Photocatalytic Degradation of Phenol Red in Water on Nb(x)/TiO2 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
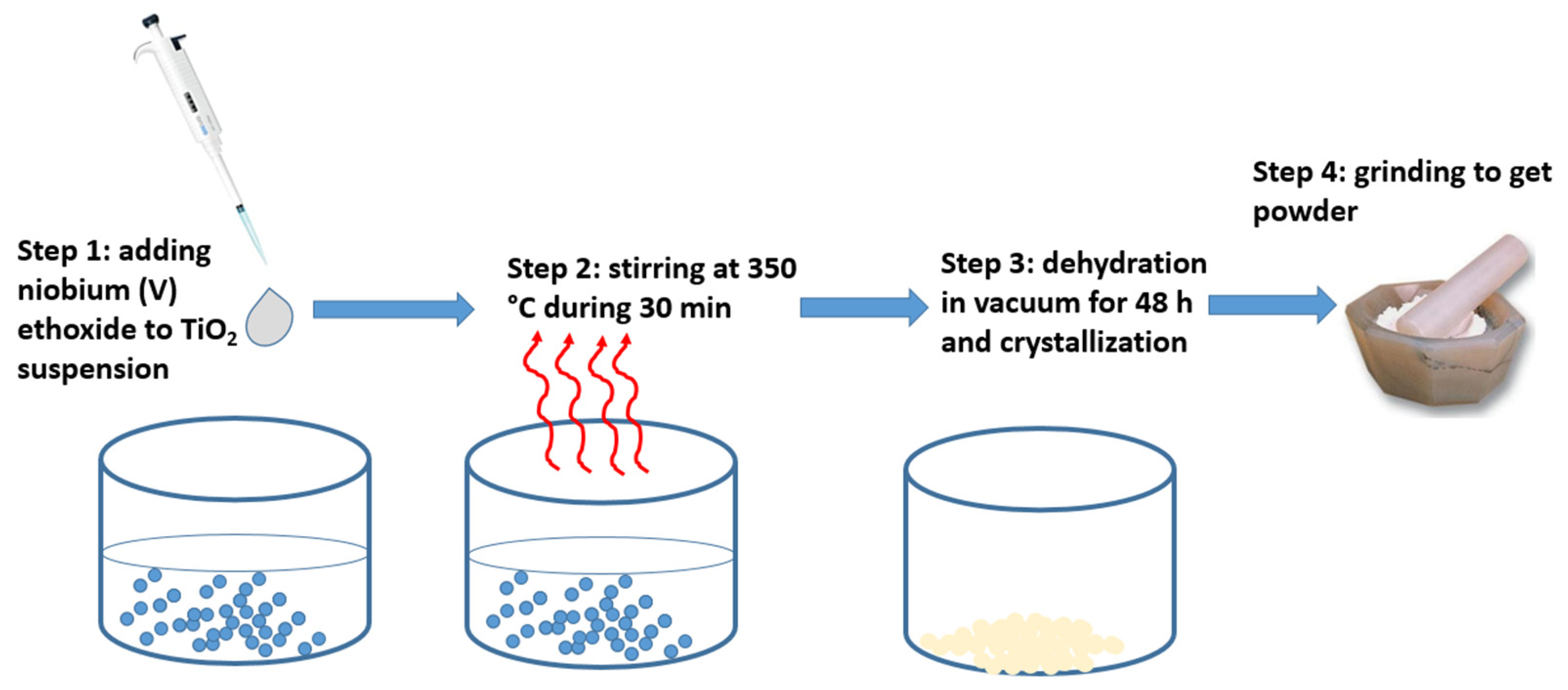
2.1. Materials Preparation
2.2. Experimental Methods
3. Results and Discussion
3.1. Structure Analysis of TiO2/xNb Nanocomposites
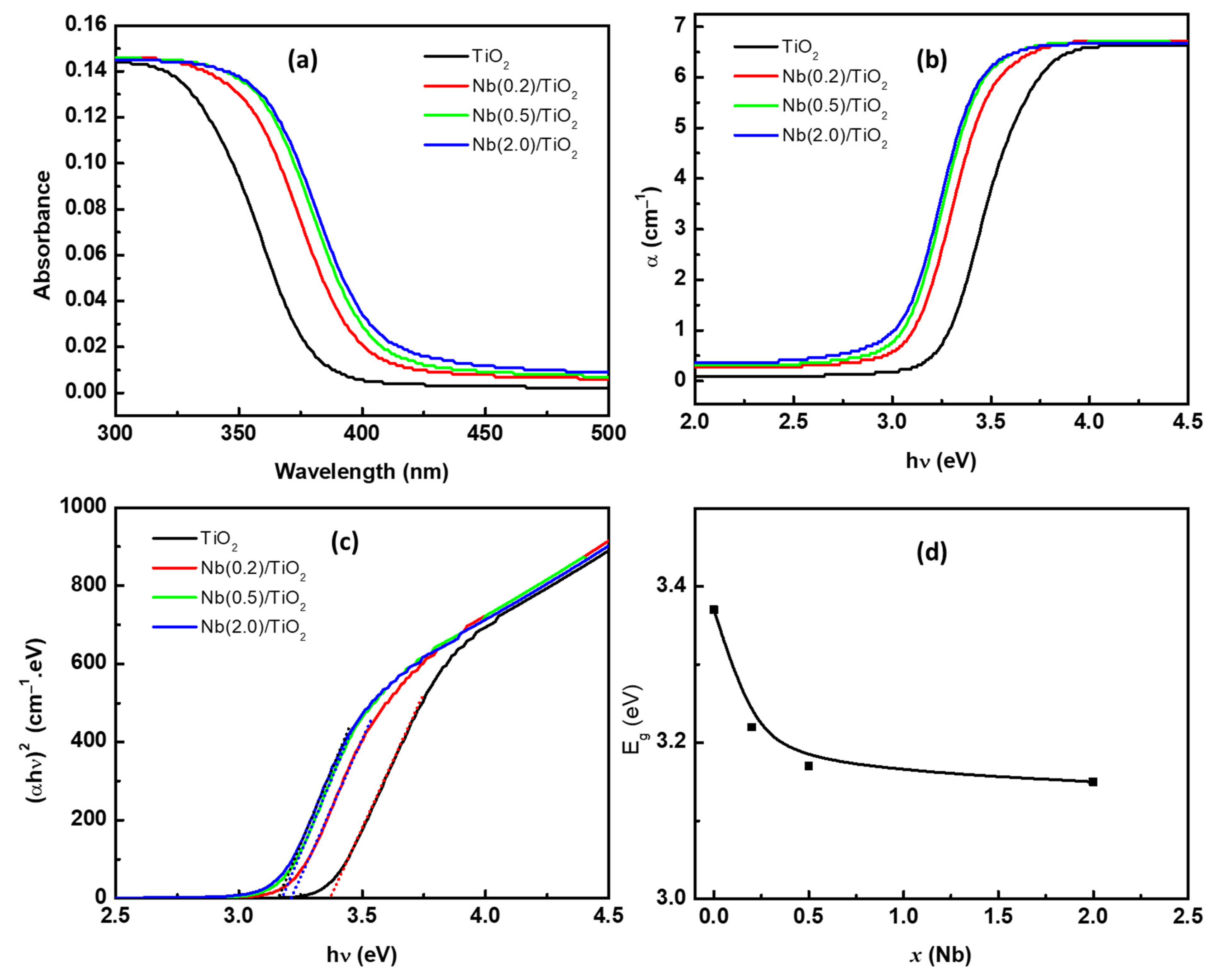
3.2. Optical Band Gap Characterizations
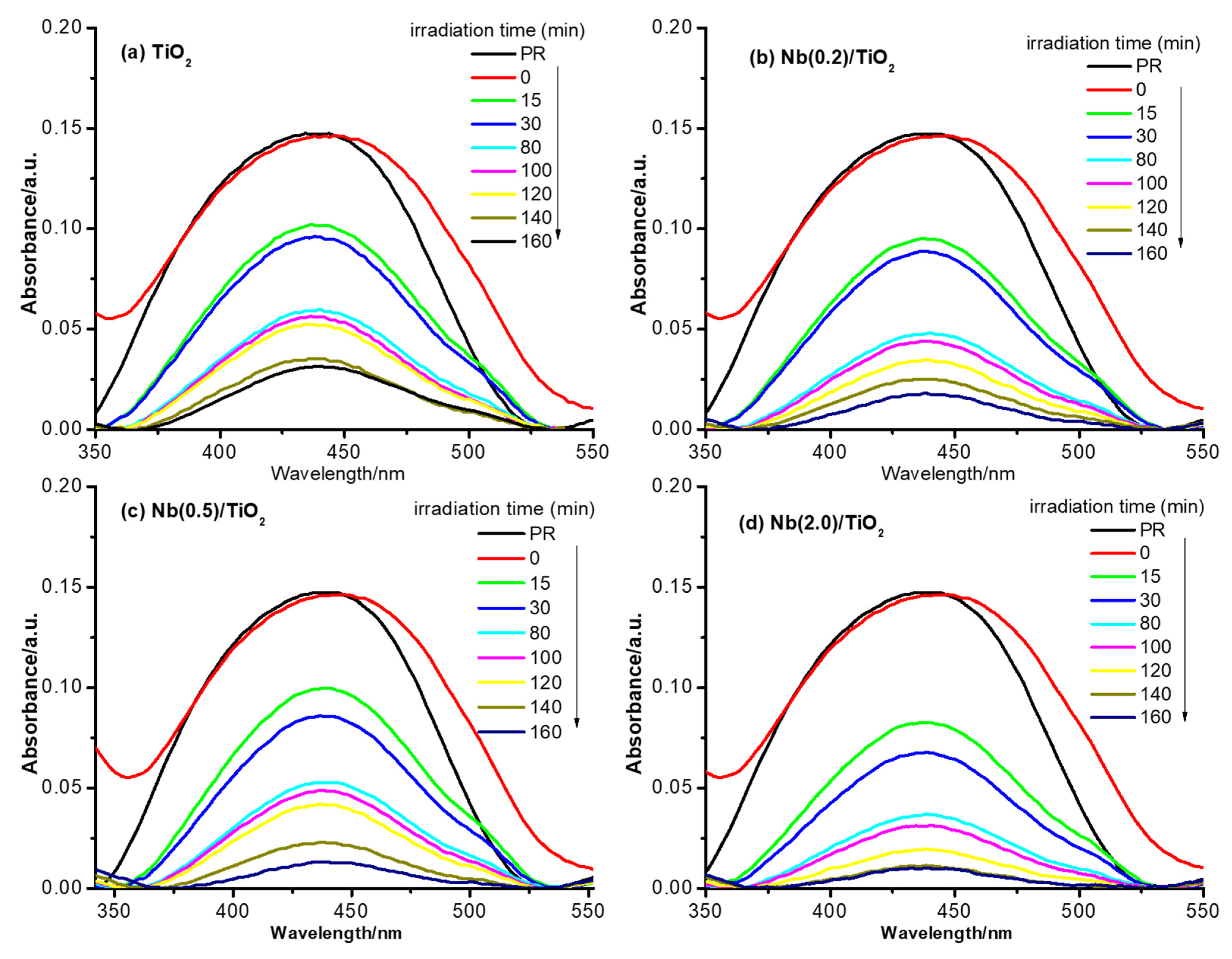
3.3. Photoluminescence Measurements
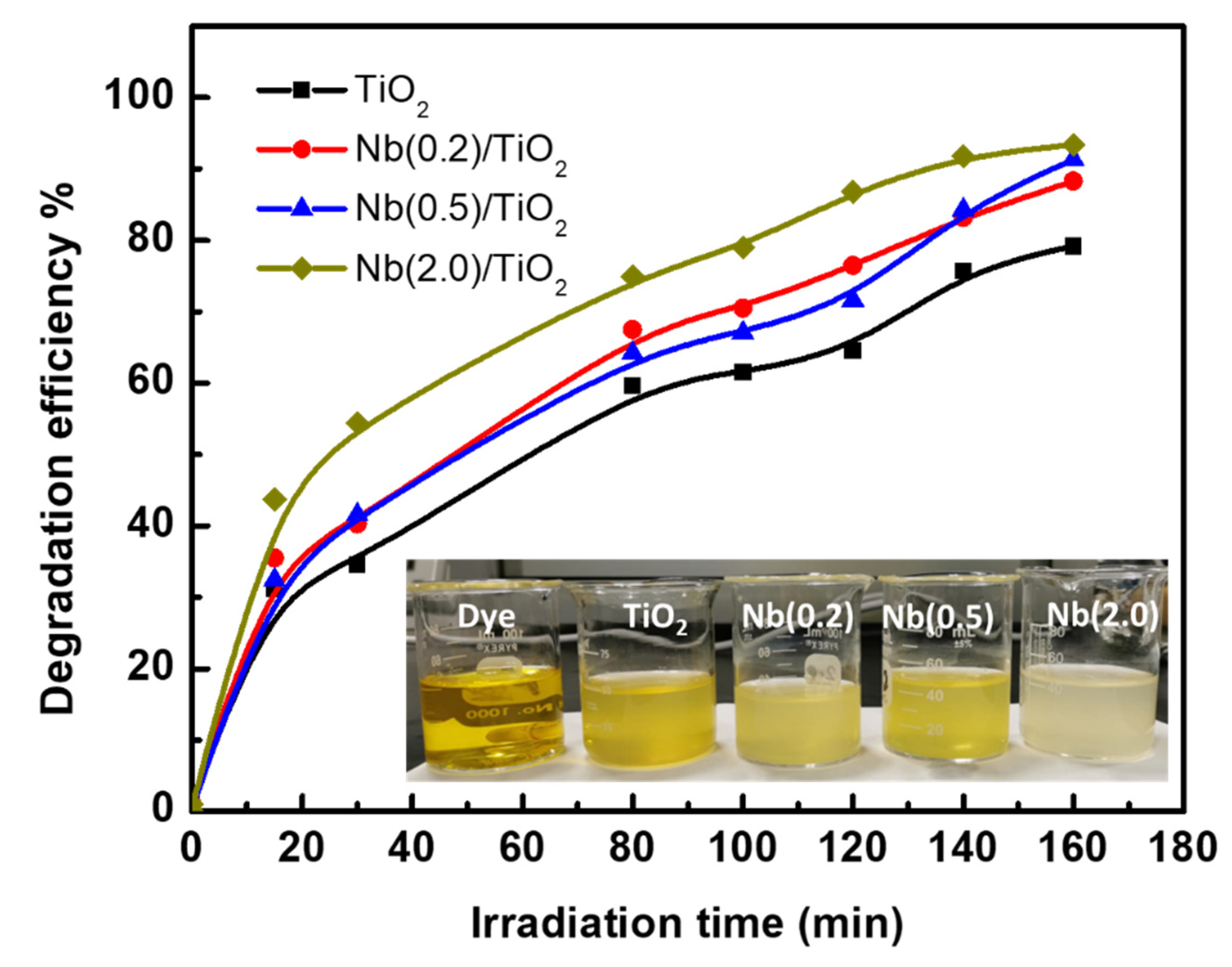
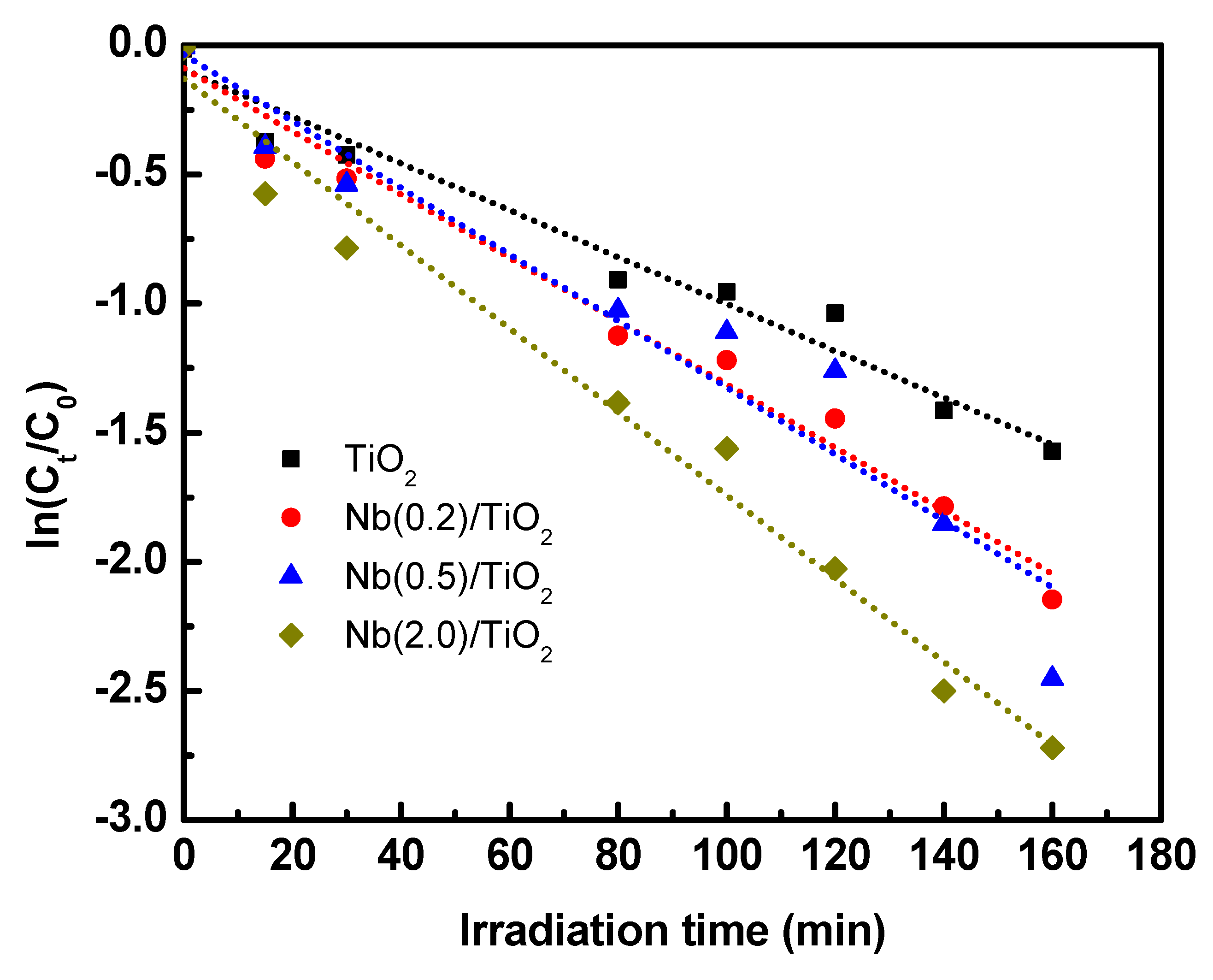
3.4. Photocatalytic Degradation of Phenol Red
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, M.; Luo, B.; Lyu, M.; Wang, S.; Wang, L. Photocatalysis: Single-Crystalline Nanomesh Tantalum Nitride Photocatalyst with Improved Hydrogen-Evolving Performance. Adv. Energy Mater. 2018, 8, 1770138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhao, Z.-J.; Wang, T.; Gong, J. Nano-Designed Semiconductors for Electro- and Photoelectro-Catalytic Conversion of Carbon Dioxide. Chem. Soc. Rev. 2018, 47, 5423–5443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Kim, J.-H.; Jo, Y.-M.; Lee, J.-H. Heterojunction between Bimetallic Metal-Organic Framework and TiO2: Band-Structure Engineering for Effective Photoelectrochemical Water Splitting. Nano Res. 2022. