The Impact of Full-Scale Substitution of Ca2+ with Ni2+ Ions on Brushite’s Crystal Structure and Phase Composition
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Synthesis of CaxNi1−xHPO4·nH2O Compounds
2.3. Characterization Techniques
3. Results and Discussion
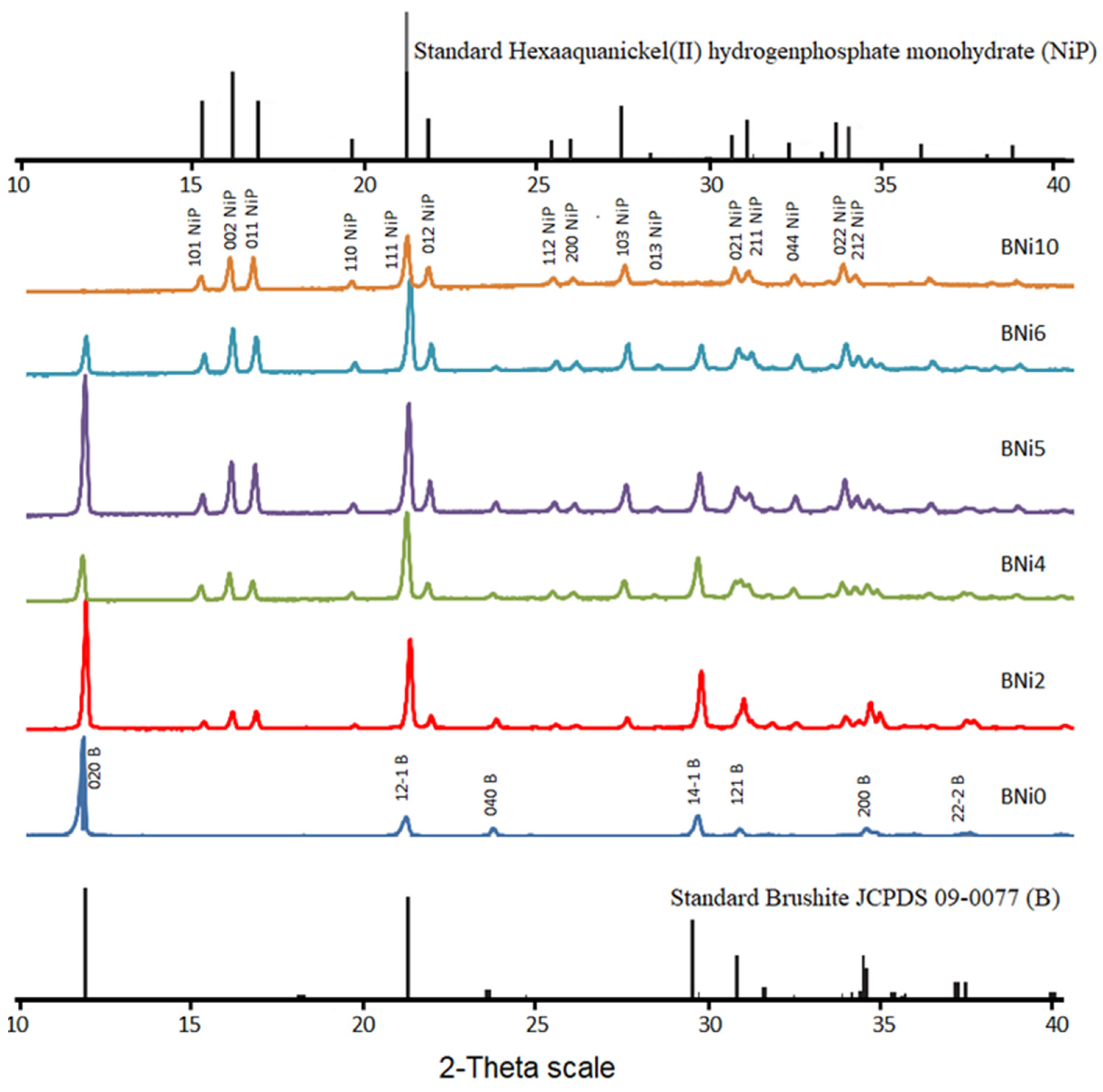
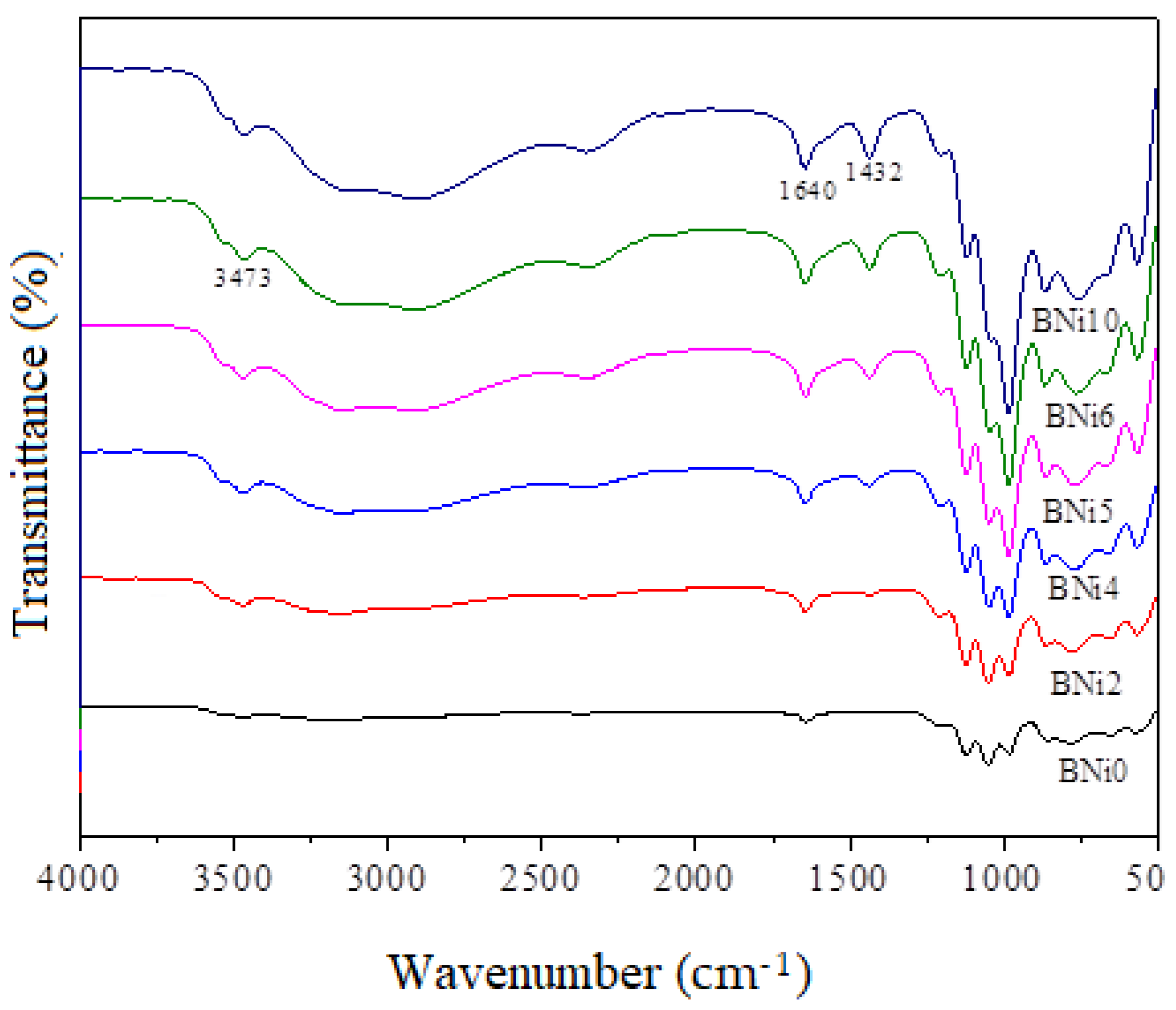
3.1. Mineralogical and Microstructural Analysis
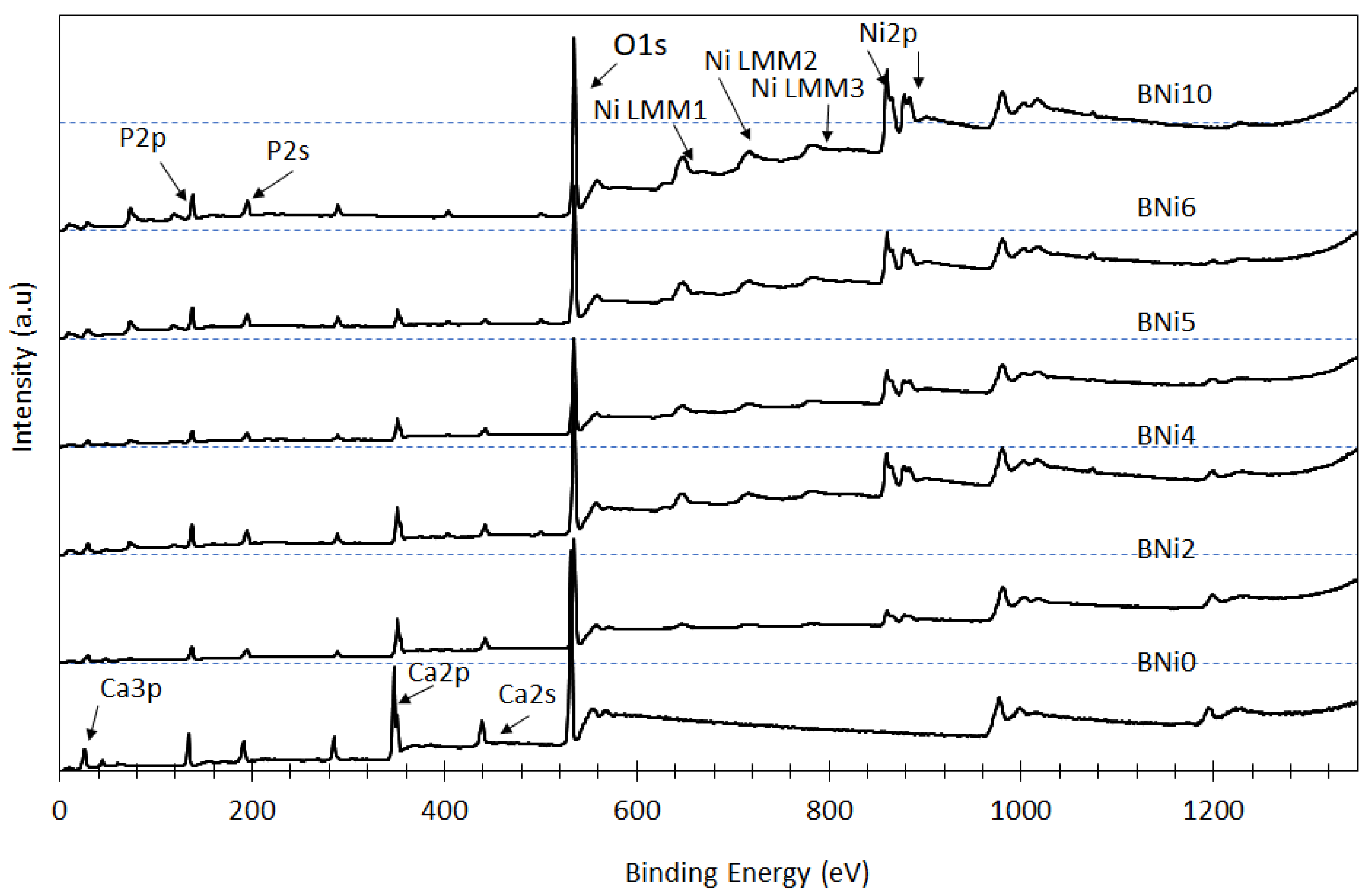
3.2. Elemental and Chemical Composition of CaxNi1−xHPO4·nH2O Compounds
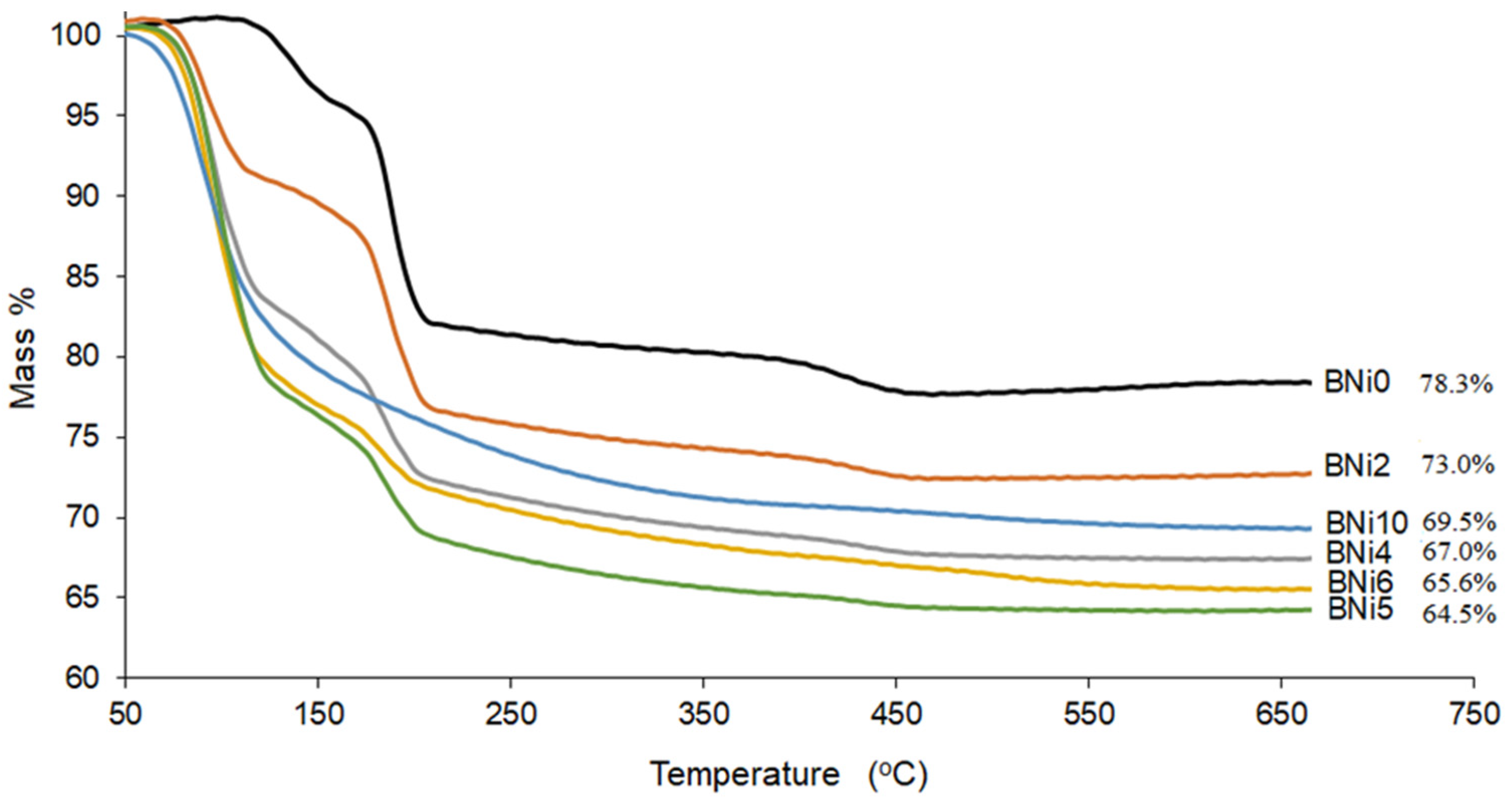
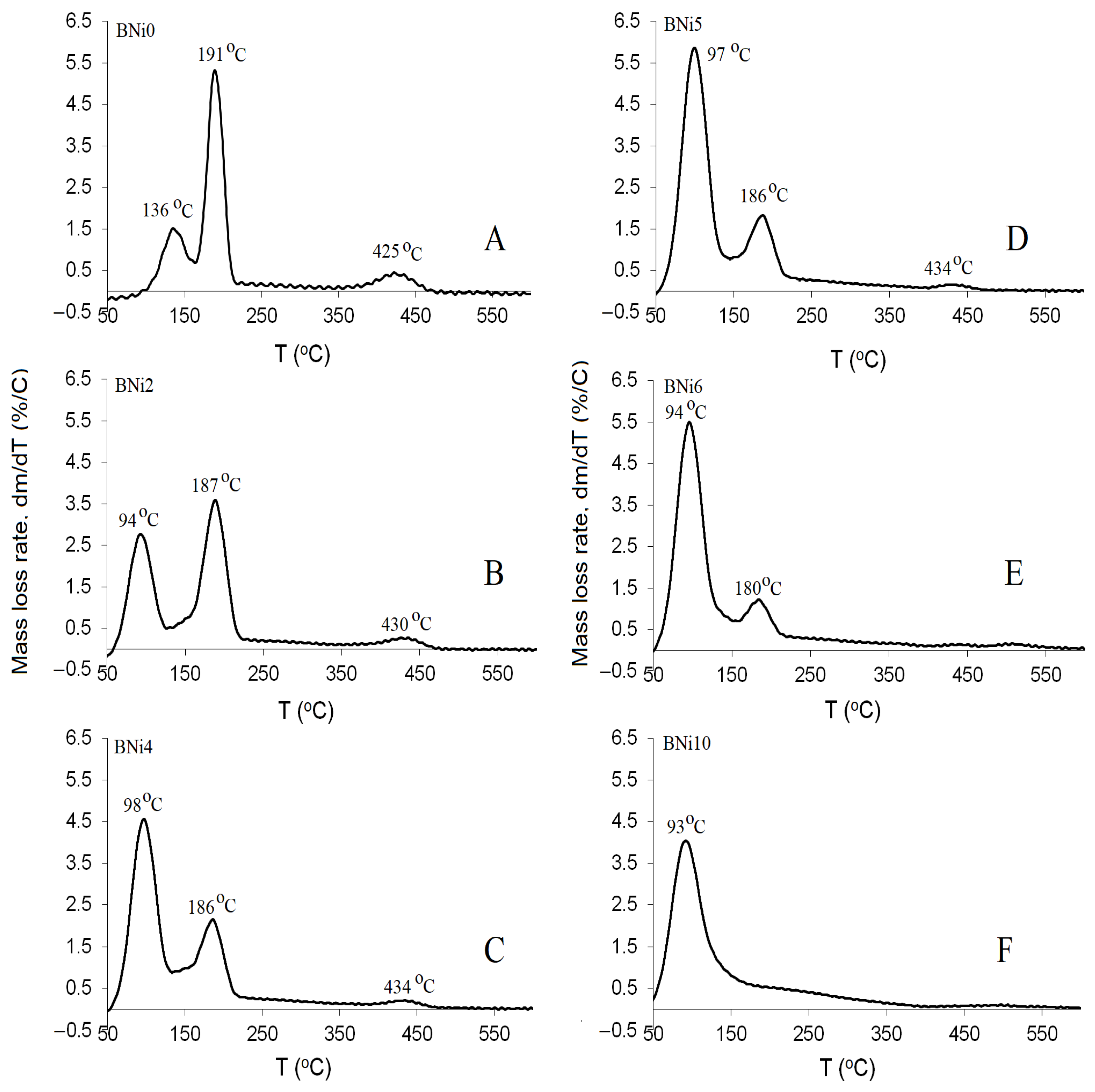
3.3. Thermogravimetric Analysis (TGA)
3.4. Phase Evolution during the Precipitation of CaxNi1−xHPO4·nH2O Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and Hydrolysis of Brushite (DCPD): The Role of Ionic Substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.; Abdeltawab, N.F.; Awad, G.A. Chitosan-calcium phosphate composite scaffolds for control of postoperative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Abdel-Fattah, E.; Saadeddin, I.; Al Battah, F.; Issa, K.I.; Saffarini, G. The effect of natural fibres template on the chemical and structural properties of Biphasic Calcium Phosphate scaffold. Mater. Res. Express 2020, 7, 065405. [Google Scholar] [CrossRef]
- Cirillo, M.; Martelli, G.; Boanini, E.; Rubini, K.; Di Filippo, M.; Torricelli, P.; Pagani, S.; Fini, M.; Bigi, A.; Giacomini, D. Strontium substituted hydroxyapatite with β-lactam integrin agonists to enhance mesenchymal cells adhesion and to promote bone regeneration. Colloids Surf. B Biointerfaces 2021, 200, 111580. [Google Scholar] [CrossRef] [PubMed]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Shyong, Y.