Study on the Effect of Different Factors on the Change of the Phosphorus-Rich Phase in High Phosphorus Steel Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
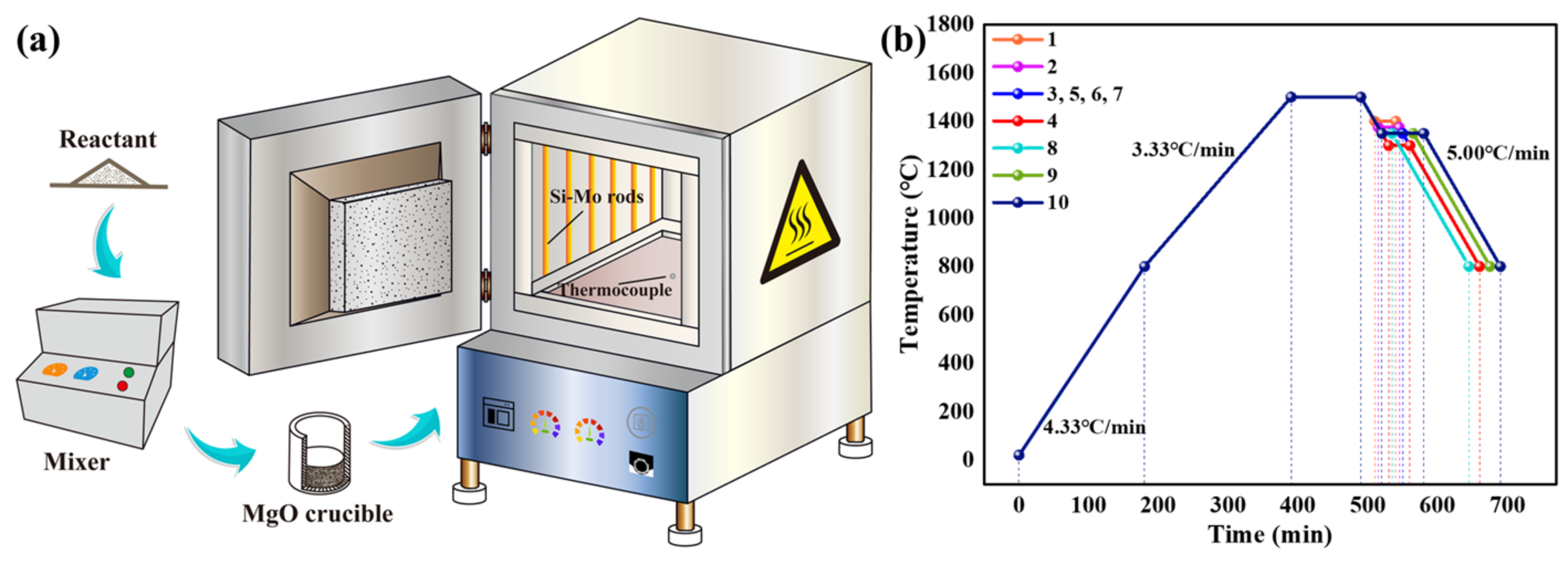
2.2. Experimental Methods
2.3. Sample Analysis
3. Results and Discussion
3.1. Pre-Experiment