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible Light Induced Photocatalytic Degradation of Organic Pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Sul, J.-H.; Thaweesak, S.; Yun, J.-H. Efficient and Rapid Photocatalytic Degradation of Methyl Orange Dye Using Al/ZnO Nanoparticles. Nanomaterials 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Pozan, G.S.; Isleyen, M.; Gokcen, S. Transition Metal Coated TiO2 Nanoparticles: Synthesis, Characterization and Their Photocatalytic Activity. Appl. Catal. B Environ. 2013, 140, 537–545. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R. V Transition Metal Ion Impregnated Mesoporous TiO2 for Photocatalytic Degradation of Organic Contaminants in Water. Ind. Eng. Chem. Res. 2006, 45, 5231–5238. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 11, 2067. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, S.W.; Keulemans, M.; Filippousi, M.; Flahaut, D.; Van Tendeloo, G.; Lacombe, S.; Martens, J.A.; Lenaerts, S. Plasmonic Gold–Silver Alloy on TiO2 Photocatalysts with Tunable Visible Light Activity. Appl. Catal. B Environ. 2014, 156, 116–121. [Google Scholar] [CrossRef]
- Yang, X.; Min, Y.; Li, S.; Wang, D.; Mei, Z.; Liang, J.; Pan, F. Conductive Nb-Doped TiO2 Thin Films with Whole Visible Absorption to Degrade Pollutants. Catal. Sci. Technol. 2018, 8, 1357–1365. [Google Scholar] [CrossRef]
