Annealing Engineering in the Growth of Perovskite Grains
Abstract
:1. Introduction
2. Conditions and Effects of Annealing
3. Growth of Perovskite
3.1. Nucleation
3.2. Crystal Growth
4. Annealing Methods
4.1. Physical Annealing
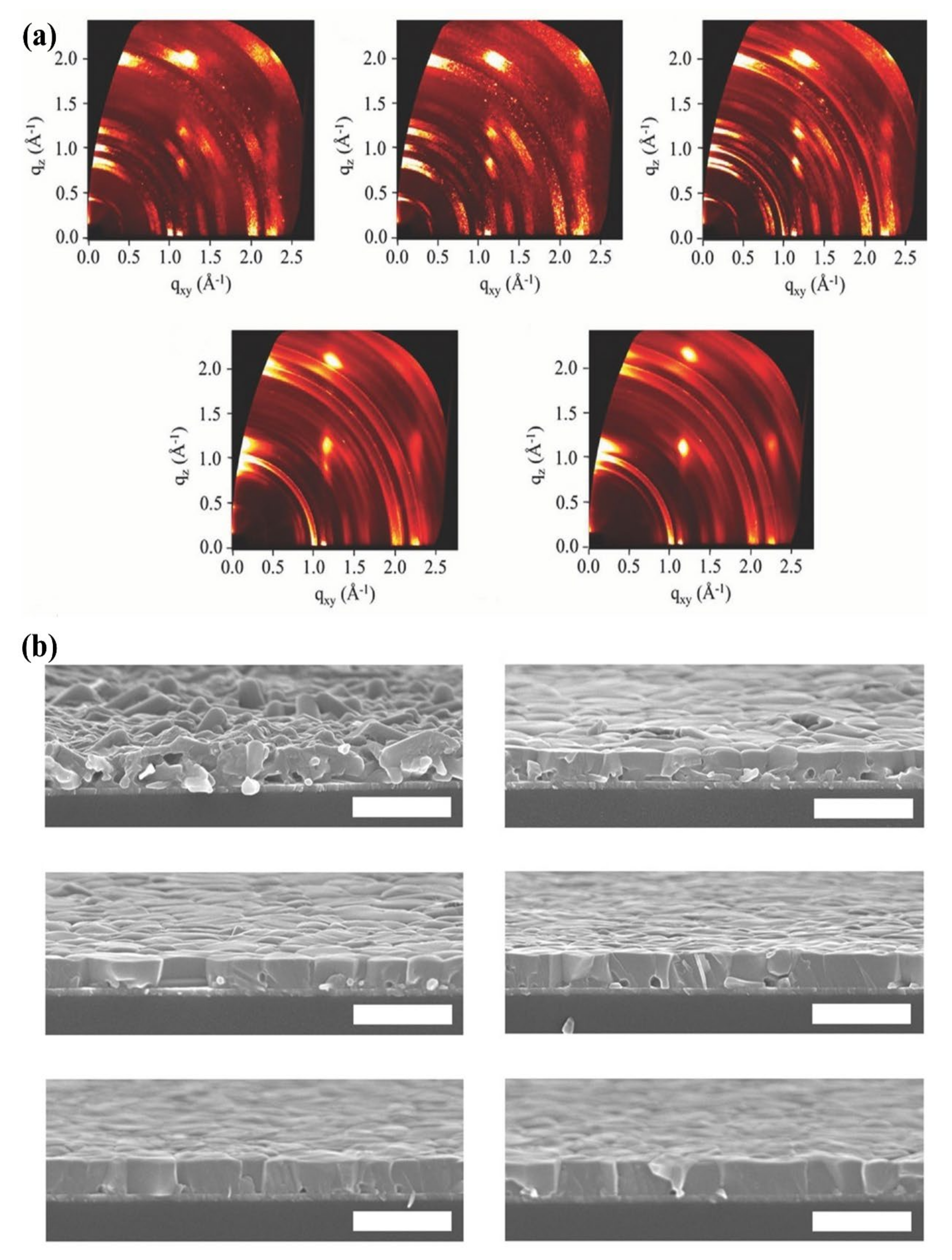
4.1.1. Thermal Annealing
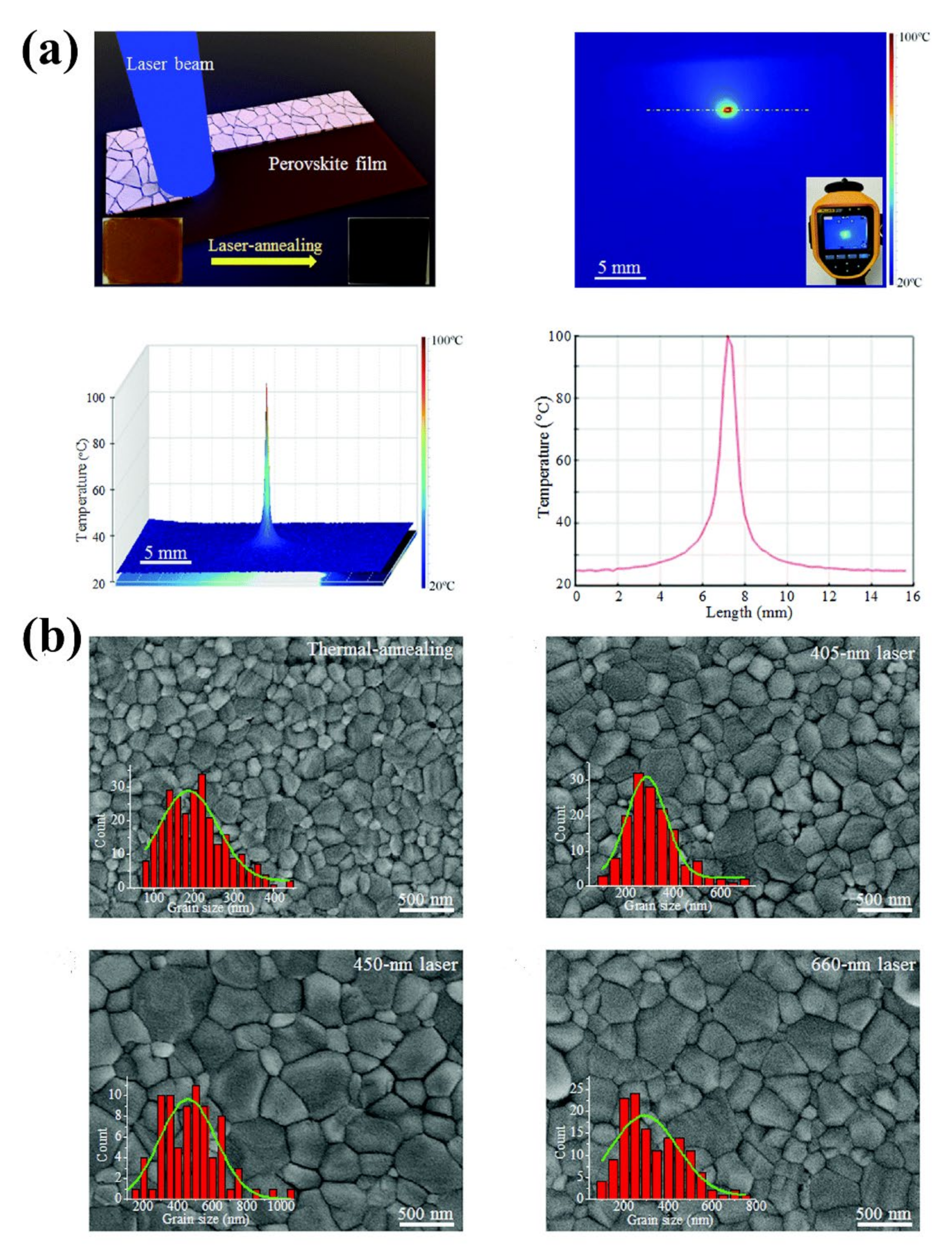
4.1.2. Electromagnetic Wave Annealing
4.1.3. Ultrasonic Annealing
4.1.4. Pressure Annealing
4.2. Chemical Annealing
4.2.1. Solvent Annealing
4.2.2. Solvent–Solvent Extraction Annealing
4.2.3. Encapsulation Layer Annealing
4.2.4. No Annealing
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Ashouri, A.; Kohnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Marquez, J.A.; Vilches, A.B.M.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.W.; Wang, M.; Zang, Z.G.; Tang, X.S.; Fang, L. Two-dimensional lead-free hybrid halide perovskite using superatom anions with tunable electronic properties. Sol. Energy Mater. Sol. Cells 2019, 191, 33–38. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lin, R.X.; Xu, J.; Wei, M.Y.; Wang, Y.R.; Qin, Z.Y.; Liu, Z.; Wu, J.L.; Xiao, K.; Chen, B.; Park, S.M.; et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 2022, 603, 73–78. [Google Scholar] [CrossRef]
- Xie, F.X.; Zhang, D.; Su, H.M.; Ren, X.G.; Wong, K.S.; Gratzel, M.; Choy, W.C.H. Vacuum-Assisted Thermal Annealing of CH3NH3PbI3 for Highly Stable and Efficient Perovskite Solar Cells. ACS Nano 2015, 9, 639–646. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Kasperovich, G.; Hausmann, J. Improvement of fatigue resistance and ductility of TiAl6V4 processed by selective laser melting. J. Mater. Processing Technol. 2015, 220, 202–214. [Google Scholar] [CrossRef]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Film. 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Wang, L.; Huang, M.Q.; Yu, X.F.; You, W.B.; Zhang, J.; Liu, X.H.; Wang, M.; Che, R.C. MOF-Derived Ni1-xCox@Carbon with Tunable Nano-Microstructure as Lightweight and Highly Efficient Electromagnetic Wave Absorber. Nano-Micro Lett. 2020, 12, 150–157. [Google Scholar] [CrossRef]
- Young, C.; Wang, J.; Kim, J.; Sugahara, Y.; Henzie, J.; Yamauchi, Y. Controlled Chemical Vapor Deposition for Synthesis of Nanowire Arrays of Metal-Organic Frameworks and Their Thermal Conversion to Carbon/Metal Oxide Hybrid Materials. Chem. Mater. 2018, 30, 3379–3386. [Google Scholar] [CrossRef]
- Duerinckx, F.; Szlufcik, J. Defect passivation of industrial multicrystalline solar cells based on PECVD silicon nitride. Sol. Energy Mater. Sol. Cells 2002, 72, 231–246. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, Y.K. Thin crystalline silicon solar cell bonded to sintered substrate with aluminum paste. Sol. Energy Mater. Sol. Cells 2005, 86, 577–584. [Google Scholar] [CrossRef]
- Li, D.; de Moraes, E.G.; Guo, P.; Zou, J.; Zhang, J.Z.; Colombo, P.; Shen, Z.J. Rapid sintering of silicon nitride foams decorated with one-dimensional nanostructures by intense thermal radiation. Sci. Technol. Adv. Mater. 2014, 15, 045003–045010. [Google Scholar] [CrossRef] [Green Version]
- Wu, W. Inorganic nanomaterials for printed electronics: A review. Nanoscale 2017, 9, 7342–7372. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, W.J.; Bai, S.X.; Liu, Z.F. Study on the sintering and contact formation process of silver front side metallization pastes for crystalline silicon solar cells. Appl. Surf. Sci. 2016, 376, 52–61. [Google Scholar] [CrossRef]
- Cao, X.B.; Zhi, L.L.; Jia, Y.; Li, Y.H.; Cui, X.; Zhao, K.; Ci, L.J.; Ding, K.X.; Wei, J.Q. High annealing temperature induced rapid grain coarsening for efficient perovskite solar cells. J. Colloid Interface Sci. 2018, 524, 483–489. [Google Scholar] [CrossRef]
- Dualeh, A.; Tetreault, N.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Effect of Annealing Temperature on Film Morphology of Organic-Inorganic Hybrid Pervoskite Solid-State Solar Cells. Adv. Funct. Mater. 2014, 24, 3250–3258. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the Efficiency Beyond 20% with a Mixed Cation Perovskite Solar Cell Fabricated in Ambient Air under Controlled Humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Dong, Q.F.; Bi, C.; Shao, Y.C.; Yuan, Y.B.; Huang, J.S. Solvent Annealing of Perovskite-Induced Crystal Growth for Photovoltaic-Device Efficiency Enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef]
- Liu, D.B.; Zeng, Q.X.; Yao, Y.Q.; Liang, H.F.; Chen, L.J.; Song, Q.L. Controllable Multistep Preparation Method for High-Efficiency Perovskite Solar Cells with Low Annealing Temperature in Glove Box. Energy Technol. 2020, 8, 2000071. [Google Scholar] [CrossRef]
- You, P.; Li, G.J.; Tang, G.Q.; Cao, J.P.; Yan, F. Ultrafast laser-annealing of perovskite films for efficient perovskite solar cells. Energy Environ. Sci. 2020, 13, 1187–1196. [Google Scholar] [CrossRef]
- Bruening, K.; Tassone, C.J. Antisolvent processing of lead halide perovskite thin films studied by in situ X-ray diffraction. J. Mater. Chem. A 2018, 6, 18865–18870. [Google Scholar] [CrossRef]
- Chen, S.S.; Xiao, X.; Chen, B.; Kelly, L.L.; Zhao, J.J.; Lin, Y.Z.; Toney, M.F.; Huang, J.S. Crystallization in one-step solution deposition of perovskite films: Upward or downward? Sci. Adv. 2021, 7, eabb2412. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Haque, F.; Wright, M.; Xu, C.; Uddin, A. Controlled nucleation assisted restricted volume solvent annealing for stable perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 167, 70–86. [Google Scholar] [CrossRef]