-J.; Chang, K.-C.; Lin, F.-H. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloids Surf. B Biointerfaces 2018, 171, 391–397. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Li, D.; Qi, C.; Han, L.; Chen, M.; Fu, F.; Yuan, J.; Li, G. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef]
- Revelo, C.F.; Colorado, H.A. A green composite material of calcium phosphate cement matrix with additions of car tire waste particles. Int. J. Appl. Ceram. Technol. 2021, 18, 182–191. [Google Scholar] [CrossRef]
- Huertas, C.F.R.; Vieira, C.M.F.; Colorado, H.A. A hybrid composite for structural applications made of rubber waste tires and calcium phosphate cement. Int. J. Appl. Ceram. Technol. 2021, 18, 1342–1353. [Google Scholar] [CrossRef]
- Boehm, A.V.; Meininger, S.; Gbureck, U.; Müller, F.A. Self-healing capacity of fiber-reinforced calcium phosphate cements. Sci. Rep. 2020, 10, 9430. [Google Scholar] [CrossRef]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar] [PubMed]
- Priya, B.A.; Senthilguru, K.; Agarwal, T.; Narayana, S.N.G.H.; Giri, S.; Pramanik, K.; Pal, K.; Banerjee, I. Nickel doped nanohydroxyapatite: Vascular endothelial growth factor inducing biomaterial for bone tissue engineering. RSC Adv. 2015, 5, 72515–72528. [Google Scholar] [CrossRef]
- Salnikow, K.; An, W.G.; Melillo, G.; Blagosklonny, M.V.; Costa, M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis 1999, 20, 1819–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civjan, S.; Huget, E.F.; DeSimon, L.B. Potential Applications of Certain Nickel-Titanium (Nitinol) Alloys. J. Dent. Res. 1975, 54, 89–96. [Google Scholar] [CrossRef]
