High-Pressure Solidification of Ternary Al-Ni-Sn Alloy
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
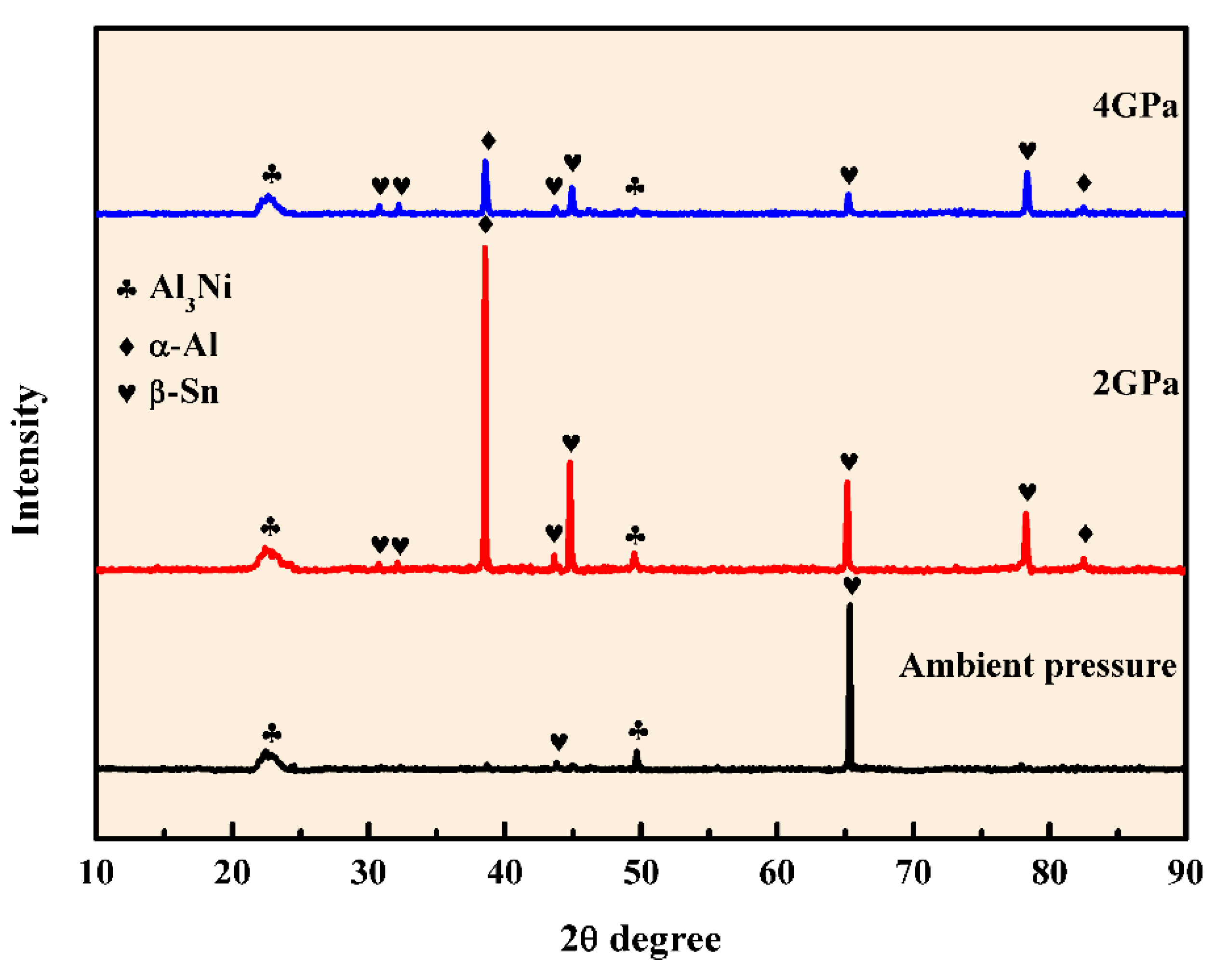
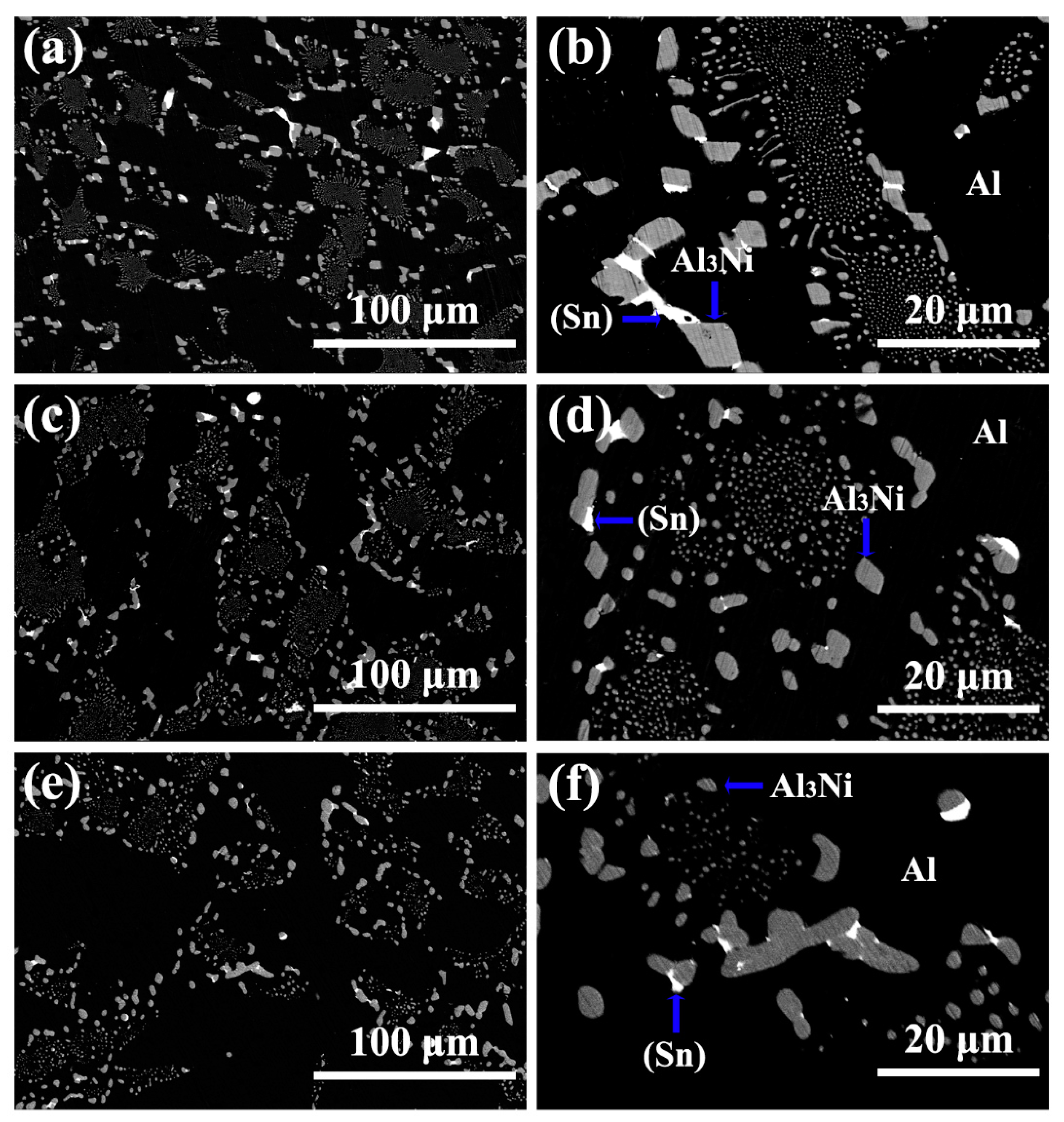
3.1. Microstructure of Al-5.4Ni-2Sn Alloy Solidified at Different Pressures
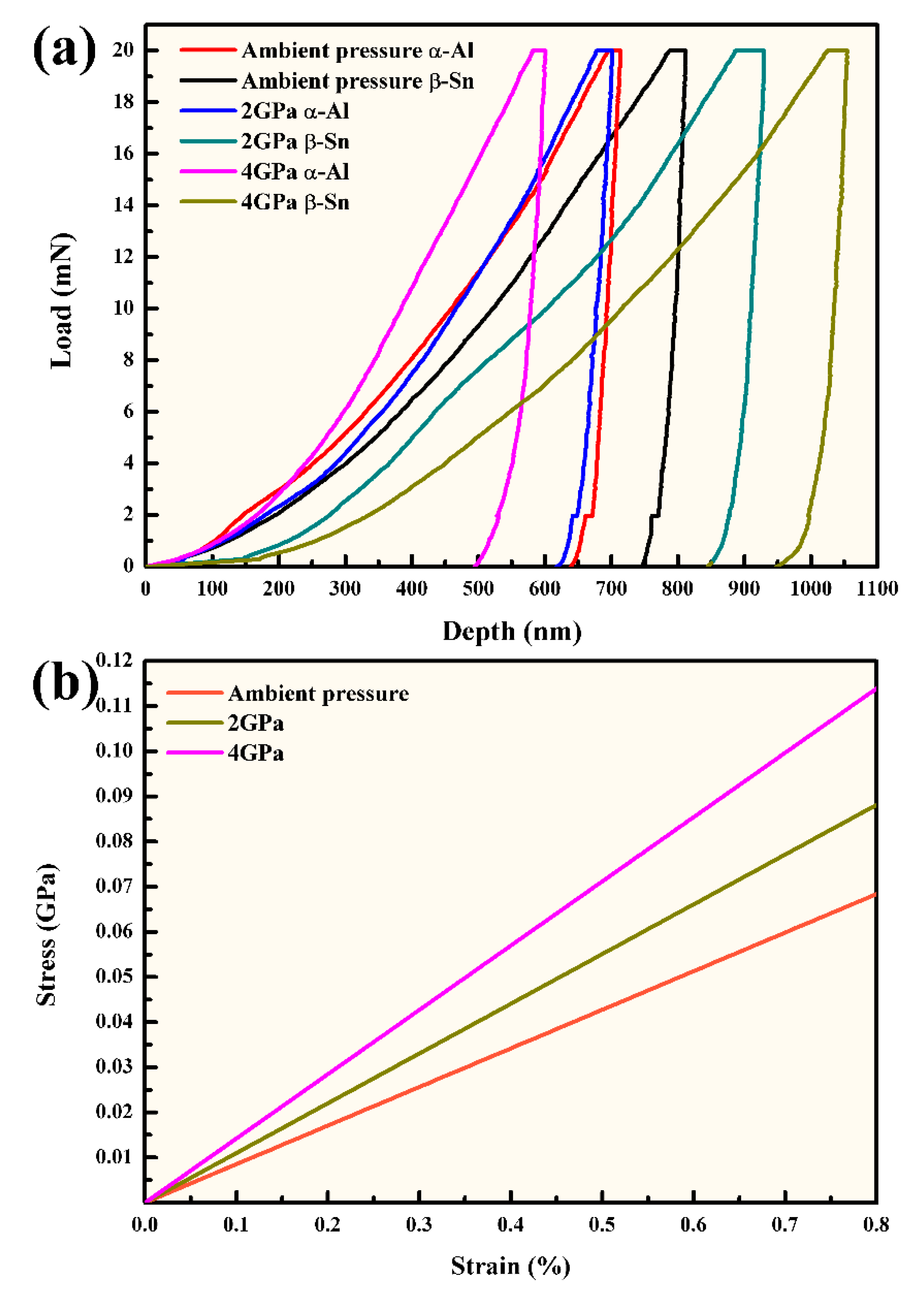
3.2. Mechanical Properties of Al-5.4Ni-2Sn Alloy Solidified at Different Pressures
4. Conclusions
- (1)
- After solidification under different pressures, the Al-5.4Ni-2Sn alloy consists of α-Al phase, bulk Al3Ni phase, interphase β-Sn and eutectic microstructure. Under high pressure, the thickness of β-Sn phase decreases obviously.
- (2)
- The precipitation sequence of the alloy during ambient pressure equilibrium solidification is L→Al3Ni→(α-Al + Al3Ni)eutectic→(α-Al + Al3Ni +β-Sn)eutectic.
- (3)
- The hardness values of α-Al phase at ambient pressure, 2 GPa and 4 GPa are 1.5 GPa, 1.62 GPa and 1.99 GPa, respectively. The tensile strength of the α-Al phase increases with the increase of solidification pressure.