- Shargaieva, O.; Nasstrom, H.; Smith, J.A.; Tobbens, D.; Munir, R.; Unger, E. Hybrid perovskite crystallization from binary solvent mixtures: Interplay of evaporation rate and binding strength of solvents. Mater. Adv. 2020, 1, 3314–3321. [Google Scholar] [CrossRef]
- Barrows, A.T.; Lilliu, S.; Pearson, A.J.; Babonneau, D.; Dunbar, A.D.F.; Lidzey, D.G. Monitoring the Formation of a CH3NH3PbI3-xClx Perovskite during Thermal Annealing Using X-Ray Scattering. Adv. Funct. Mater. 2016, 26, 4934–4942. [Google Scholar] [CrossRef] [Green Version]
- Suchan, K.; Just, J.; Becker, P.; Unger, E.L.; Unold, T. Optical in situ monitoring during the synthesis of halide perovskite solar cells reveals formation kinetics and evolution of optoelectronic properties. J. Mater. Chem. A 2020, 8, 10439–10449. [Google Scholar] [CrossRef]
- Serpetzoglou, E.; Konidakis, I.; Maksudov, T.; Panagiotopoulos, A.; Kymakis, E.; Stratakis, E. In situ monitoring of the charge carrier dynamics of CH3NH3PbI3 perovskite crystallization process. J. Mater. Chem. C 2019, 7, 12170–12179. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.W.; Li, Y.F.; Li, X.Y.; Wang, H.Q. Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites. Mater. Horiz. 2015, 2, 378–405. [Google Scholar] [CrossRef]
- Seo, J.Y.; Matsui, T.; Luo, J.S.; Correa-Baena, J.P.; Giordano, F.; Saliba, M.; Schenk, K.; Ummadisingu, A.; Domanski, K.; Hadadian, M.; et al. Ionic Liquid Control Crystal Growth to Enhance Planar Perovskite Solar Cells Efficiency. Adv. Energy Mater. 2016, 6, 1600767. [Google Scholar] [CrossRef]
- Wang, M.H.; Wang, W.; Ma, B.; Shen, W.; Liu, L.H.; Cao, K.; Chen, S.F.; Huang, W. Lead-Free Perovskite Materials for Solar Cells. Nano-Micro Lett. 2021, 13, 21. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.C.; Lyu, M.Q.; Yun, J.H.; Wang, L.Z. In Situ Growth of 2D Perovskite Capping Layer for Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.X.; Isikgor, F.H.; Xue, D.J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H.; et al. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef]
- Moore, D.T.; Sai, H.; Tan, K.W.; Estroff, L.A.; Wiesner, U. Impact of the organic halide salt on final perovskite composition for photovoltaic applications. APL Mater. 2014, 2, 081802. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; McCleese, C.; Kolodziej, C.; Samia, A.C.S.; Zhao, Y.X.; Burda, C. Identification and characterization of the intermediate phase in hybrid organic-inorganic MAPbI(3) perovskite. Dalton Trans. 2016, 45, 3806–3813. [Google Scholar] [CrossRef]
- Liu, Y.H.; Akin, S.; Hinderhofer, A.; Eickemeyer, F.T.; Zhu, H.W.; Seo, J.Y.; Zhang, J.H.; Schreiber, F.; Zhang, H.; Zakeeruddin, S.M.; et al. Stabilization of Highly Efficient and Stable Phase-Pure FAPbI(3)Perovskite Solar Cells by Molecularly Tailored 2D-Overlayers. Angew. Chem. Int. Ed. 2020, 59, 15688–15694. [Google Scholar] [CrossRef]
- Zhang, Y.; Seo, S.; Lim, S.Y.; Kim, Y.; Kim, S.G.; Lee, D.K.; Lee, S.H.; Shin, H.; Cheong, H.; Park, N.G. Achieving Reproducible and High-Efficiency (>21%) Perovskite Solar Cells with a Presynthesized FAPbI(3) Powder. Acs Energy Lett. 2020, 5, 360–366. [Google Scholar] [CrossRef]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly Efficient All-Inorganic Planar Heterojunction Perovskite Solar Cells Produced by Thermal Coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2017, 8, 67–72. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, J.; Niu, G.D.; Tang, J. Inorganic CsPbI3 Perovskite-Based Solar Cells: A Choice for a Tandem Device. Sol. Rrl 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Xia, X.; Ding, Y.; Arain, Z.; An, S.J.; Liu, X.P.; Cristina, R.C.; Dai, S.Y.; Nazeeruddin, M.K. Soft Template-Controlled Growth of High-Quality CsPbI3 Films for Efficient and Stable Solar Cells. Adv. Energy Mater. 2020, 10, 1903751. [Google Scholar] [CrossRef]
- Dinegar, V.K.L.R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Sun, Y.G. Controlled synthesis of colloidal silver nanoparticles in organic solutions: Empirical rules for nucleation engineering. Chem. Soc. Rev. 2013, 42, 2497–2511. [Google Scholar] [CrossRef]
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef]
- Chen, J.W.; Hillig, W.B.; Sears, G.W. The molecular mechanism of solidifition. Acta Metall. 1964, 12, 1421–1439. [Google Scholar] [CrossRef]
- Cahn, J.W. Theory of crystal growth and interface motion. Acta Metall. 1960, 8, 554–562. [Google Scholar] [CrossRef]
- Jie, W. Principle and Technology of Crystal Growth; Science Press: Beijing, China, 2009. [Google Scholar]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the Law of Bravais. Am. Mineral. 1937, 22, 446–467. [Google Scholar]
- Hua, J.C.; Deng, X.; Niu, C.; Huang, F.Z.; Peng, Y.; Li, W.N.; Ku, Z.L.; Cheng, Y.B. A pressure-assisted annealing method for high quality CsPbBr3 film deposited by sequential thermal evaporation. RSC Adv. 2020, 10, 8905–8909. [Google Scholar] [CrossRef] [Green Version]