- Fini, M.; Aldini, N.N.; Torricelli, P.; Giavaresi, G.; Borsari, V.; Lenger, H.; Bernauer, J.; Giardino, R.; Chiesa, R.; Cigada, A. A new austenitic stainless steel with negligible nickel content: An in vitro and in vivo comparative investigation. Biomaterials 2003, 24, 4929–4939. [Google Scholar] [CrossRef]
- Sayahi, M.; Santos, J.; El-Feki, H.; Charvillat, C.; Bosc, F.; Karacan, I.; Milthorpe, B.; Drouet, C. Brushite (Ca,M)HPO4, 2H2O doping with bioactive ions (M = Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): A new path to functional biomaterials? Mater. Today Chem. 2020, 16, 100230. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabarcos, E.L. Strontium Ions Substitution in Brushite Crystals: The Role of Strontium Chloride. J. Funct. Biomater. 2011, 2, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Wang, Z.; Sun, A.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO4·2H2O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C 2020, 111, 110782. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.Y.; Roh, Y.; Lee, J.; Kim, J.; Lee, Y.; Bang, J.; Lee, Y.J. Optimizing Calcium Phosphates by the Control of pH and Temperature via Wet Precipitation. J. Nanosci. Nanotechnol. 2015, 15, 10008–10016. [Google Scholar] [CrossRef]
- Alshaaer, M.; Al-Kafawein, J.; Qazaq, A.; Issa, K.; Saffarini, G. Effects of magnetite incorporation in a chemically bonded phosphate ceramic. J. Phys. Chem. Solids 2022, 162, 110531. [Google Scholar] [CrossRef]