- (4)
- The compressive strength and strain of the Al-5.4Ni-2Sn alloy under high pressure are obviously improved compared with those of the samples solidified under ambient pressure. When the deformation is 30%, the compressive strength at ambient pressure, 2 GPa and 4 GPa is 538.1 MPa, 1403.2 MPa and 1547.9 MPa respectively. The compressive strength under high pressure increased by 160.85% and 187.7%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jambur, V.; Tangpatjaroen, C.; Xi, J.; Tarnsangpradit, J.; Gao, M.; Sheng, H.; Perepezko, J.H.; Szlufarska, I. Effects of minor alloying on the mechanical properties of Al based metallic glasses. J. Alloys Compd. 2021, 854, 157266. [Google Scholar] [CrossRef]
- Das, S.; Mondal, D.P.; Sawla, S.; Ramakrishnan, N. Synergic effect of reinforcement and heat treatment on the two body abrasive wear of an Al–Si alloy under varying loads and abrasive sizes. Wear 2008, 264, 47–59. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, X.; Zu, Q.; Han, B.H.; Han, X.; Cui, C.X. Significantly improved particle strengthening of Al-Sc alloy by high Sc composition design and rapid solidification. Mater. Sci. Eng. A 2021, 800, 140304. [Google Scholar] [CrossRef]
- Nie, Z.R. Function and development of alloy elements in aluminum. Chin. J. Nonferr. Metal. 2009, 22, 56–57. [Google Scholar]
- Yun, K.; Li, S.R.; Li, W.X. Effects of transition metals, Fe, Ni, Sc and Zr, on the microstructure and mechanical properties of 2618 aluminum alloy. Rare Met. Mater. Eng. 2000, 29, 177–181. [Google Scholar]
- Nandi, P.; Suwas, S.; Subodh, K. The Effect of minor addition of Ni on the microstructural evolution and mechanical properties of suction cast Al-0.14 at.pct Sc binary alloy. Metall. Mater. Trans. A 2013, 44, 2591–2603. [Google Scholar] [CrossRef]
- Adachi, H.; Osamura, K.; Ochiai, S.; Jun, K.S.; Kazuhiko, Y. Mechanical property of nanoscale precipitate hardening aluminum alloys. Scr. Mater. 2001, 44, 1489–1492. [Google Scholar] [CrossRef]
- Saadat, Z.; Moghanaki, S.K.; Kazeminezhad, M.; Goodarzi, M.; Afjeh, S.M.B.G. Effect of two steps annealing on the microstructure and dynamic strain aging behavior of Al-6Mg alloy. Mater. Sci. Eng. A 2020, 798, 140097. [Google Scholar] [CrossRef]
- Compton, D.N.; Cornish, L.A.; Witcomb, M.J. The effect of microstructure on hardness measurements in the aluminium-rich corner of the Al–Ni–Cr system. J. Alloys Compd. 2001, 317–318, 372–378. [Google Scholar] [CrossRef]
- Belov, N.A.; Zolotorevskiy, V.S. The Effect of Nickel on the Structure, Mechanical and Casting Properties of Aluminium Alloy of 7075 Type. Mater. Sci. Forum 2002, 396–402, 935–940. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Wang, Z.L.; Zhang, L.B. Effect of Ni on properties of ZL101A aluminum alloy. Spec. Cast. Nonferr. Alloy. 2013, 33, 191. [Google Scholar]
- Chen, D.H.; Chen, C.; Zhu, X.R. Squeeze casting and strengthening mechanism of high strength Al-Ni cast aluminum alloy. Rare Met. Mater. Eng. 2017, 46, 3525–3531. [Google Scholar]
- Li, X.; Xie, H.; Zhang, Y.L.; Wei, X. Power Factor Optimization and Thermoelectric Properties of Mg2Si1-xSnx Alloys by Directional Solidification. Rare Met. Mater. Eng. 2020, 49, 2779–2785. [Google Scholar]
- Xu, Z.; Utton, C.; Tsakiropoulos, P.A. Study of the Effect of 5 at.% Sn on the Micro-Structure and Isothermal Oxidation at 800 and 1200 degrees C of Nb-24Ti-18Si Based Alloys with Al and/or Cr Additions. Materials 2020, 13, 245. [Google Scholar] [CrossRef] [Green Version]
- Billur, C.A.; Saatçi, B. Experimental determination of interfacial energy for solid Al in the Al-Sn-Mg eutectic system with Bridgman-type apparatus. Phys. Lett. A 2019, 383, 2652–2657. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.F.; Xu, J.Y.; Feng, J.K.; Zhao, Y.; Jiang, B.; Pan, F.S. Improvement of mechanical properties of hot extruded and age treated Mg–Zn–Mn–Ca alloy through Sn addition. J. Alloys Compd. 2020, 850, 156711. [Google Scholar] [CrossRef]