- Ghahremani, A.H.; Ratnayake, D.; Sherehiy, A.; Popa, D.O.; Druffel, T. Automated Fabrication of Perovskite Photovoltaics Using Inkjet Printing and Intense Pulse Light Annealing. Energy Technol. 2021, 9, 2100452. [Google Scholar] [CrossRef]
- Bi, C.; Shao, Y.C.; Yuan, Y.B.; Xiao, Z.G.; Wang, C.G.; Gao, Y.L.; Huang, J.S. Understanding the formation and evolution of interdiffusion grown organolead halide perovskite thin films by thermal annealing. J. Mater. Chem. A 2014, 2, 18508–18514. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.B.; Ge, Z.Y. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 2020, 49, 1653–1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Chen, Y.Q.; Li, R.Y.; Xu, Y.B.; Feng, J.S.; Yang, D.; Yuan, N.Y.; Zhang, W.H.; Liu, S.; Ding, J.N. Superior Textured Film and Process Tolerance Enabled by Intermediate-State Engineering for High-Efficiency Perovskite Solar Cells. Adv. Sci. 2020, 7, 1903009. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.C.; Tse, K.F.; Lau, T.K.; Li, Y.H.; Su, C.J.; Yang, G.; Chen, J.H.; Zhu, J.Y.; Jeng, U.S.; Li, G.; et al. Manipulating the Mixed-Perovskite Crystallization Pathway Unveiled by In Situ GIWAXS. Adv. Mater. 2019, 31, 1901284. [Google Scholar] [CrossRef]
- Kim, G.; Moon, C.S.; Yang, T.-Y.; Kim, Y.Y.; Chung, J.; Jung, E.H.; Shin, T.J.; Jeon, N.J.; Park, H.H.; Seo, J. A Thermally Induced Perovskite Crystal Control Strategy for Efficient and Photostable Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2020, 4, 2000033. [Google Scholar] [CrossRef]
- Van Franeker, J.J.; Hendriks, K.H.; Bruijnaers, B.J.; Verhoeven, M.; Wienk, M.M.; Janssen, R.A.J. Monitoring Thermal Annealing of Perovskite Solar Cells with In Situ Photoluminescence. Adv. Energy Mater. 2017, 7, 1601822. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, G.H.; Oh, K.S.; Jo, Y.; Yoon, H.; Kim, K.H.; Lee, H.; Kim, J.Y.; Kim, D.S. High-Temperature-Short-Time Annealing Process for High-Performance Large-Area Perovskite Solar Cells. Acs Nano 2017, 11, 6057–6064. [Google Scholar] [CrossRef]
- Shargaieva, O.; Lang, F.; Rappich, J.; Dittrich, T.; Kalus, M.; Meixner, M.; Genzel, C.; Nickel, N.H. Influence of the Grain Size on the Properties of CH3NH3PbI3 Thin Films. Acs Appl. Mater. Interfaces 2017, 9, 38428–38435. [Google Scholar] [CrossRef]
- Lin, P.A.; Zhang, W.F.; Tian, L.W.; Zhang, F.; Zhou, S.H.; Liu, R.; Hu, T.T.; Zhang, M.; Du, L.; Wen, F.; et al. Remanent solvent management engineering of perovskite films for PEDOT: PSS-based inverted solar cells. Sol. Energy 2021, 216, 530–536. [Google Scholar] [CrossRef]
- Huang, L.K.; Hu, Z.Y.; Xu, J.; Zhang, K.; Zhang, J.; Zhu, Y.J. Multi-step slow annealing perovskite films for high performance planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2015, 141, 377–382. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, W.S.; Yu, W.; Yu, S.W.; Wu, Y.L.; Guo, X.; Liu, S.Z.; Li, C. A Two-Stage Annealing Strategy for Crystallization Control of CH3NH3PbI3 Films toward Highly Reproducible Perovskite Solar Cells. Small 2018, 14, 1800181. [Google Scholar] [CrossRef]
- Fan, B.B.; Peng, D.H.; Lin, S.W.; Wang, N.; Zhao, Y.; Sun, Y.M. Enhanced efficiency of planar-heterojunction perovskite solar cells through a thermal gradient annealing process. Rsc Adv. 2015, 5, 58041–58045. [Google Scholar] [CrossRef]
- Xi, J.H.; Yuan, J.F.; Yan, X.Q.; Binks, D.; Tian, J.J. Gradient Annealing of Halide Perovskite Films for Improved Performance of Solar Cells. Acs Appl. Energy Mater. 2020, 3, 8130–8134. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Meng, Y.Q.; Gao, H.L.; Chen, Y.C.; Meng, Q.; Bai, Y.J.; Wang, H.; Zhang, Y.Z.; Yan, H.; Han, C.B. Flexible perovskite solar cells fabricated by a gradient heat treatment process. Sustain. Energy Fuels 2020, 4, 824–831. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, H.Y.; Xu, G.Y.; Xue, R.M.; Wang, S.H.; Li, Y.W.; Li, Y.F. Precise Control of Crystal Growth for Highly Efficient CsPbI2Br Perovskite Solar Cells. Joule 2019, 3, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Liang, C.J.; Zhang, H.M.; Ji, C.; Sun, M.J.; Sun, F.L.; Jing, X.P.; You, F.T.; Lu, Y.W.; He, Z.Q. Additional Organic-Solvent-Rinsing Process to Enhance Perovskite Photovoltaic Performance. Adv. Electron. Mater. 2019, 5, 1900244. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hassanzadeh, H.; Harding, T.G. Thermal analysis of high frequency electromagnetic heating of lossy porous media. Chem. Eng. Sci. 2017, 172, 13–22. [Google Scholar] [CrossRef]
- Ankireddy, K.; Ghahremani, A.H.; Martin, B.; Gupta, G.; Druffel, T. Rapid thermal annealing of CH3NH3PbI3 perovskite thin films by intense pulsed light with aid of diiodomethane additive. J. Mater. Chem. A 2018, 6, 9378–9383. [Google Scholar] [CrossRef]
- Nam, J.B.; Jang, Y.R.; Hwang, Y.T.; Kim, H.H.; Jung, I.H.; Kim, H.S. Intense Pulsed Light Sintering of Screen-Printed Paste Electrode on Silicon Solar Cell for High Throughput and Cost-Effective Low Temperature Metallization. Int. J. Precis. Eng. Manuf. -Green Technol. 2022, 9, 523–535. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, S.; Pareek, S.; Agasti, A.; Mallick, S.; Kabra, D.; Bhargava, P. Radiative and conductive thermal annealing of hybrid organic-inorganic perovskite layer. Sol. Energy Mater. Sol. Cells 2019, 195, 353–357. [Google Scholar] [CrossRef]