- Luo, J.; Engqvist, H.; Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 2018, 81, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Mert, I.; Mandel, S.; Tas, A.C. Do cell culture solutions transform brushite (CaHPO4 2H2O) to octacalium phosphate (Ca8(HPO4)2(PO4)4 5H2O)? In Advances in Bioceramics and Porous Ceramics IV; Narayan, R., Colombo, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 79–94. [Google Scholar]
- Hurle, K.; Oliveira, J.; Reis, R.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Guerra-López, J.R.; Güida, J.A.; Ramos, M.A.; Punte, G. The influence of nickel on brushite structure stabilization. J. Mol. Struct. 2017, 1137, 720–724. [Google Scholar] [CrossRef]
- Guerra-López, J.; Güida, J.; Bianchi, A.; Punte, G. Influence of carbonate and nickel(II) concentration on the synthesis of calcium phosphates. J. Solid State Chem. 2018, 267, 98–105. [Google Scholar] [CrossRef]
- Patil, S.B.; Jena, A.; Bhargava, P. Influence of Ethanol Amount During Washing on Deagglomeration of Co-Precipitated Calcined Nanocrystalline 3YSZ Powders. Int. J. Appl. Ceram. Technol. 2012, 10, E247–E257. [Google Scholar] [CrossRef]
- Piva, R.H.; Piva, D.H.; Pierri, J.; Montedo, O.R.K.; Morelli, M.R. Azeotropic distillation, ethanol washing, and freeze drying on coprecipitated gels for production of high surface area 3Y–TZP and 8YSZ powders: A comparative study. Ceram. Int. 2015, 41, 14148–14156. [Google Scholar] [CrossRef]
- Lu, B.-Q.; Willhammar, T.; Sun, B.-B.; Hedin, N.; Gale, J.D.; Gebauer, D. Introducing the crystalline phase of dicalcium phosphate monohydrate. Nat. Commun. 2020, 11, 1546. [Google Scholar] [CrossRef]
