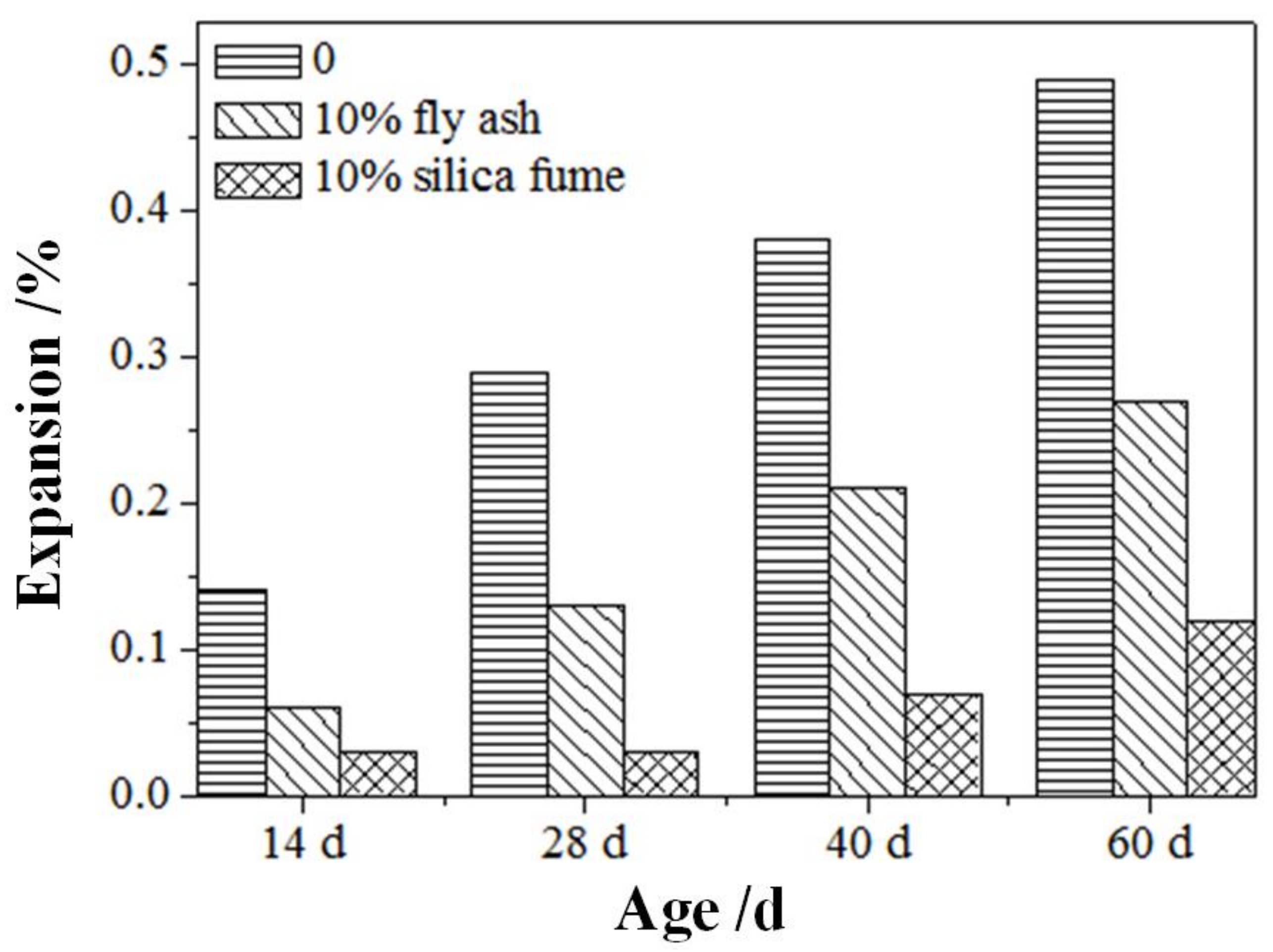
3.2. Inhibitory Effect of Fly Ash on Aggregate Alkali Activity
3.3. Inhibitory Effect of Silica Fume on Aggregate Alkali Activity
3.4. Comparison of Inhibition Effect between Fly Ash and Silica Fume
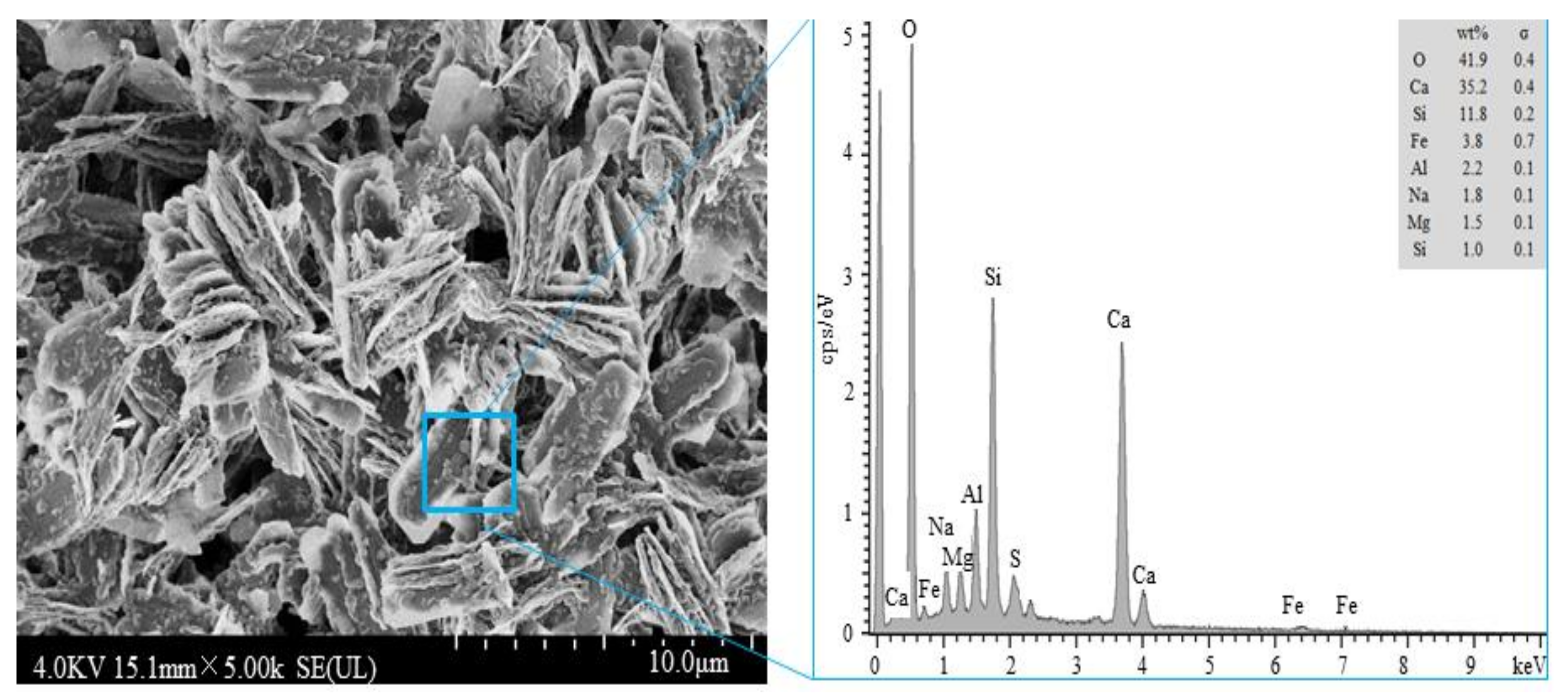
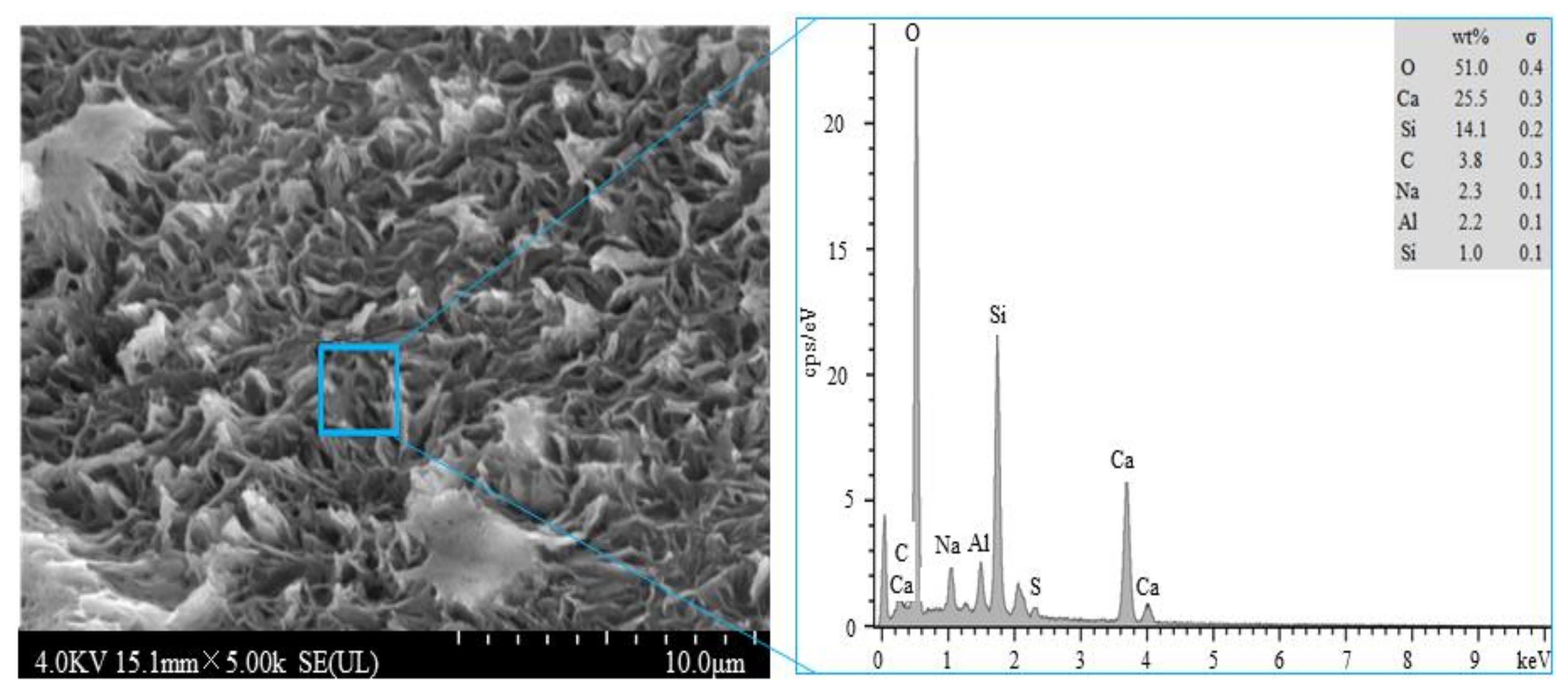
3.5. Analysis of Inhibition Mechanisms
4. Conclusions
- Aggregates in Northwest China are generally alkaline-active and exhibit serious, harmful ASRs. Therefore, it is necessary to implement measures to prevent alkali aggregate damage in concrete structures in the concrete mix design process.