- Girolami, M.; Bellucci, A.; Mastellone, M.; Serpente, V.; Orlando, S.; Valentini, V.; Palma, A.L.; Di Carlo, A.; Trucchi, D.M. Improving the Performance of Printable Carbon Electrodes by Femtosecond Laser Treatment. C-J. Carbon Res. 2020, 6, 48. [Google Scholar] [CrossRef]
- Druffel, T.; Dharmadasa, R.; Lavery, B.W.; Ankireddy, K. Intense pulsed light processing for photovoltaic manufacturing. Sol. Energy Mater. Sol. Cells 2018, 174, 359–369. [Google Scholar] [CrossRef]
- Ghahremani, A.H.; Pishgar, S.; Bahadur, J.; Druffel, T. Intense Pulse Light Annealing of Perovskite Photovoltaics Using Gradient Flashes. Acs Appl. Energy Mater. 2020, 3, 11641–11654. [Google Scholar] [CrossRef]
- Lavery, B.W.; Kumari, S.; Konermann, H.; Draper, G.L.; Spurgeon, J.; Druffel, T. Intense Pulsed Light Sintering of CH3NH3PbI3 Solar Cells. Acs Appl. Mater. Interfaces 2016, 8, 8419–8426. [Google Scholar] [CrossRef]
- Piper, R.T.; Daunis, T.B.; Xu, W.J.; Schroder, K.A.; Hsu, J.W.P. Photonic Curing of Nickel Oxide Transport Layer and Perovskite Active Layer for Flexible Perovskite Solar Cells: A Path Towards High-Throughput Manufacturing. Front. Energy Res. 2021, 9, 640960. [Google Scholar] [CrossRef]
- Xu, W.J.; Daunis, T.B.; Piper, R.T.; Hsu, J.W.P. Effects of Photonic Curing Processing Conditions on MAPbI(3) Film Properties and Solar Cell Performance. Acs Appl. Energy Mater. 2020, 3, 8636–8645. [Google Scholar] [CrossRef]
- Bahadur, J.; Ghahremani, A.H.; Gupta, S.; Druffel, T.; Sunkara, M.K.; Pal, K. Enhanced moisture stability of MAPbI(3) perovskite solar cells through Barium doping. Sol. Energy 2019, 190, 396–404. [Google Scholar] [CrossRef]
- Muydinov, R.; Seeger, S.; Kumar, S.; Klimm, C.; Kraehnert, R.; Wagner, M.R.; Szyszka, B. Crystallisation behaviour of CH3NH3PbI3 films: The benefits of sub-second flash lamp annealing. Thin Solid Film. 2018, 653, 204–214. [Google Scholar] [CrossRef]
- Troughton, J.; Carnie, M.J.; Davies, M.L.; Charbonneau, C.; Jewell, E.H.; Worsley, D.A.; Watson, T.M. Photonic flash-annealing of lead halide perovskite solar cells in 1 ms. J. Mater. Chem. A 2016, 4, 3471–3476. [Google Scholar] [CrossRef]
- Sanchez, S.; Christoph, N.; Grobety, B.; Phung, N.; Steiner, U.; Saliba, M.; Abate, A. Efficient and Stable Inorganic Perovskite Solar Cells Manufactured by Pulsed Flash Infrared Annealing. Adv. Energy Mater. 2018, 8, 1802060. [Google Scholar] [CrossRef] [Green Version]
- Feleki, B.; Bex, G.; Andriessen, R.; Galagan, Y.; Di Giacomo, F. Rapid and low temperature processing of mesoporous TiO2 for perovskite solar cells on flexible and rigid substrates. Mater. Today Commun. 2017, 13, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, G.L.; Xi, X.; Zhu, B.J.; Li, S.M.; Shao, J.B.; Zhu, F.; Feng, H.Y. Infrared photon-assisted annealing for crystal engineering in perovskite solar cells. Bull. Mater. Sci. 2022, 45, 54. [Google Scholar] [CrossRef]
- Gunzler, A.; Bermudez-Urena, E.; Muscarella, L.A.; Ochoa, M.; Ochoa-Martinez, E.; Ehrler, B.; Saliba, M.; Steiner, U. Shaping Perovskites: In Situ Crystallization Mechanism of Rapid Thermally Annealed, Prepatterned Perovskite Films. ACS Appl Mater Interfaces 2021, 13, 6854–6863. [Google Scholar] [CrossRef]
- Huang, S.H.; Guan, C.K.; Lee, P.H.; Huang, H.C.; Li, C.F.; Huang, Y.C.; Su, W.F. Toward All Slot-Die Fabricated High Efficiency Large Area Perovskite Solar Cell Using Rapid Near Infrared Heating in Ambient Air. Adv. Energy Mater. 2020, 10, 2001567. [Google Scholar] [CrossRef]
- Sánchez, S.; Vallés-Pelarda, M.; Alberola-Borràs, J.-A.; Vidal, R.; Jerónimo-Rendón, J.J.; Saliba, M.; Boix, P.P.; Mora-Seró, I. Flash infrared annealing as a cost-effective and low environmental impact processing method for planar perovskite solar cells. Mater. Today 2019, 31, 39–46. [Google Scholar] [CrossRef]
- Ling, P.S.V.; Hagfeldt, A.; Sanchez, S. Flash Infrared Annealing for Perovskite Solar Cell Processing. J. Vis. Exp. 2021, 168, e61730. [Google Scholar] [CrossRef]
- Sanchez, S.; Hua, X.; Phung, N.; Steiner, U.; Abate, A. Flash Infrared Annealing for Antisolvent-Free Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702915. [Google Scholar] [CrossRef]
- Troughton, J.; Charbonneau, C.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Rapid processing of perovskite solar cells in under 2.5 s. J. Mater. Chem. A 2015, 3, 9123–9127. [Google Scholar] [CrossRef]
- Qiu, Z.W.; Gong, H.B.; Zheng, G.H.J.; Yuan, S.A.; Zhang, H.L.; Zhu, X.M.; Zhou, H.P.; Cao, B.Q. Enhanced physical properties of pulsed laser deposited NiO films via annealing and lithium doping for improving perovskite solar cell efficiency. J. Mater. Chem. C 2017, 5, 7084–7094. [Google Scholar] [CrossRef]
- Wilkes, G.C.; Deng, X.Y.; Choi, J.J.; Gupta, M.C. Laser Annealing of TiO2 Electron-Transporting Layer in Perovskite Solar Cells. Acs Appl. Mater. Interfaces 2018, 10, 41312–41317. [Google Scholar] [CrossRef]
- Xia, R.; Yin, G.Y.; Wang, S.M.; Dong, W.W.; You, L.B.; Meng, G.; Fang, X.D.; Nazeeruddin, M.K.; Fei, Z.F.; Dyson, P.J. Precision excimer laser annealed Ga-doped ZnO electron transport layers for perovskite solar cells. Rsc Adv. 2018, 8, 17694–17701. [Google Scholar] [CrossRef] [Green Version]
- Jeon, T.; Jin, H.M.; Lee, S.H.; Lee, J.M.; Park, H.I.; Kim, M.K.; Lee, K.J.; Shin, B.; Kim, S.O. Laser Crystallization of Organic-Inorganic Hybrid Perovskite Solar Cells. Acs Nano 2016, 10, 7907–7914. [Google Scholar] [CrossRef]