- Alshaaer, M.; Afify, A.S.; Moustapha, M.E.; Hamad, N.; Hammouda, G.A.; Rocha, F. Effects of the full-scale substitution of strontium for calcium on the microstructure of brushite: (CaxSr1−x)HPO4.nH2O system. Clay Miner. 2020, 55, 366–374. [Google Scholar] [CrossRef]
- Nosrati, H.; Le, D.Q.S.; Emameh, R.Z.; Perez, M.C.; Bünger, C.E. Nucleation and growth of brushite crystals on the graphene sheets applicable in bone cement. Bol. Soc. Esp. Cerám. Vidr. 2022, 61, 27–34. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium–magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Alshaaer, M.; Issa, K.; Alanazi, A.; Mallouh, S.; Afify, A.; Moustapha, M.; Komnitsas, K. Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials. Minerals 2021, 11, 284. [Google Scholar] [CrossRef]
- Alshaaer, M.; Al-Kafawein, J.; Afify, A.S.; Hamad, N.; Saffarini, G.; Issa, K. Effect of Ca2+ Replacement with Cu2+ Ions in Brushite on the Phase Composition and Crystal Structure. Minerals 2021, 11, 1028. [Google Scholar] [CrossRef]
- Girisken, G.; Tas, A.C. Development of biomineralization solutions to facilitate the transformation of brushite (CaHPO4·2H2O) into octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O). In Proceedings of the 15th National Biomedical Engineering Meeting (BIYOMUT), Antalya, Turkey, 21–24 April 2010. [Google Scholar]
- Tamimi, F.; Le Nihouannen, D.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J.; Tamimi, F. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Madhurambal, G.; Subha, R.; Mojumdar, S.C. Crystallization and thermal characterization of calcium hydrogen phosphate dihydrate crystals. J. Therm. Anal. 2009, 96, 73–76. [Google Scholar] [CrossRef]