- Ahmed, F.; Kanoun, M.B.; Awada, C.; Jonin, C.; Brevet, P.-F. An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals. Crystals 2021, 11, 1488. [Google Scholar] [CrossRef]
- Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple Preparation of Ceramic-like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals 2020, 10, 1024. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Kou, Y.; Yang, J.; Li, B.; Fu, S. Solar Photocatalytic Activities of Porous Nb-Doped TiO2 Microspheres by Coupling with Tungsten Oxide. Mater. Res. Bull. 2015, 63, 105–111. [Google Scholar] [CrossRef]
- Wu, M.-C.; Lin, T.-H.; Chih, J.-S.; Hsiao, K.-C.; Wu, P.-Y. Niobium Doping Induced Morphological Changes and Enhanced Photocatalytic Performance of Anatase TiO2. Jpn. J. Appl. Phys. 2017, 56, 04CP07. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 Catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Jin, H.-J.; Jung, H.R.; Kim, J.; Nguyen, B.P.; Kim, J.; Jo, W. Reduced Extrinsic Recombination Process in Anatase and Rutile TiO2 Epitaxial Thin Films for Efficient Electron Transport Layers. Sci. Rep. 2021, 11, 6810. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, C.; Sun, P.; Wang, L.; Xia, B.; Liu, Y. Solar Photocatalytic Activities of Porous Nb-Doped TiO2 Microspheres Prepared by Ultrasonic Spray Pyrolysis. Solid State Sci. 2012, 14, 139–144. [Google Scholar] [CrossRef]