- He, C.Y.; Wang, J.G.; Chen, Y.L.; Yu, W.; Tang, D. Effects of Sn and Sb on the Hot Ductility of Nb plus Ti Micro-alloyed Steels. Metals 2020, 10, 1679. [Google Scholar] [CrossRef]
- Ma, J.X.; Wei, Q.; Le, T.H.; Wang, J.H.; Jin, P.P. Effect of Sn addition on the microstructure and mechanical properties of AZ31 alloys. Mater. Res. Express 2020, 7, 126507. [Google Scholar] [CrossRef]
- Ma, P.; Zou, C.M.; Wang, H.W.; Scudino, S.; Fu, B.G.; Wei, Z.J.; Kuhn, U.; Eckert, J. Effects of High Pressure and SiC Content on Microstructure and Precipitation Kinetics of Al–20Si alloy. J. Alloys Compd. 2014, 586, 639–644. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.; Jia, Y.D.; Wei, Z.J.; Suo, C.J.; Ji, P.C.; Shi, X.R.; Yu, Z.S.; Prashanth, K.G. Solidification of Al-xCu alloy under high pressures. J. Mater. Res. Technol. 2020, 9, 2983–2991. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhu, D.D.; Zou, C.M.; Wei, Z.J. Evolution of the microstructure and nanohardness of Ti–48 at.%Al alloy solidified under high pressure. Mater. Des. 2012, 38, 1790–1796. [Google Scholar] [CrossRef]
- Emuna, M.; Greenberg, Y.; Hevroni, R.; Korover, I.; Makov, G. Phase Diagrams of Binary Alloys under Pressure. J. Alloys Compd. 2016, 687, 360–369. [Google Scholar] [CrossRef]
- Wang, S.M.; He, D.W.; Zou, Y.T.; Wei, J.J.; Lei, L.; Li, Y.J.; Wang, J.H.; Wang, W.D.; Kou, Z.L. High-pressure and high-temperature sintering of nanostructured bulk NiAl materials. J. Mater. Res. 2011, 24, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Jie, J.C.; Zou, C.M.; Brosh, E.; Wang, H.W.; Wei, Z.J.; Li, T.J. Microstructure and mechanical properties of an Al–Mg alloy solidified under high pressures. J. Alloys Compd. 2013, 578, 394–404. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, X.; Xu, R.; Dong, Y.; Wang, L.; Zhao, S.S. Electron back-scattering diffraction preliminary analysis of heterogeneous nuclei in magnesium alloy during solidification process under GPa high pressure. J. Rare Earth 2018, 36, 184–189. [Google Scholar] [CrossRef]
- Fu, H.; Liu, N.; Zhang, Z.W.; Peng, Q.M. Effect of super-high pressure on microstructure and mechanical properties of Mg97Zn1Y2 alloys. J. Magnes. Alloy. 2016, 4, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Lin, X.P.; Xu, R.; Zheng, R.G.; Fan, Z.B.; Liu, S.J.; Wang, Z. Microstructure and compression deformation behavior in the quasicrystal-reinforced Mg-8Zn-1Y alloy solidified under super-high pressure. J. Rare Earth 2014, 32, 1048–1055. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Xu, Y.; Liang, Y.; Lin, J. Phase transformations and phase equilibria of a Ti-46.5Al-16.5Nb alloy. Mater. Des. 2016, 101, 88–94. [Google Scholar] [CrossRef]



| Al | Ni | Sn |
|---|---|---|
| 92.63 | 5.39 | 1.98 |
| Phase | Atom | Ambient Pressure | 2 GPa | 4 GPa |
|---|---|---|---|---|
| Black phase | Ni (wt.%) | 0.17 | 0.25 | 0.31 |
| Al (wt.%) | 97.59 | 97.26 | 96.74 | |
| Sn (wt.%) | 2.23 | 2.49 | 2.95 | |
| White phase | Ni (wt.%) | 3.49 | 3.02 | 2.7 |
| Al (wt.%) | 36.43 | 23.07 | 9.68 | |
| Sn (wt.%) | 60.07 | 73.91 | 87.62 |
| Phase | Ambient Pressure | 2 GPa | 4 GPa |
|---|---|---|---|
| α-Al | 1.5 | 1.62 | 1.99 |
| β-Sn | 1.09 | 0.86 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Lv, F.; Wang, X.; Zhang, Z.; Zhu, D.; Chen, Y.; Ge, J.; Tang, J. High-Pressure Solidification of Ternary Al-Ni-Sn Alloy. Crystals 2022, 12, 1025. https://doi.org/10.3390/cryst12081025
Yang X, Lv F, Wang X, Zhang Z, Zhu D, Chen Y, Ge J, Tang J. High-Pressure Solidification of Ternary Al-Ni-Sn Alloy. Crystals. 2022; 12(8):1025. https://doi.org/10.3390/cryst12081025
Chicago/Turabian StyleYang, Xiaohong, Feng Lv, Xiaohong Wang, Zhengzhong Zhang, Dongdong Zhu, Yuan Chen, Jianya Ge, and Jinhua Tang. 2022. "High-Pressure Solidification of Ternary Al-Ni-Sn Alloy" Crystals 12, no. 8: 1025. https://doi.org/10.3390/cryst12081025
APA StyleYang, X., Lv, F., Wang, X., Zhang, Z., Zhu, D., Chen, Y., Ge, J., & Tang, J. (2022). High-Pressure Solidification of Ternary Al-Ni-Sn Alloy. Crystals, 12(8), 1025. https://doi.org/10.3390/cryst12081025