- Rajendran, K.; Keefe, C.D. Growth and characterization of calcium hydrogen phosphate dihydrate crystals from single diffusion gel technique. Cryst. Res. Technol. 2010, 45, 939–945. [Google Scholar] [CrossRef]
- Eldrehmy, E.; Alghamdi, Y.; Amer, H.; Yassin, M.; Mostafa, S.; Moustapha, M.E.; Menazea, A. Hydroxyapatite-based bio-ceramic of ternary nanocomposites containing cuprous oxide/graphene oxide for biomedical applications. Diam. Relat. Mater. 2022, 126, 109121. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Wang, J.; Ide, Y.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Nanjundan, A.K.; Golberg, D.; et al. Self-assembly of nickel phosphate-based nanotubes into two-dimensional crumpled sheet-like architectures for high-performance asymmetric supercapacitors. Nano Energy 2019, 67, 104270. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Gao, T.; Shen, W.; Dong, X.; Naito, S.; Qin, L.; Huang, Y. Unusual adsorption and desorption behaviors of NO and CO on nanoporous nickel phosphate VSB-5: In situ FT-IR and TPD study. Catal. Today 2015, 258, 199–204. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Li, B.; Zhang, D.; Zhao, N.; Tang, S. Comparison of different K-struvite crystallization processes for simultaneous potassium and phosphate recovery from source-separated urine. Sci. Total Environ. 2019, 651, 787–795. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Hulliger, J. Growth of strontium hydrogen phosphate/gelatin composites: A biomimetic approach. New J. Chem. 2016, 40, 5495–5500. [Google Scholar] [CrossRef] [Green Version]