3.2. Effects of Different Factors on Phosphorus-Rich Phase
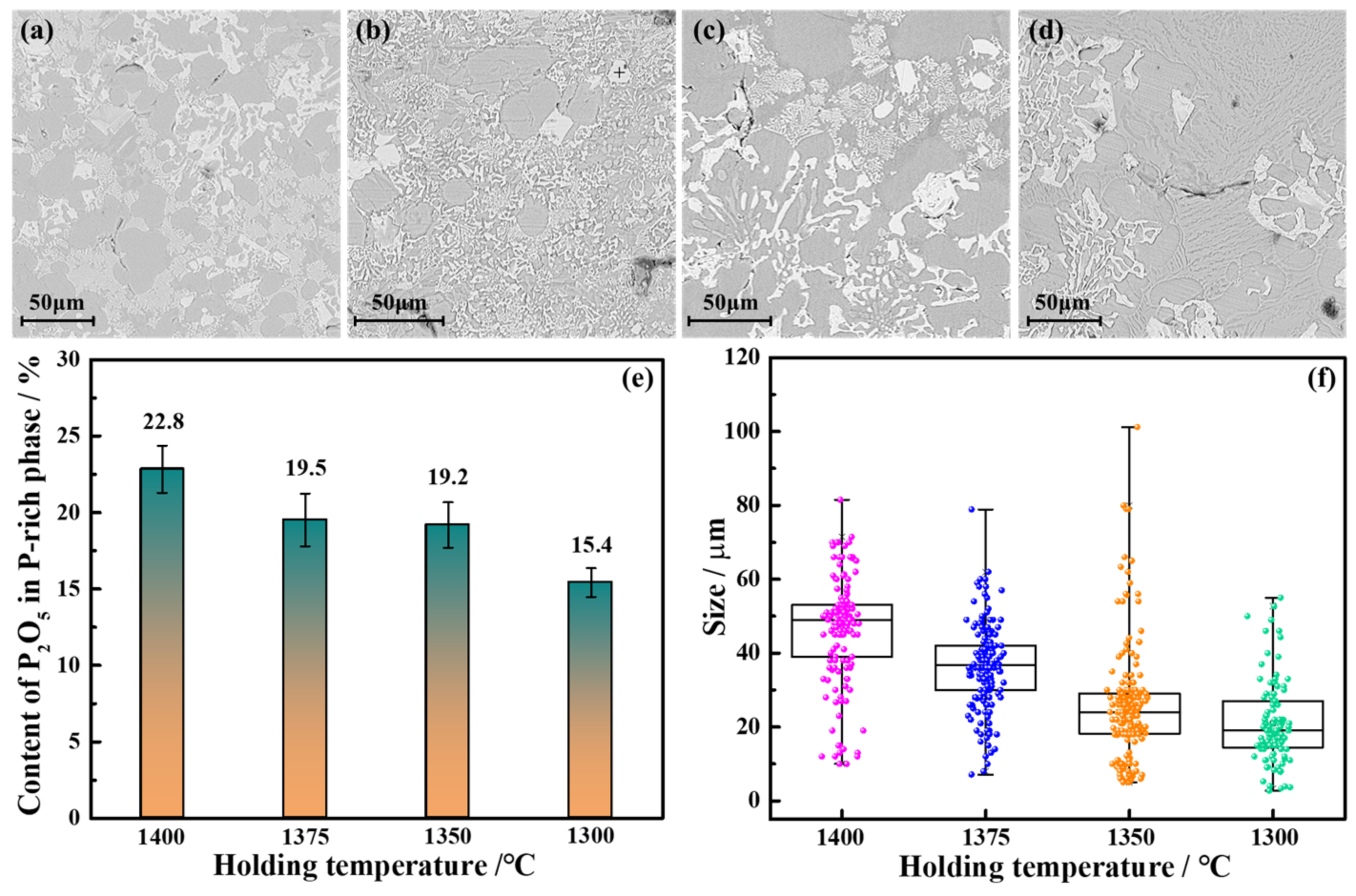
3.2.1. Holding Temperature