- Standard GB/T 50733-2011 was used to evaluate the inhibitory effects of the admixtures on the activity of the aggregate base. The results demonstrate that when fly ash and silica fume dosages are more 20% and 10%, respectively, the 14 d expansion rates of the mortar bar are less than 0.03%, thereby effectively preventing the ASR of the aggregate; this provides a reference value for optimizing the mix ratio of high-performance concrete in the northwest regions.
- The effects of fly ash and silica ash on inhibiting the alkaline activity expansion differ. For the same amounts of fly ash and silica ash (for example, 10%), silica fume exhibits a superior inhibitory effect over the fly ash. Fly ash and silica fume adsorb alkali ions by forming hydration products with a lower ca/si ratio, so as to reduce their expansion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafaatian, S.M.H.; Akhavan, A.; Maraghechi, H.; Rajabipour, F. How does fly ash mitigate alkali–silica reaction (ASR) in accelerated mortar bar test (ASTM C1567)? Cem. Concr. Compos. 2013, 37, 143–153. [Google Scholar] [CrossRef]
- Saccani, A.; Manzi, S. The Role of Microstructure in Alkali–Silica Reaction Tests. Crystals 2022, 12, 646. [Google Scholar] [CrossRef]
- Yi, L.; Mao, Z.; Deng, M.; Liu, X.; Fan, Z.; Huang, X.; Zhang, T.; Tang, M. Rapid Test Method for Evaluating Inhibiting Effectiveness of Supplementary Cementitious Materials on Alkali–Silica Reaction Expansion of Concrete. Materials 2022, 15, 3202. [Google Scholar] [CrossRef] [PubMed]
- Juenger, M.C.G.; Ostertag, C.P. Alkali–silica reactivity of large silica fume-derived particles. Cem. Concr. Res. 2004, 34, 1389–1402. [Google Scholar] [CrossRef]
- Maas, A.J.; Ideker, J.H.; Juenger, M.C.G. Alkali silica reactivity of agglomerated silica fume. Cem. Concr. Res. 2007, 37, 166–174. [Google Scholar] [CrossRef]
- Glasser, L.S.; Kataoka, N. The Chemistry of ‘Alkali-Aggregate’ Reaction. Cem. Concr. Res. 1981, 11, 1–9. [Google Scholar] [CrossRef]
- Mo, X.Y.; Yua, C.J.; Xu, Z.Z. Long-term Effectiveness and Mechanism of LiOH in Inhibiting Alkali–Silica Reaction. Cem. Concr. Res. 2003, 33, 115–119. [Google Scholar] [CrossRef]
- Jing, L.; Masoud, M.; Yubao, Z.; Pengqiang, Z.; Maria, R.; Peyman, M. Utilizing Artificial Intelligence to Predict the Superplasticizer Demand of Self-Consolidating Concrete Incorporating Pumice, silica fume, and Fly Ash Powders. Materials 2021, 14, 6792. [Google Scholar]
- Liu, X.; Mao, Z.; Yi, L.; Fan, Z.; Zhang, T.; Huang, X.; Deng, M.; Tang, M. Experimental Method for Evaluating the Reactivity of Alkali-Carbonate Reaction Activity. Materials 2022, 15, 2853. [Google Scholar] [CrossRef]
- Tang, M. Classification of Alkali-aggregate Reaction. In Proceedings of the 9th International Conference on Alkali-Aggregate Reaction in Concrete, London, UK, 27–31 July 1992; Concrete Society: Camberley, UK, 1992; pp. 648–653. [Google Scholar]
- Stanton, T.E. Expansion of Concrete Through Reaction Between Cement and Aggregate. Proc. ASCE 1940, 66, 1781–1811. [Google Scholar] [CrossRef]
- Chen, B.; Deng, M.; Huang, X.; Mo, L.; Huang, B.; Lan, X. Siliceous and Dolomitic-bearing Aggregates Reaction in Tetramethyl Ammonium Hydroxide. Constr. Build. Mater. 2021, 299, 123948. [Google Scholar] [CrossRef]
- Lima, S.; Gomes, T.; Moraes, J. Novel One-part Alkali-Activated Binder Produced with Coffee Husk Ash. Mater. Lett. 2022, 313, 131733. [Google Scholar] [CrossRef]
- Yurt, Ü.; Bekar, F. Comparative Study of Hazelnut-Shell Biomass Ash and Metakaolin to Improve the Performance of Alkali-Activated Concrete: A Sustainable Greener Alternative. Constr. Build. Mater. 2022, 320, 126230. [Google Scholar] [CrossRef]
- Wang, C.; He, H.; Wang, Y.; Xue, W. Effects of Fluorogypsum and Flue-gas Desulfurization Gypsum on the Hydration and Hardened Properties of AlkaliSlag Cement. Crystals 2021, 11, 1475. [Google Scholar] [CrossRef]