- Tortet, L.; Gavarri, J.R.; Nihoul, G.; Dianoux, A. Study of Protonic Mobility in CaHPO4·2H2O (Brushite) and CaHPO4(Monetite) by Infrared Spectroscopy and Neutron Scattering. J. Solid State Chem. 1997, 132, 6–16. [Google Scholar] [CrossRef]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite, CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineral. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Thermal stability of the ‘cave’ mineral brushite CaHPO4·2H2O—Mechanism of formation and decomposition. Thermochim. Acta 2011, 521, 14–17. [Google Scholar] [CrossRef] [Green Version]



| Product ID | NaH2PO4·2H2O | Ca(NO3)2·4H2O | Ni(NO3)2·6H2O | Ni/Ca Molar Ratio |
|---|---|---|---|---|
| BNi0 | 1 | 1 | 0 | 0 |
| BNi2 | 1 | 0.8 | 0.2 | 0.25 |
| BNi4 | 1 | 0.6 | 0.4 | 0.67 |
| BNi5 | 1 | 0.5 | 0.5 | 1.0 |
| BNi6 | 1 | 0.4 | 0.6 | 1.5 |
| BNi10 | 1 | 0 | 1 | - |
| Product ID | Brushite wt % | a (Å) | b (Å) | c (Å) | βο | V (Å3) |
|---|---|---|---|---|---|---|
| Standard Brushite | 100 | 5.812 | 15.18 | 6.239 | 116.43 | 492.91 |
| BNi0 | 100 | 5.8145 | 15.1693 | 6.2399 | 116.43 | 492.8455 |
| BNi2 | 93 | 5.81143 | 15.17852 | 6.23839 | 116.43 | 492.7654 |
| BNi4 | 25.5 | 5.8195 | 15.19959 | 6.24705 | 116.43 | 494.8206 |
| BNi5 | 34.5 | 5.82386 | 15.21097 | 6.25173 | 116.43 | 495.9334 |
| BNi6 | 14.6 | 5.81326 | 15.18328 | 6.24035 | 116.43 | 493.2301 |
| BNi10 | 0 | - | - | - | - | - |
| Product ID | Brushite wt % | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|
| Standard HNiP | 100 | 6.916 | 6.1032 | 11.1679 | 471.394 |
| BNi0 | 0 | - | - | - | - |
| BNi2 | 7 | 6.91997 | 6.09953 | 11.14389 | 470.3676 |
| BNi4 | 74.5 | 6.93074 | 6.11028 | 11.17562 | 473.2737 |