- Konidakis, I.; Maksudov, T.; Serpetzoglou, E.; Kakavelakis, G.; Kymakis, E.; Stratakis, E. Improved Charge Carrier Dynamics of CH3NH3PbI3 Perovskite Films Synthesized by Means of Laser-Assisted Crystallization. Acs Appl. Energy Mater. 2018, 1, 5101–5111. [Google Scholar] [CrossRef]
- Song, C.P.; Tong, L.; Liu, F.; Ye, L.; Cheng, G.J. Addressing the Reliability and Electron Transport Kinetics in Halide Perovskite Film via Pulsed Laser Engineering. Adv. Funct. Mater. 2020, 30, 1906781. [Google Scholar] [CrossRef]
- Trinh, X.L.; Tran, N.H.; Seo, H.; Kim, H.C. Enhanced performance of perovskite solar cells via laser-induced heat treatment on perovskite film. Sol. Energy 2020, 206, 301–307. [Google Scholar] [CrossRef]
- Yang, H.R.; Song, C.P.; Xia, T.C.; Li, S.F.; Sun, D.Y.; Liu, F.; Cheng, G.J. Ultrafast transformation of PbI2 in two-step fabrication of halide perovskite films for long-term performance and stability via nanosecond laser shock annealing. J. Mater. Chem. C 2021, 9, 12819–12827. [Google Scholar] [CrossRef]
- Li, F.M.; Zhu, W.D.; Bao, C.X.; Yu, T.; Wang, Y.Q.; Zhou, X.X.; Zou, Z.G. Laser-assisted crystallization of CH3NH3PbI3 films for efficient perovskite solar cells with a high open-circuit voltage. Chem. Commun. 2016, 52, 5394–5397. [Google Scholar] [CrossRef]
- Jones, T.W.; Osherov, A.; Alsari, M.; Sponseller, M.; Duck, B.C.; Jung, Y.K.; Settens, C.; Niroui, F.; Brenes, R.; Stan, C.V.; et al. Lattice strain causes non-radiative losses in halide perovskites. Energy Environ. Sci. 2019, 12, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Niu, X.X.; Fu, Y.H.; Li, N.X.; Hu, C.; Chen, Y.H.; He, X.; Na, G.R.; Liu, P.F.; Zai, H.C.; et al. Strain engineering in perovskite solar cells and its impacts on carrier dynamics. Nat. Commun. 2019, 10, 815. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Kim, S.; Bae, S.; Cho, K.; Chung, T.; Mundt, L.E.; Lee, S.; Park, S.; Park, H.; Schubert, M.C.; et al. UV Degradation and Recovery of Perovskite Solar Cells. Sci. Rep. 2016, 6, 38150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, Q.; Iftikhar, F.J.; Khan, M.E.; Ullah, A.; Iqbal, Y.; Jose, R. Advances in stability of perovskite solar cells. Org. Electron. 2020, 78, 105590. [Google Scholar] [CrossRef]
- Ouyang, Z.L.; Abrams, H.; Bergstone, R.; Li, Q.T.; Zhu, F.; Li, D.W. Rapid Layer-Specific Annealing Enabled by Ultraviolet LED with Estimation of Crystallization Energy for High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902898. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.L.; Yue, Q.Y. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Motshekga, S.C.; Pillai, S.K.; Ray, S.S.; Jalama, K.; Krause, R.W.M. Recent Trends in the Microwave-Assisted Synthesis of Metal Oxide Nanoparticles Supported on Carbon Nanotubes and Their Applications. J. Nanomater. 2012, 2012, 691503. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, M.S.; Raghavan, G.S.V. An overview of microwave processing and dielectric properties of agri-food materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Z.Y.; Jia, X.Y.; Huang, L.K.; Huang, X.K.; Wang, L.M.; Wang, P.; Zhang, H.C.; Zhang, J.; Zhang, J.J.; et al. A rapid annealing technique for efficient perovskite solar cells fabricated in air condition under high humidity. Org. Electron. 2016, 34, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ma, T.T.; Wang, F.F.; Liu, Y.; Liu, S.Z.; Wang, J.A.; Cheng, Z.C.; Chang, Q.; Yang, R.; Huang, W.C.; et al. Rapid Microwave-Annealing Process of Hybrid Perovskites to Eliminate Miscellaneous Phase for High Performance Photovoltaics. Adv. Sci. 2020, 7, 2000480. [Google Scholar] [CrossRef]
- Brites, M.J.; Barreiros, M.A.; Corregidor, V.; Alves, L.C.; Pinto, J.V.; Mendes, M.J.; Fortunato, E.; Martins, R.; Mascarenhas, J. Ultrafast Low-Temperature Crystallization of Solar Cell Graded Formamidinium-Cesium Mixed-Cation Lead Mixed-Halide Perovskites Using a Reproducible Microwave-Based Process. Acs Appl. Energy Mater. 2019, 2, 1844–1853. [Google Scholar] [CrossRef]
- Maitani, M.M.; Iso, D.; Kim, J.; Tsubaki, S.; Wada, Y. Microwave Application to Efficient Annealing Process of CH3NH3PbI3 Perovskite Crystalline Films. Electrochemistry 2017, 85, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.P.; Yang, S.W.; Gao, Q.Q.; Lei, L.; Yu, Y.; Shao, J.; Liu, Y. Fast and Controllable Crystallization of Perovskite Films by Microwave Irradiation Process. Acs Appl. Mater. Interfaces 2016, 8, 7854–7861. [Google Scholar] [CrossRef]
- Ahmadian-Yazdi, M.R.; Eslamian, M. Toward scale-up of perovskite solar cells: Annealing-free perovskite layer by low-cost ultrasonic substrate vibration of wet films. Mater. Today Commun. 2018, 14, 151–159. [Google Scholar] [CrossRef]
- Zhang, X.; Zabihi, F.; Xiong, H.; Eslamian, M.; Hou, C.Y.; Zhu, M.F.; Wang, H.Z.; Zhang, Q.H. Highly efficient flexible perovskite solar cells made via ultrasonic vibration assisted room temperature cold sintering. Chem. Eng. J. 2020, 394, 124887. [Google Scholar] [CrossRef]
- Takizawa, K.; Fukudome, H.; Kozaki, Y.; Ando, S. Pressure-Induced Changes in Crystalline Structures of Polyimides Analyzed by Wide-Angle X-ray Diffraction at High Pressures. Macromolecules 2014, 47, 3951–3958. [Google Scholar] [CrossRef]