- Lo, K.Y.; Hefny, A.M. Measurements of Residual Expansion Rates Resulting from Alkali-Aggregate Reaction in Existing Concrete Dams. ACI Mater. J. 1999, 96, 339–345. [Google Scholar]
- Pantazopoulou, S.J.; Thomas, M.D.A. Modeling Stress-Strain Behavior of Concrete Damaged by Alkali-Aggregate Reaction (AAR). ACI Struct. J. 1999, 96, 790–798. [Google Scholar]
- Tang, M.; Deng, M. Recent Progress of Studies on Alkali Aggregate Reaction. J. Build. Mater. 2003, 6, 1–8. [Google Scholar]
- Lu, D.; Lu, Y.; Xu, Z.; Tang, M. Effectiveness of Fly Ash on Suppressing ASR Expansion and its Evaluation. J. Chin. Silic. Soc. 2003, 31, 498–593. [Google Scholar]
- Mo, X. Laboratory Study of LiOH in Inhibiting Alkali–Silica Reaction at 20 °C: A Contribution. Cem. Concr. Res. 2005, 35, 499–504. [Google Scholar] [CrossRef]
- Mo, I.X.Y.; Xu, Z.Z.; Wu, K.R.; Tang, M.S. Effectiveness of LiOH in Inhibiting Alkali-Aggregate Reaction and its Mechanism. Mater. Struct. 2005, 38, 57–61. [Google Scholar] [CrossRef]
- Mo, X.; Jin, T.; Li, G.; Wang, K.; Xu, Z.; Tang, M. Alkali-Aggregate Reaction Suppressed by Chemical Admixture at 80 °C. Constr. Build. Mater. 2005, 19, 473–479. [Google Scholar] [CrossRef]
- Thomas, M.; Dunster, A.; Nixon, P.; Blackwell, B. Effect of Fly Ash on the Expansion of Concrete due to Alkali-Silica Reaction–Exposure Site Studies. Cem. Concr. Compos. 2011, 33, 359–367. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, C.; Wei, X. Influence of Lithium Sulfate Addition on the Properties of Portland Cement Paste. Constr. Build. Mater. 2014, 50, 457–462. [Google Scholar] [CrossRef]
- Pan, J.; Feng, Y.T.; Jin, F. Meso-Scale Particle Modeling of Concrete Deterioration Caused by Alkali-Aggregate Reaction. Int. J. Numer. Anal. Met. 2012, 37, 2690–2705. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.N.N.; Sarker, P.K.; Shaikh, F.A.; Pramanik, A. The ASR Mechanism of Reactive Aggregates in Concrete and its Mitigation by Fly Ash: A Critical Review. Constr. Build. Mater. 2018, 171, 743–758. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Z.; Tang, M. ASR Models for Siliceous Aggregates with Different Texture and Structure Characteristics. J. Chin. Silic. Soc. 2002, 30, 149–154. [Google Scholar]
- Du, J.; Zhang, X.; Huang, T.; Li, M.; Ga, Z.; Ge, H.; Wang, Z.; Gao, H.; Ma, J. Trade-Driven Black Carbon Climate Forcing and Environmental Equality Under China’s West-East Energy Transmission. J. Clean. Prod. 2021, 313, 127896. [Google Scholar] [CrossRef]
- Zheng, W.; Chang, C.; Xiao, X.; Zhou, Y.; Dong, J.; Li, Y.; Wen, J.; Huang, Q.; Man, Y.A.D. Study on Applicability of Foaming Agent to Magnesium Oxychloride Cement. J. Salt Lake Res. 2019, 27, 62–69. [Google Scholar]
- Zheng, W.X.; Xiao, X.Y.; Chang, C.G.; Dong, J.D.; Wen, J.; Huang, Q.; Zhou, Y.; Li, Y. Characterizing Properties of Magnesium Oxychloride Cement Concrete Pavement. J. Cent. South Univ. 2019, 26, 3410–3419. [Google Scholar] [CrossRef]
- Cheng, J.L.; Yang, B.; Zhou, W.J.; Shang, Z.K.; Yao, L.J.; Hang, R.M.; Gao, C.; Xu, L.; Zhang, Y.M.; Yang, Q.S.; et al. GB/T14684-2011; Sand of Construction. Standardization Administration: Beijing, China, 2012.
- Huang, Q.; Zheng, W.; Xiao, X.; Dong, J.; Wen, J.; Chang, C. Effects of Fly Ash, Phosphoric Acid, and Nano-silica on the Properties of Magnesium Oxychloride Cement. Ceram. Int. 2021, 47, 34341–34351. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, W.; Xiao, X.; Dong, J.; Wen, J.; Chang, C. A study on the Salt Attack Performance of Magnesium Oxychloride Cement in Different Salt Environments. Constr. Build. Mater. 2022, 320, 126224. [Google Scholar] [CrossRef]
- Ding, W.; Len, F.G.; Lu, D.Y.; Wang, L.; Fen, H.M.; Zhou, Y.X.; Hao, T.Y.; Xie, Y.J.; Li, P.X.; Zhang, J.B.; et al. GB/T50733-2011; Technical Code for Prevention of Alkali-Aggregate Reaction in Concrete. Ministry of Housing and Urban-Rural Development: Beijing, China, 2012.