- Barbosa López, A.L.; Castro, I.M. Niobium-Titanium-Based Photocatalysts: Its Potentials for Free Cyanide Oxidation in Residual Aqueous Effluent. Front. Chem. 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, D.; Li, K. Single-Crystalline Nanoporous Nb2O5 Nanotubes. Nanoscale Res. Lett. 2011, 6, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulle, A.; Chartier, A.; Crocombette, J.; Jourdan, T.; Pellegrino, S.; Debelle, A. Strain and Damage Build-up in Irradiated Crystals: Coupling X-ray Diffraction with Numerical Simulations. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 458, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. Repeatability of Indium Oxide Gas Sensors for Detecting Methane at Low Temperature. Mater. Sci. Semicond. Process. 2016, 56, 260–264. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Chaoui, N.; Genet, M.; Gaigneaux, E.M.; Robert, D. Effect of Compressive Stress Inducing a Band Gap Narrowing on the Photoinduced Activities of Sol–Gel TiO2 Films. Thin Solid Film. 2011, 520, 1147–1154. [Google Scholar] [CrossRef]
- Orendorz, A.; Brodyanski, A.; Lösch, J.; Bai, L.H.; Chen, Z.H.; Le, Y.K.; Ziegler, C.; Gnaser, H. Phase Transformation and Particle Growth in Nanocrystalline Anatase TiO2 Films Analyzed by X-ray Diffraction and Raman Spectroscopy. Surf. Sci. 2007, 601, 4390–4394. [Google Scholar] [CrossRef]
- Cheng, G.; Akhtar, M.S.; Yang, O.-B.; Stadler, F.J. Structure Modification of Anatase TiO2 Nanomaterials-Based Photoanodes for Efficient Dye-Sensitized Solar Cells. Electrochim. Acta 2013, 113, 527–535. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman Scattering Study on Anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Hendi, A.A.; Rashad, M. Photo-Induced Changes in Nano-Copper Oxide for Optoelectronic Applications. Phys. B Condens. Matter. 2018, 538, 185–190. [Google Scholar] [CrossRef]
- El-Menyawy, E.M.; Elagamey, A.A.; Elgogary, S.R.; Abu El-Enein, R.A.N. Synthesis, Crystal Structure and Thin-Film-Optical Properties of 3-Amino-2-(2-Nitrophenyl)Diazinyl-3-(Morpholin-1-Yl)Acrylonitrile. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 75–81. [Google Scholar] [CrossRef]
- Mathew, S.; kumar Prasad, A.; Benoy, T.; Rakesh, P.P.; Hari, M.; Libish, T.M.; Radhakrishnan, P.; Nampoori, V.P.N.; Vallabhan, C.P.G. UV-Visible Photoluminescence of TiO2 Nanoparticles Prepared by Hydrothermal Method. J. Fluoresc. 2012, 22, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.M.