- Tan, X.; Wang, K.; Li, S.; Yuan, H.; Yan, T.; Liu, J.; Yang, K.; Liu, B.; Zou, G.; Zou, B. Exploration of the pyrazinamide polymorphism at high pressure. J. Phys. Chem. B 2012, 116, 14441–14450. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yang, K.; Liu, B.; Zou, B. High-Pressure-Induced Polymorphic Transformation of Maleic Hydrazide. J. Phys. Chem. C 2014, 118, 8122–8127. [Google Scholar] [CrossRef]
- Yu, J.; Tonpheng, B.; Andersson, O. High-Pressure-Induced Microstructural Evolution and Enhancement of Thermal Properties of Nylon-6. Macromolecules 2010, 43, 10512–10520. [Google Scholar] [CrossRef]
- Liu, M.Y.; Chen, Z.M.; Xue, Q.F.; Cheung, S.H.; So, S.K.; Yip, H.L.; Cao, Y. High performance low-bandgap perovskite solar cells based on a high-quality mixed Sn-Pb perovskite film prepared by vacuum-assisted thermal annealing. J. Mater. Chem. A 2018, 6, 16347–16354. [Google Scholar] [CrossRef]
- Lu, J.J.; Wan, M.X.; Wen, P.; Luo, F.; Liu, X.; Wen, J.; Hu, C.Y.; Guo, J. Investigation on the high pressure annealing induced re crystallization mechanism of CH3NH3PbI3 film. J. Alloy. Compd. 2017, 694, 1365–1370. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, N.; Peng, Y.Z.; Tang, L.L.; Zhang, J.J.; Cai, H.K.; Ni, J.; Sun, Y.Y.; Li, J. The Effect of Annealing Pressure on Perovskite Films and Its Thin-Film Field-Effect Transistors’ Performance. Phys. Status Solidi A-Appl. Mater. Sci. 2019, 216, 1900434. [Google Scholar] [CrossRef]
- Liang, C.; Li, P.W.; Gu, H.; Zhang, Y.Q.; Li, F.Y.; Song, Y.L.; Shao, G.S.; Mathews, N.; Xing, G.C. One-Step Inkjet Printed Perovskite in Air for Efficient Light Harvesting. Sol. Rrl 2018, 2, 1700217. [Google Scholar] [CrossRef]
- Jiang, J.J.; Tang, W.C.; Yang, X.M.; Sun, X.F.; Yang, J.C.; Cai, H.L.; Zhang, F.M.; Wu, X.S. Improvement of quality and stability of MAPbI(3) films grown by post annealing under high pressure argon atmosphere. J. Phys. D-Appl. Phys. 2021, 54, 075101. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Low temperature formation of CH3NH3PbI3 perovskite films in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104604. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wu, Z.L.; Dou, Y.X.; Zhang, J.W.; Bu, T.L.; Zhang, K.C.; Fang, D.; Ku, Z.L.; Huang, F.Z.; Cheng, Y.B.; et al. Formamidinium-Based Perovskite Solar Cells with Enhanced Moisture Stability and Performance via Confined Pressure Annealing. J. Phys. Chem. C 2020, 124, 12249–12258. [Google Scholar] [CrossRef]
- Ge, Q.Q.; Ding, J.; Liu, J.; Ma, J.Y.; Chen, Y.X.; Gao, X.X.; Wan, L.J.; Hu, J.S. Promoting crystalline grain growth and healing pinholes by water vapor modulated post-annealing for enhancing the efficiency of planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 13458–13467. [Google Scholar] [CrossRef]
- Wang, G.; Liao, L.P.; Chen, L.J.; Xu, C.Y.; Yao, Y.Q.; Liu, D.B.; Li, P.; Deng, J.D.; Song, Q.L. Perovskite solar cells fabricated under ambient air at room temperature without any post-treatment. Org. Electron. 2020, 86, 105918. [Google Scholar] [CrossRef]
- Yang, Z.J.; Pan, J.L.; Liang, Y.Q.; Li, Q.; Xu, D.S. Ambient Air Condition for Room-Temperature Deposition of MAPbI(3) Films in Highly Efficient Solar Cells. Small 2018, 14, 1802240. [Google Scholar] [CrossRef]
- Kong, J.M.; Wang, H.Y.; Rohr, J.A.; Fishman, Z.S.; Zhou, Y.Y.; Li, M.X.; Cotlet, M.; Kim, G.; Karpovich, C.; Antonio, F.; et al. Perovskite Solar Cells with Enhanced Fill Factors Using Polymer-Capped Solvent Annealing. Acs Appl. Energy Mater. 2020, 3, 7231–7238. [Google Scholar] [CrossRef]
- Kim, G.H.; Jeong, J.; Jang, H.; Kim, J.W.; Kim, J.Y. Fast vaporizing anti-solvent for high crystalline perovskite to achieve high performance perovskite solar cells. Thin Solid Film. 2018, 661, 122–127. [Google Scholar] [CrossRef]
- Liu, J.; Gao, C.; He, X.L.; Ye, Q.Y.; Ouyang, L.Q.; Zhuang, D.M.; Liao, C.; Mei, J.; Lau, W.M. Improved Crystallization of Perovskite Films by Optimized Solvent Annealing for High Efficiency Solar Cell. Acs Appl. Mater. Interfaces 2015, 7, 24008–24015. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.F.; Chang, J.J.; Yang, H.F.; Xi, H.; Lu, G.; Chen, D.Z.; Lin, Z.H.; Lu, X.L.; Zhang, J.C.; et al. Mixed-solvent-vapor annealing of perovskite for photovoltaic device efficiency enhancement. Nano Energy 2016, 28, 417–425. [Google Scholar] [CrossRef]
- Sutanto, A.A.; Lan, S.; Cheng, C.F.; Mane, S.B.; Wu, H.P.; Leonardus, M.; Xie, M.Y.; Yeh, S.C.; Tseng, C.W.; Chen, C.T.; et al. Solvent-assisted crystallization via a delayed-annealing approach for highly efficient hybrid mesoscopic/planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 270–276. [Google Scholar] [CrossRef]
- Tian, L.W.; Zhang, W.F.; Yu, H.; Peng, C.T.; Mao, H.Y.; Li, Y.P.; Wang, Q.Y.; Huang, Y.L. Post-treatment of Perovskite Films toward Efficient Solar Cells via Mixed Solvent Annealing. Acs Appl. Energy Mater. 2019, 2, 4954–4963. [Google Scholar] [CrossRef]
- Zhang, L.X.; Tian, S.; Yu, Z.H.; Zhang, F.; Niu, F.F.; Li, X.C.; Song, J.; Zeng, P.J.; Lian, J.R. Rational Solvent Annealing for Perovskite Film Formation in Air Condition. Ieee J. Photovolt. 2017, 7, 1338–1341. [Google Scholar] [CrossRef]