- Li, X.; Yan, P.Y. Influence of Fly Ash Content on Alkalinity of Pore Solution and Microstructure of Cement Pastes. J. Build. Mater. 2010, 13, 787–791. [Google Scholar]
- Wang, W.C. Effects of Fly Ash and Lithium Compounds on the Water-Soluble Alkali and Lithium Content of Cement Specimens. Constr. Build. Mater. 2014, 50, 727–735. [Google Scholar] [CrossRef]
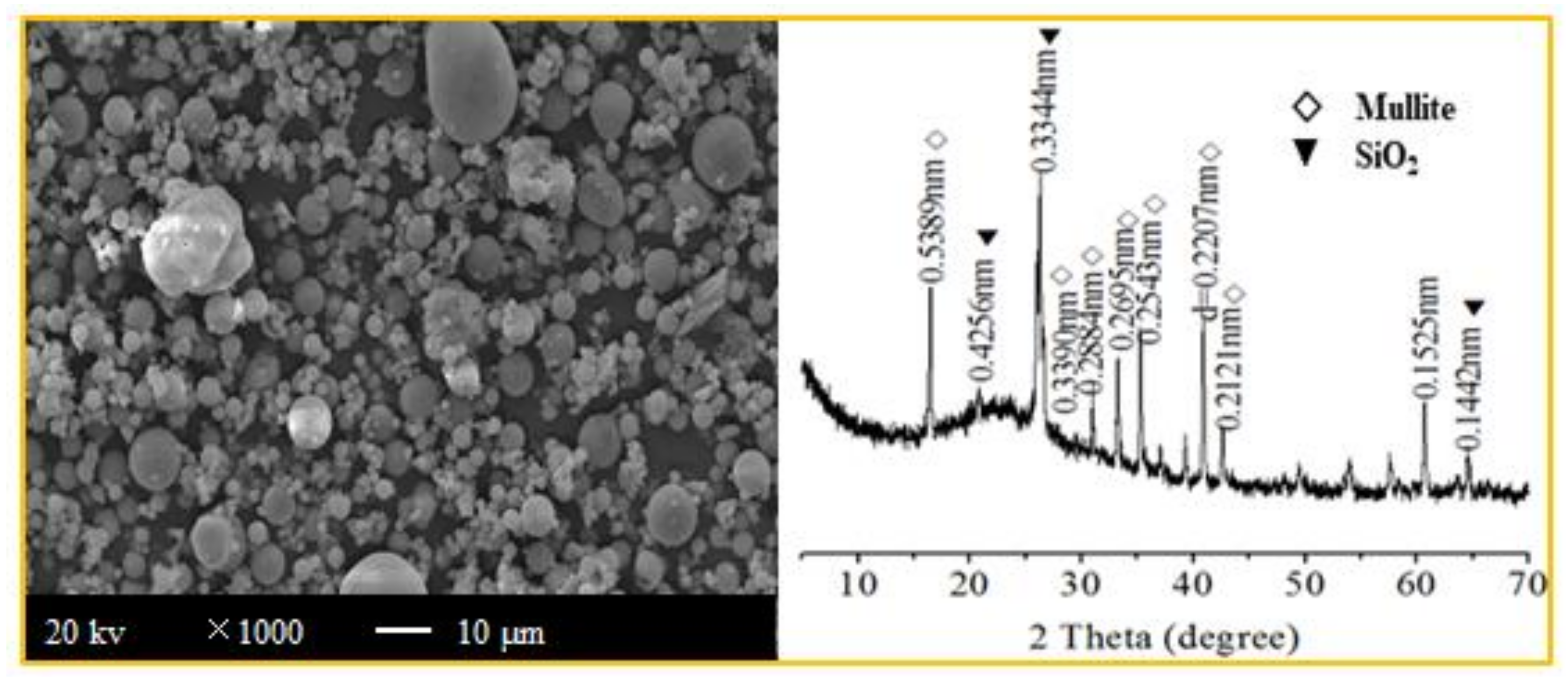

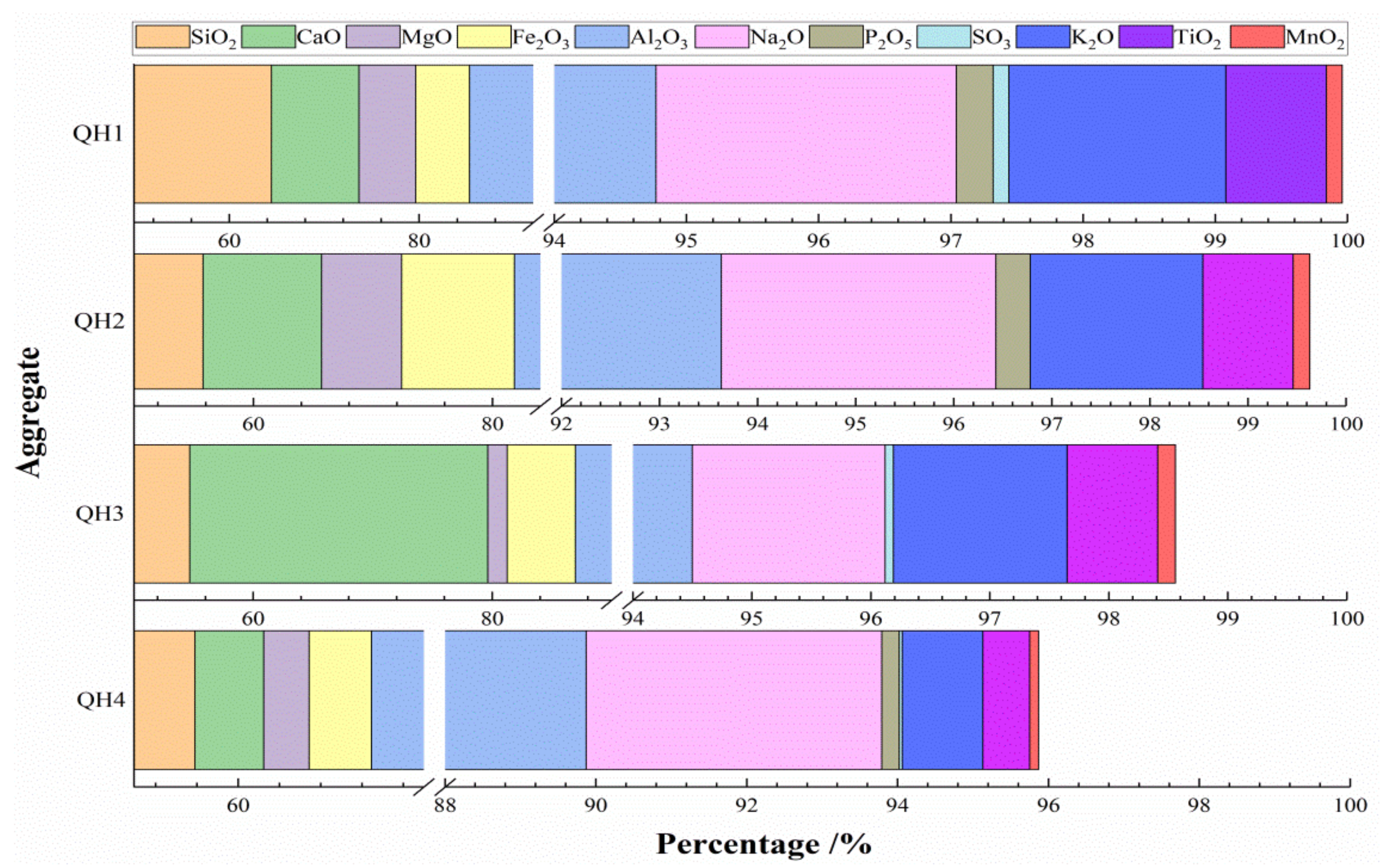
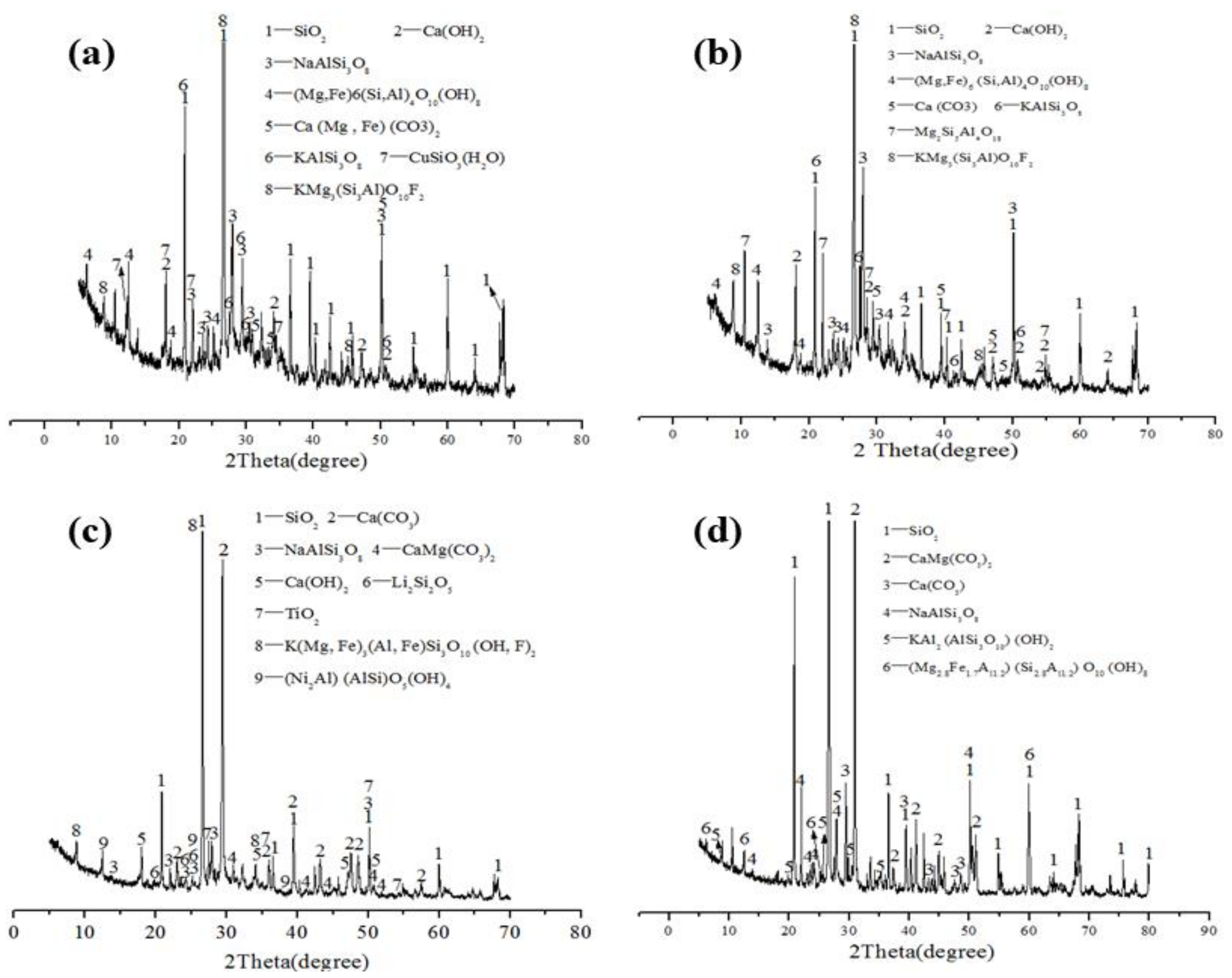