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Masoud, M.S. Graphene Oxide–Metal Oxide Nanocomposites: Fabrication, Characterization and Removal of Cationic Rhodamine B Dye. RSC Adv. 2018, 8, 13323–13332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asiri, A.M.; Al-Amoudi, M.S.; Al-Talhi, T.A.; Al-Talhi, A.D. Photodegradation of Rhodamine 6G and Phenol Red by Nanosized TiO2 under Solar Irradiation. J. Saudi Chem. Soc. 2011, 15, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of Azo Dye Acid Red 14 in Water on ZnO as an Alternative Catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation Enhancement of Methylene Blue on ZnO Nanocombs Synthesized by Thermal Evaporation Technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Yan, H.; Hou, J.; Fu, Z.; Yang, B.; Yang, P.; Liu, K.; Wen, M.; Chen, Y.; Fu, S.; Li, F. Growth and Photocatalytic Properties of One-Dimensional ZnO Nanostructures Prepared by Thermal Evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Al-Aoh, H.A.; Mihaina, I.A.M.; Alsharif, M.A.; Darwish, A.A.A.; Rashad, M.; Mustafa, S.K.; Aljohani, M.M.H.; Al-Duais, M.A.; Al-Shehri, H.S. Removal of Methylene Blue from Synthetic Wastewater by the Selected Metallic Oxides Nanoparticles Adsorbent: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. Commun. 2020, 207, 1719–1735. [Google Scholar] [CrossRef]