- Zhao, X.M.; Liu, T.R.; Kaplan, A.B.; Yao, C.; Loo, Y.L. Accessing Highly Oriented Two-Dimensional Perovskite Films via Solvent-Vapor Annealing for Efficient and Stable Solar Cells. Nano Lett. 2020, 20, 8880–8889. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Huang, L.M.; Mei, X.F.; Zhao, Y.; Lin, Z.H.; Zhen, H.Y.; Ling, Q.D. Highly reproducible and photocurrent hysteresis-less planar perovskite solar cells with a modified solvent annealing method. Sol. Energy 2016, 136, 210–216. [Google Scholar] [CrossRef]
- Raghav, A.; Singh, S.; Moghe, D.; Sharma, S.; Kabra, D.; Satapathi, S. Charge carrier dynamics study and morphology optimization in solvent annealed CH3NH3PbI3 perovskite for air processed stable solar cell application. Chem. Phys. 2019, 526, 110408. [Google Scholar] [CrossRef]
- Wenderott, J.K.; Raghav, A.; Shtein, M.; Green, P.F.; Satapathi, S. Local Optoelectronic Characterization of Solvent-Annealed, Lead-Free, Bismuth-Based Perovskite Films. Langmuir 2018, 34, 7647–7654. [Google Scholar] [CrossRef]
- Li, J.J.; Ma, J.Y.; Hu, J.S.; Wang, D.; Wan, L.J. Influence of N,N-Dimethylformamide Annealing on the Local Electrical Properties of Organometal Halide Perovskite Solar Cells: An Atomic Force Microscopy Investigation. Acs Appl. Mater. Interfaces 2016, 8, 26002–26007. [Google Scholar] [CrossRef]
- Peng, H.X.; Lan, C.F.; Chen, S.T.; Fan, P.; Liang, G.X.; Lan, H.B. N,N-dimethylformamide vapor effect on microstructural and optical properties of CH3NH3PbI3 film during solvent annealing. Surf. Coat. Technol. 2019, 359, 162–168. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, S.B.; Zhang, P.; Liu, D.T.; Gu, X.L.; Sarvari, H.; Ye, Z.B.; Wu, J.; Wang, Z.M.; Chen, Z.D. Solvent annealing of PbI2 for the high-quality crystallization of perovskite films for solar cells with efficiencies exceeding 18%. Nanoscale 2016, 8, 19654–19661. [Google Scholar] [CrossRef]
- Zhu, L.Z.; Yuh, B.; Schoen, S.; Li, X.P.; Aldighaithir, M.; Richardson, B.J.; Alamer, A.; Yu, Q.M. Solvent-molecule-mediated manipulation of crystalline grains for efficient planar binary lead and tin triiodide perovskite solar cells. Nanoscale 2016, 8, 7621–7630. [Google Scholar] [CrossRef]
- Yang, X.M.; Wei, Y.L.; Huang, F.Y.; Jin, S.; Luo, D.; Fang, Y.; Zhao, Y.Z.; Guo, Q.Y.; Huang, Y.F.; Fan, L.Q.; et al. Mixed-steam annealing treatment for perovskite films to improve solar cells performance. Sol. Energy 2019, 177, 299–305. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.J.; Ma, D.H.; Jiang, Z.Y.; Fan, Z.Q.; Ma, Q.; Xi, Y.L. Improvement of photovoltaic performance of the inverted planar perovskite solar cells by using CH3NH3PbI3-xBrx films with solvent annealing. Superlattices Microstruct. 2018, 113, 1–12. [Google Scholar] [CrossRef]
- Kim, G.H.; Jeong, J.; Yoon, Y.J.; Jang, H.; Kim, S.; Seo, J.; Kim, J.Y. The optimization of intermediate semi-bonding structure using solvent vapor annealing for high performance p-i-n structure perovskite solar cells. Org. Electron. 2019, 65, 300–304. [Google Scholar] [CrossRef]
- Wang, B.H.; Wong, K.Y.; Yang, S.F.; Chen, T. Crystallinity and defect state engineering in organo-lead halide perovskite for high-efficiency solar cells. J. Mater. Chem. A 2016, 4, 3806–3812. [Google Scholar] [CrossRef]
- Lei, J.; Wang, H.X.; Gao, F.; Liu, S.Z. Improving the Quality of CH3NH3PbI3 Films via Chlorobenzene Vapor Annealing. Phys. Status Solidi A-Appl. Mater. Sci. 2018, 215, 1700959. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Yi, C.; Shi, X.J.; Smith, A.W.; Gong, X.; Heeger, A.J. Efficient Perovskite Hybrid Photovoltaics via Alcohol-Vapor Annealing Treatment. Adv. Funct. Mater. 2016, 26, 101–110. [Google Scholar] [CrossRef]
- Mou, J.P.; Song, J.; Che, M.; Liu, Y.; Qin, Y.S.; Liu, H.M.; Zhu, L.; Zhao, Y.L.; Qiang, Y.H. Butanol-assisted solvent annealing of CH3NH3PbI3 film for high-efficient perovskite solar cells. J. Mater. Sci.-Mater. Electron. 2019, 30, 746–752. [Google Scholar] [CrossRef]
- Zheng, H.F.; Liu, Y.Q.; Sun, J. Micron-sized columnar grains of CH3NH3PbI3 grown by solvent-vaporassisted low-temperature (75 degrees C) solid-state reaction: The role of non-coordinating solvent-vapor. Appl. Surf. Sci. 2018, 437, 82–91. [Google Scholar] [CrossRef]
- Fan, H.C.; Huang, J.H.; Chen, L.S.; Zhang, Y.; Wang, Y.; Gao, C.Y.; Wang, P.C.; Zhou, X.Q.; Jiang, K.J.; Song, Y.L. Methylamine-assisted secondary grain growth for CH3NH3PbI3 perovskite films with large grains and a highly preferred orientation. J. Mater. Chem. A 2021, 9, 7625–7630. [Google Scholar] [CrossRef]
- Hong, L.; Hu, Y.; Mei, A.Y.; Sheng, Y.S.; Jiang, P.; Tian, C.B.; Rong, Y.G.; Han, H.W. Improvement and Regeneration of Perovskite Solar Cells via Methylamine Gas Post-Treatment. Adv. Funct. Mater. 2017, 27, 1703060. [Google Scholar] [CrossRef]
- Jiang, Y.; Juarez-Perez, E.J.; Ge, Q.Q.; Wang, S.H.; Leyden, M.R.; Ono, L.K.; Raga, S.R.; Hu, J.S.; Qi, Y.B. Post-annealing of MAPbI(3) perovskite films with methylamine for efficient perovskite solar cells. Mater. Horiz. 2016, 3, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.W.; Wen, F.; Zhang, W.F.; Zhang, H.C.; Yu, H.; Lin, P.A.; Liu, X.; Zhou, S.H.; Zhou, X.Q.; Jiang, Y.T.; et al. Rising from the Ashes: Gaseous Therapy for Robust and Large-Area Perovskite Solar Cells. Acs Appl. Mater. Interfaces 2020, 12, 49648–49658. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Jasti, N.P.; Hadke, S.; Raghavan, S.; Avasthi, S. Large grained and high charge carrier lifetime CH3NH3PbI3 thin-films: Implications for perovskite solar cells. Curr. Appl. Phys. 2017, 17, 1335–1340. [Google Scholar] [CrossRef]