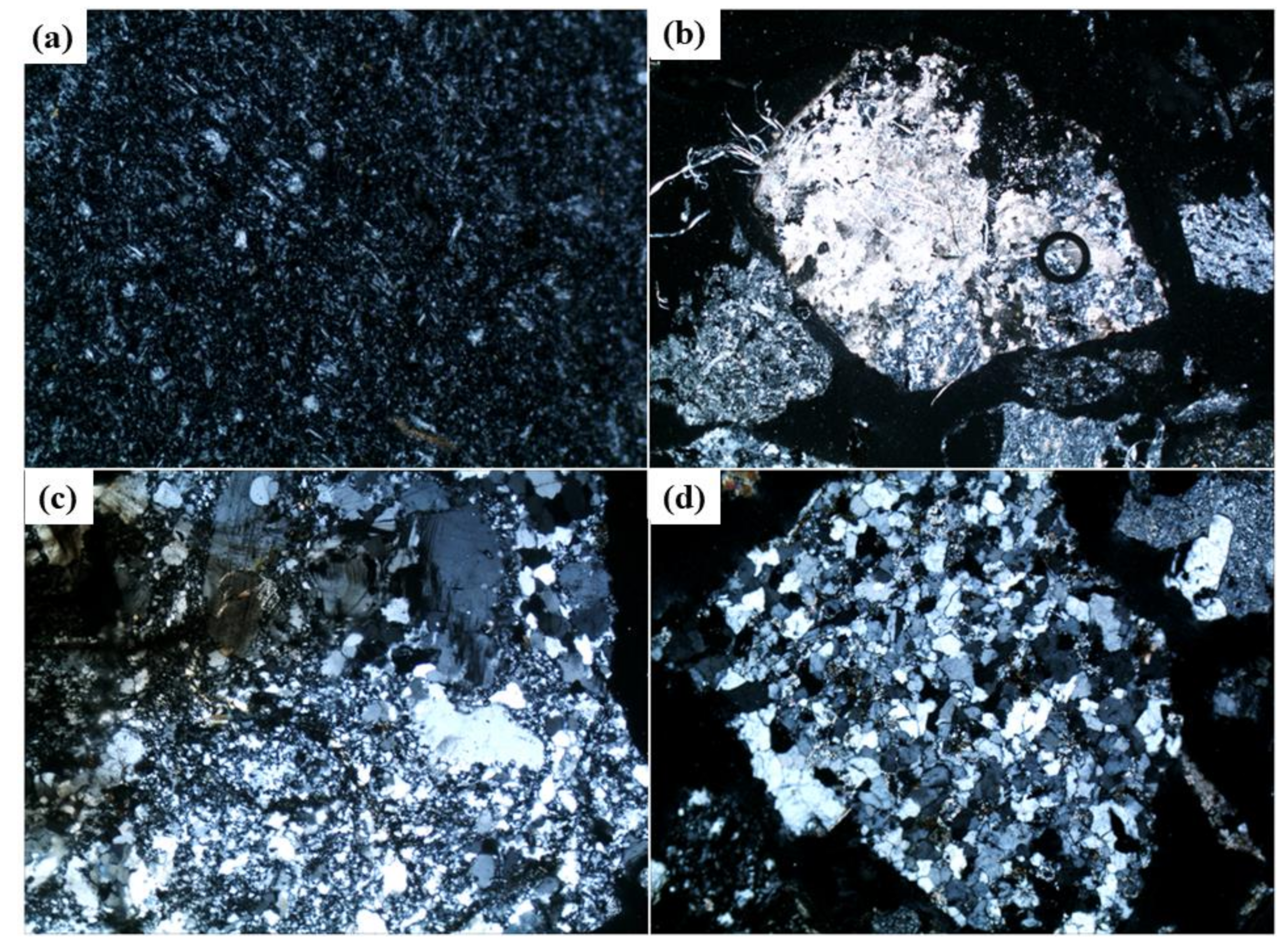


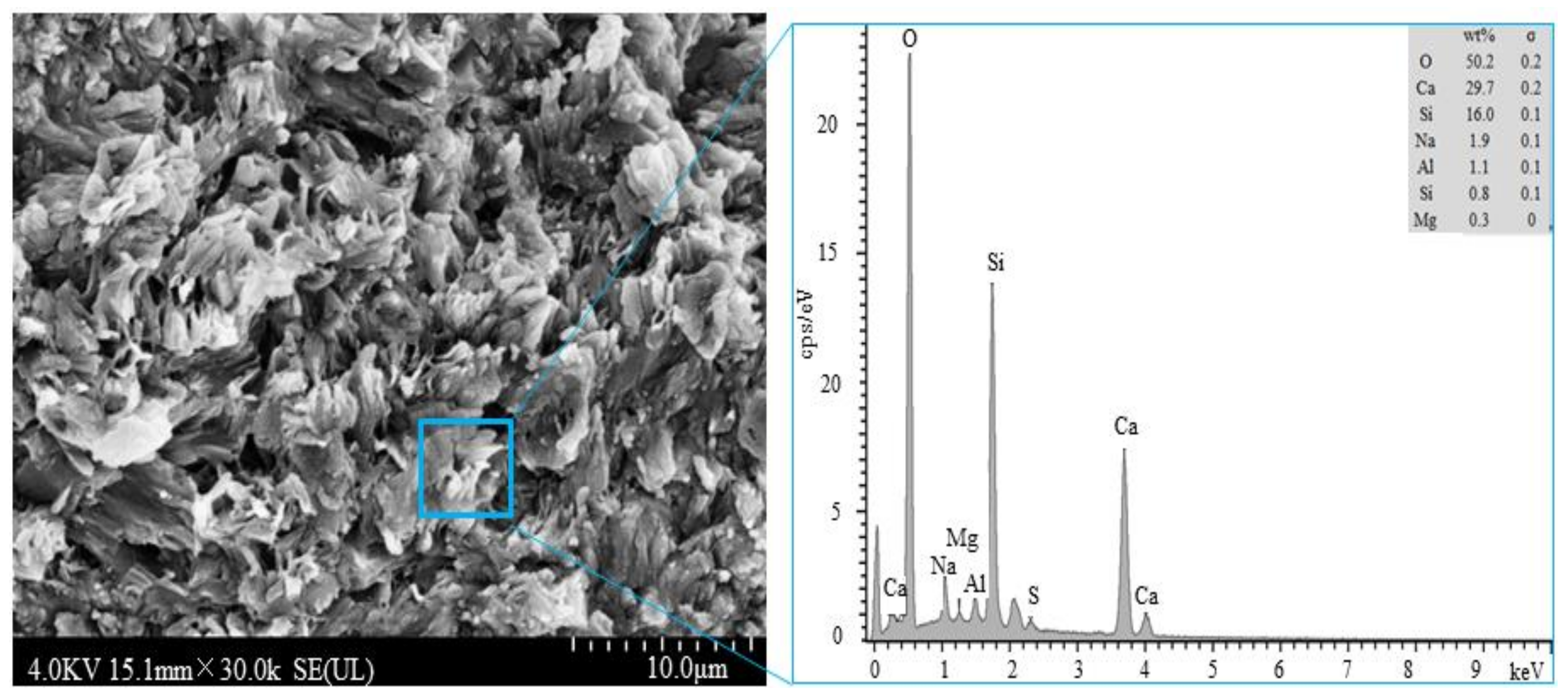
| Content/wt% | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | R2O | LOI |
|---|---|---|---|---|---|---|---|---|
| Cement | 63.82 | 20.43 | 4.83 | 2.79 | 1.53 | 2.05 | 0.88 | 2.89 |
| Fly ash | 4.77 | 54.88 | 26.89 | 6.49 | 1.31 | 1.16 | 1.57 | 3.10 |
| Silica fume | 1.72 | 92.00 | 0.78 | 0.79 | 2.71 | 1.16 | - | 4.67 |
| Region | Datong | Ledu | Xining | Minhe |
|---|---|---|---|---|
| Abbreviation | QH1 | QH2 | QH3 | QH4 |
| Gradations/mm | 4.75~2.36 | 2.36~1.18 | 1.18~0.60 | 0.60~0.30 | 0.30~0.15 |
|---|---|---|---|---|---|
| Contents/wt% | 10 | 25 | 25 | 25 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Dong, J.; Chang, C.; Xiao, X.; Zheng, W. Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China. Crystals 2022, 12, 1013. https://doi.org/10.3390/cryst12071013
Wen J, Dong J, Chang C, Xiao X, Zheng W. Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China. Crystals. 2022; 12(7):1013. https://doi.org/10.3390/cryst12071013
Chicago/Turabian StyleWen, Jing, Jinmei Dong, Chenggong Chang, Xueying Xiao, and Weixin Zheng. 2022. "Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China" Crystals 12, no. 7: 1013. https://doi.org/10.3390/cryst12071013
APA StyleWen, J., Dong, J., Chang, C., Xiao, X., & Zheng, W. (2022). Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China. Crystals, 12(7), 1013. https://doi.org/10.3390/cryst12071013