- Rashad, M. Performance Efficiency and Kinetic Studies of Water Purification Using ZnO and MgO Nanoparticles for Potassium Permanganate. Opt. Quantum Electron. 2019, 51, 291. [Google Scholar] [CrossRef]





| Sample | qe mg/g | k | K |
|---|---|---|---|
| TiO2 | 158 | 0.01 | 0.63 |
| Nb(0.2)/TiO2 | 176 | 0.02 | 0.59 |
| Nb(0.5)/TiO2 | 183 | 0.05 | 0.23 |
| Nb(2.0)/TiO2 | 187 | 0.19 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almulhem, N.; Awada, C.; Shaalan, N.M. Photocatalytic Degradation of Phenol Red in Water on Nb(x)/TiO2 Nanocomposites. Crystals 2022, 12, 911. https://doi.org/10.3390/cryst12070911
Almulhem N, Awada C, Shaalan NM. Photocatalytic Degradation of Phenol Red in Water on Nb(x)/TiO2 Nanocomposites. Crystals. 2022; 12(7):911. https://doi.org/10.3390/cryst12070911
Chicago/Turabian StyleAlmulhem, Najla, Chawki Awada, and Nagih M. Shaalan. 2022. "Photocatalytic Degradation of Phenol Red in Water on Nb(x)/TiO2 Nanocomposites" Crystals 12, no. 7: 911. https://doi.org/10.3390/cryst12070911
APA StyleAlmulhem, N., Awada, C., & Shaalan, N. M. (2022). Photocatalytic Degradation of Phenol Red in Water on Nb(x)/TiO2 Nanocomposites. Crystals, 12(7), 911. https://doi.org/10.3390/cryst12070911