| BNi5 | 65.5 | 6.94044 | 6.11663 | 11.18034 | 474.629 |
| BNi6 | 85.4 | 6.9211 | 6.10223 | 11.16156 | 471.3989 |
| BNi10 | 100 | 6.9356 | 6.10915 | 11.17447 | 473.4692 |
| Ni/Ca Ratio | Crystal Structure | Crystal Size (µm) | Compounds Formed |
|---|---|---|---|
| 0 < 0.25 | Monoclinic | ~10 | Brushite |
| 0.25 ≤ x ≤ 1.5 | Monoclinic/orthorhombic | ~5 + 0.5 | Brushite + HNiP |
| >1.5 | Orthorhombic | ~0.5 | HNiP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaaer, M.; Issa, K.; Afify, A.S.; Moustapha, M.E.; Alanazi, A.A.; Elsanousi, A.; Qahtan, T.F. The Impact of Full-Scale Substitution of Ca2+ with Ni2+ Ions on Brushite’s Crystal Structure and Phase Composition. Crystals 2022, 12, 940. https://doi.org/10.3390/cryst12070940
Alshaaer M, Issa K, Afify AS, Moustapha ME, Alanazi AA, Elsanousi A, Qahtan TF. The Impact of Full-Scale Substitution of Ca2+ with Ni2+ Ions on Brushite’s Crystal Structure and Phase Composition. Crystals. 2022; 12(7):940. https://doi.org/10.3390/cryst12070940
Chicago/Turabian StyleAlshaaer, Mazen, Khalil Issa, Ahmed S. Afify, Moustapha E. Moustapha, Abdulaziz A. Alanazi, Ammar Elsanousi, and Talal F. Qahtan. 2022. "The Impact of Full-Scale Substitution of Ca2+ with Ni2+ Ions on Brushite’s Crystal Structure and Phase Composition" Crystals 12, no. 7: 940. https://doi.org/10.3390/cryst12070940
APA StyleAlshaaer, M., Issa, K., Afify, A. S., Moustapha, M. E., Alanazi, A. A., Elsanousi, A., & Qahtan, T. F. (2022). The Impact of Full-Scale Substitution of Ca2+ with Ni2+ Ions on Brushite’s Crystal Structure and Phase Composition. Crystals, 12(7), 940. https://doi.org/10.3390/cryst12070940