- Venkatesan, S.; Hao, F.; Kim, J.Y.; Rong, Y.G.; Zhu, Z.; Liang, Y.L.; Bao, J.M.; Yao, Y. Moisture-driven phase transition for improved perovskite solar cells with reduced trap-state density. Nano Res. 2017, 10, 1413–1422. [Google Scholar] [CrossRef]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; van Franeker, J.J.; Dequilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The Importance of Moisture in Hybrid Lead Halide Perovskite Thin Film Fabrication. Acs Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef]
- Raga, S.R.; Jung, M.C.; Lee, M.V.; Leyden, M.R.; Kato, Y.; Qi, Y.B. Influence of Air Annealing on High Efficiency Planar Structure Perovskite Solar Cells. Chem. Mater. 2015, 27, 1597–1603. [Google Scholar] [CrossRef]
- Yin, X.T.; Guo, Y.X.; Liu, J.; Chen, P.; Chen, W.; Que, M.D.; Que, W.X.; Niu, C.M.; Bian, J.H.; Yang, Y.D. Moisture annealing effect on CH3NH3PbI3 films deposited by solvent engineering method. Thin Solid Film. 2017, 636, 664–670. [Google Scholar] [CrossRef]
- Zhou, X.; Xin, C.G.; Hou, F.H.; Shi, B.; Pan, S.J.; Hou, S.X.; Zhang, J.; Wang, P.Y.; Ren, H.Z.; Zhao, Y.; et al. Role of Moisture in the Preparation of Efficient Planar Perovskite Solar Cells. Acs Sustain. Chem. Eng. 2019, 7, 17691–17696. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Song, S.; Pyeon, L.; Kang, G.; Lee, G.Y.; Park, T. Simple post annealing-free method for fabricating uniform, large grain-sized, and highly crystalline perovskite films. Nano Energy 2017, 34, 181–187. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Park, E.Y.; Yang, T.Y.; Noh, J.H.; Shin, T.J.; Jeon, N.J.; Seo, J. Fast two-step deposition of perovskite via mediator extraction treatment for large-area, high-performance perovskite solar cells. J. Mater. Chem. A 2018, 6, 12447–12454. [Google Scholar] [CrossRef]
- Li, N.X.; Niu, X.X.; Li, L.; Wang, H.; Huang, Z.J.; Zhang, Y.; Chen, Y.H.; Zhang, X.; Zhu, C.; Zai, H.C.; et al. Liquid medium annealing for fabricating durable perovskite solar cells with improved reproducibility. Science 2021, 373, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.S.; Xie, F.X.; Chen, H.; Yang, X.D.; Ye, F.; Bi, E.B.; Wu, Y.Z.; Cai, M.T.; Han, L.Y. Annealing-free perovskite films by instant crystallization for efficient solar cells. J. Mater. Chem. A 2016, 4, 8548–8553. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, M.J.; Wu, W.W.; Vasiliev, A.L.; Zhu, K.; Padture, N.P. Room-temperature crystallization of hybrid-perovskite thin films via solvent-solvent extraction for high-performance solar cells. J. Mater. Chem. A 2015, 3, 8178–8184. [Google Scholar] [CrossRef]
- Cronin, H.M.; Jayawardena, K.; Stoeva, Z.; Shkunov, M.; Silva, S.R.P. Effects of ambient humidity on the optimum annealing time of mixed-halide Perovskite solar cells. Nanotechnology 2017, 28, 114004. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, Q.X.; Song, J.; Hayase, S.; Qu, J.L.; Shen, Q. A New Strategy for Increasing the Efficiency of Inverted Perovskite Solar Cells to More than 21%: High-Humidity Induced Self-Passivation of Perovskite Films. Sol. RRL 2020, 4, 2000149. [Google Scholar] [CrossRef]
- Cao, X.B.; Zhi, L.L.; Li, Y.H.; Fang, F.; Cui, X.; Ci, L.J.; Ding, K.X.; Wei, J.Q. Fabrication of Perovskite Films with Large Columnar Grains via Solvent-Mediated Ostwald Ripening for Efficient Inverted Perovskite Solar Cells. Acs Appl. Energy Mater. 2018, 1, 868–875. [Google Scholar] [CrossRef]
- Fang, X.; Wu, Y.H.; Lu, Y.T.; Sun, Y.; Zhang, S.; Zhang, J.; Zhang, W.H.; Yuan, N.Y.; Ding, J.N. Annealing-free perovskite films based on solvent engineering for efficient solar cells. J. Mater. Chem. C 2017, 5, 842–847. [Google Scholar] [CrossRef]
- Dong, H.; Pang, S.Z.; He, F.Q.; Yang, H.F.; Zhu, W.D.; Chen, D.Z.; Xi, H.; Zhang, J.C.; Hao, Y.; Zhang, C.F. Annealing-Free, High-Performance Perovskite Solar Cells by Controlling Crystallization via Guanidinium Cation Doping. Sol. RRL 2021, 5, 2100097. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhao, Y.X.; Liang, Z.Q. Non-Thermal Annealing Fabrication of Efficient Planar Perovskite Solar Cells with Inclusion of NH4Cl. Chem. Mater. 2015, 27, 1448–1451. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, G.; Xi, X.; Yang, G.; Hu, L.; Zhu, B.; He, Y.; Liu, Y.; Qian, H.; Zhang, S.; et al. Annealing Engineering in the Growth of Perovskite Grains. Crystals 2022, 12, 894. https://doi.org/10.3390/cryst12070894
Wang L, Liu G, Xi X, Yang G, Hu L, Zhu B, He Y, Liu Y, Qian H, Zhang S, et al. Annealing Engineering in the Growth of Perovskite Grains. Crystals. 2022; 12(7):894. https://doi.org/10.3390/cryst12070894
Chicago/Turabian StyleWang, Lan, Guilin Liu, Xi Xi, Guofeng Yang, Lifa Hu, Bingjie Zhu, Yifeng He, Yushen Liu, Hongqiang Qian, Shude Zhang, and et al. 2022. "Annealing Engineering in the Growth of Perovskite Grains" Crystals 12, no. 7: 894. https://doi.org/10.3390/cryst12070894
APA StyleWang, L., Liu, G., Xi, X., Yang, G., Hu, L., Zhu, B., He, Y., Liu, Y., Qian, H., Zhang, S., & Zai, H. (2022). Annealing Engineering in the Growth of Perovskite Grains. Crystals, 12(7), 894. https://doi.org/10.3390/cryst12070894