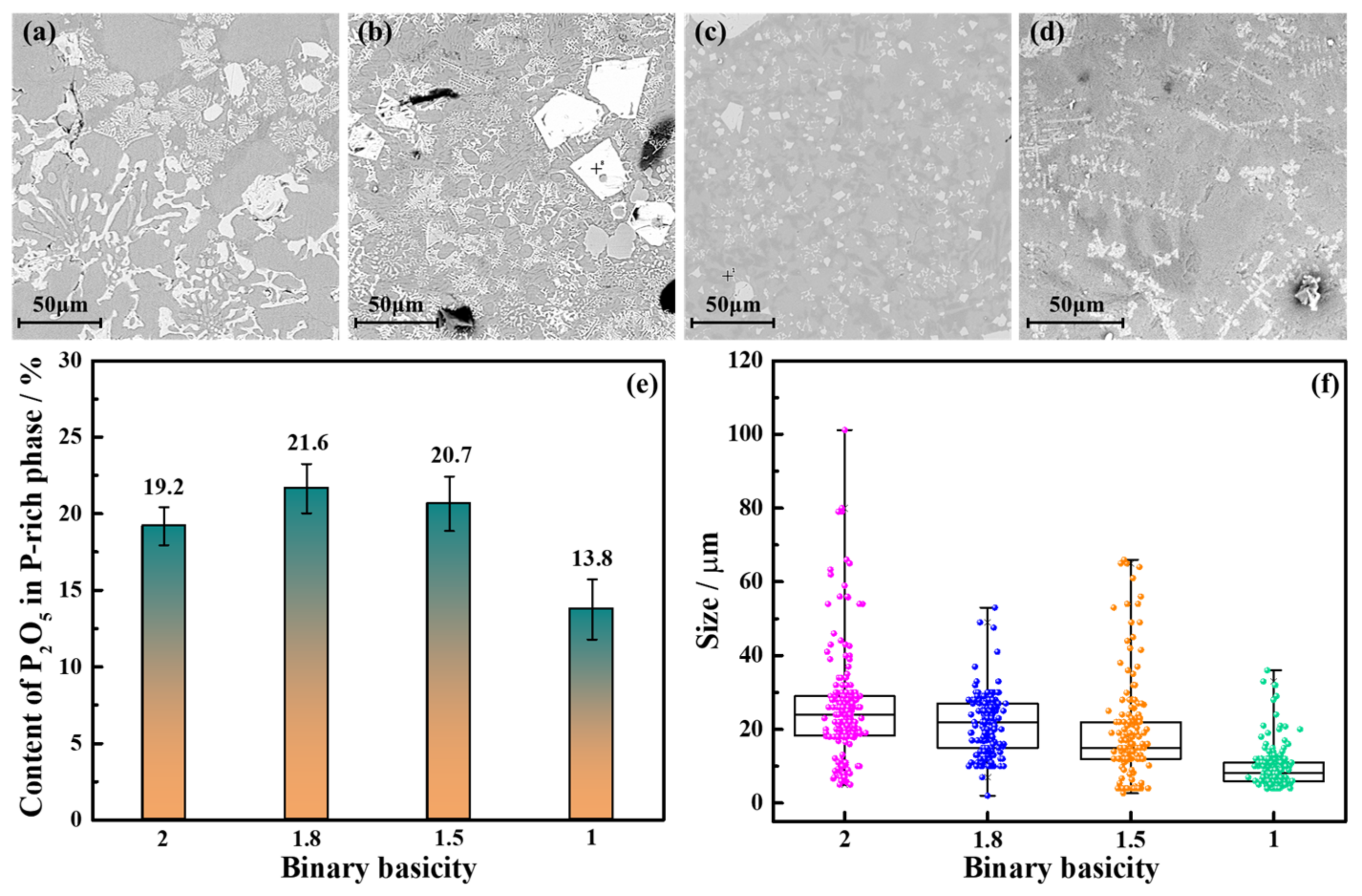
3.2.2. Binary Basicity
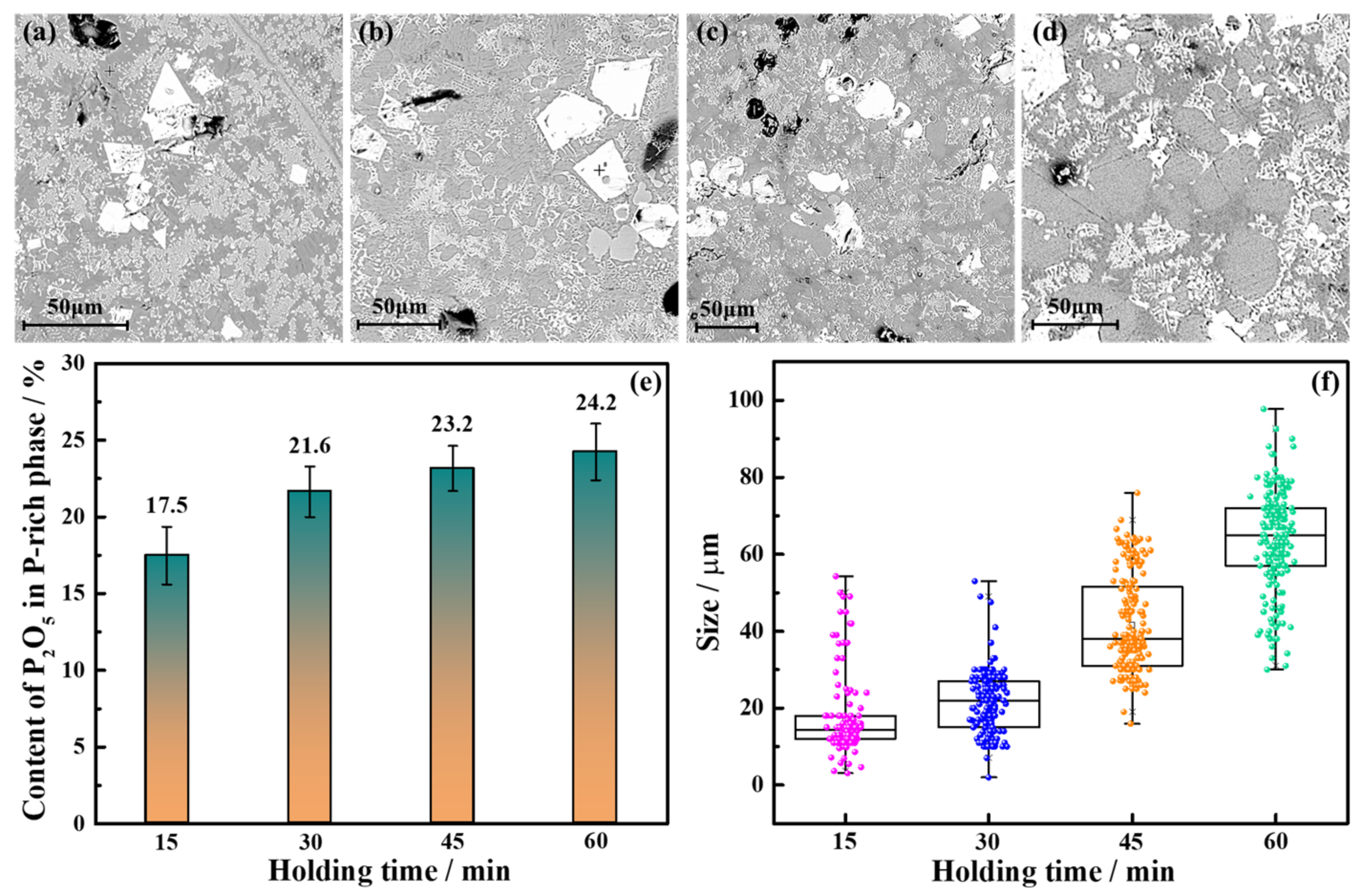
3.2.3. Holding Time
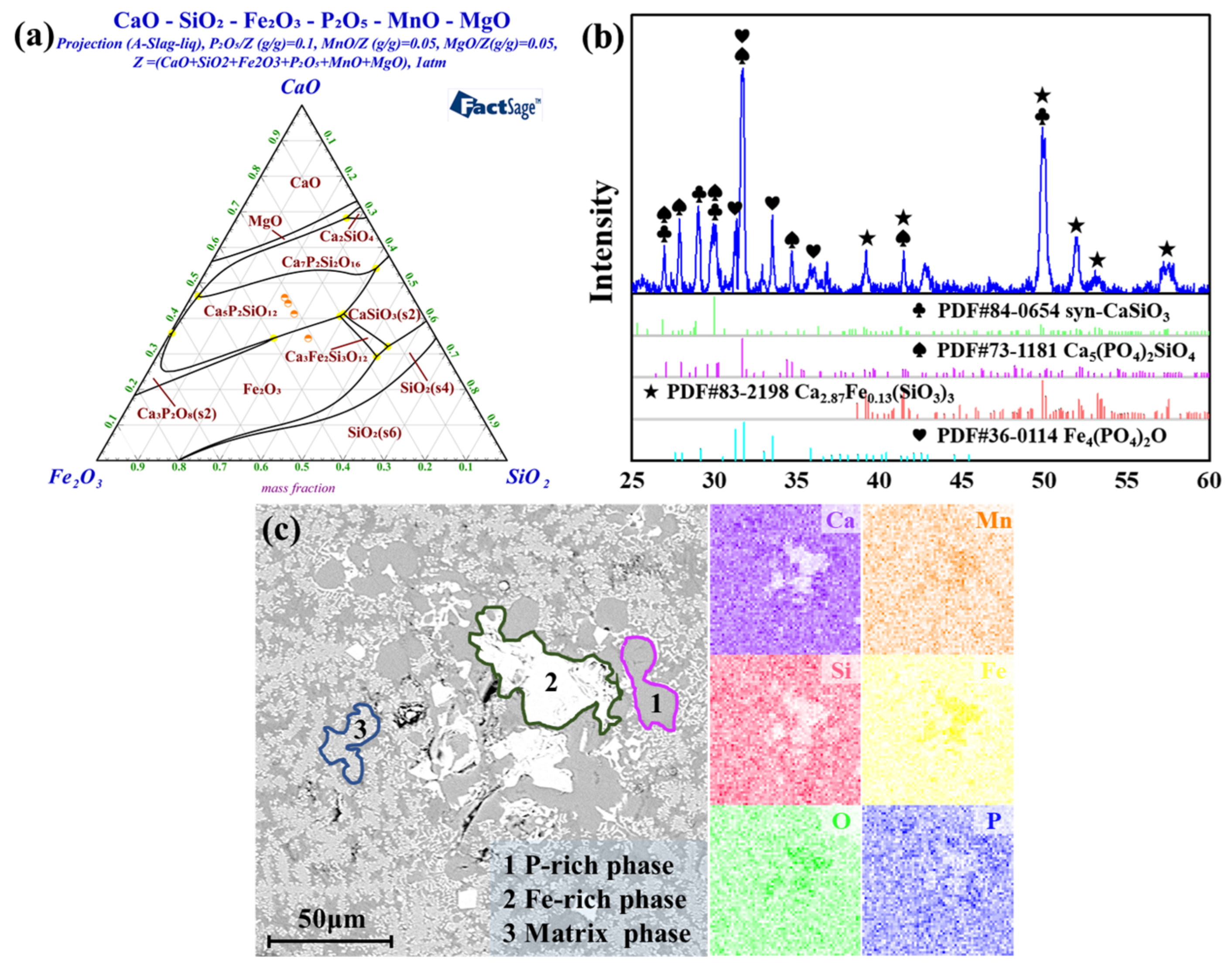
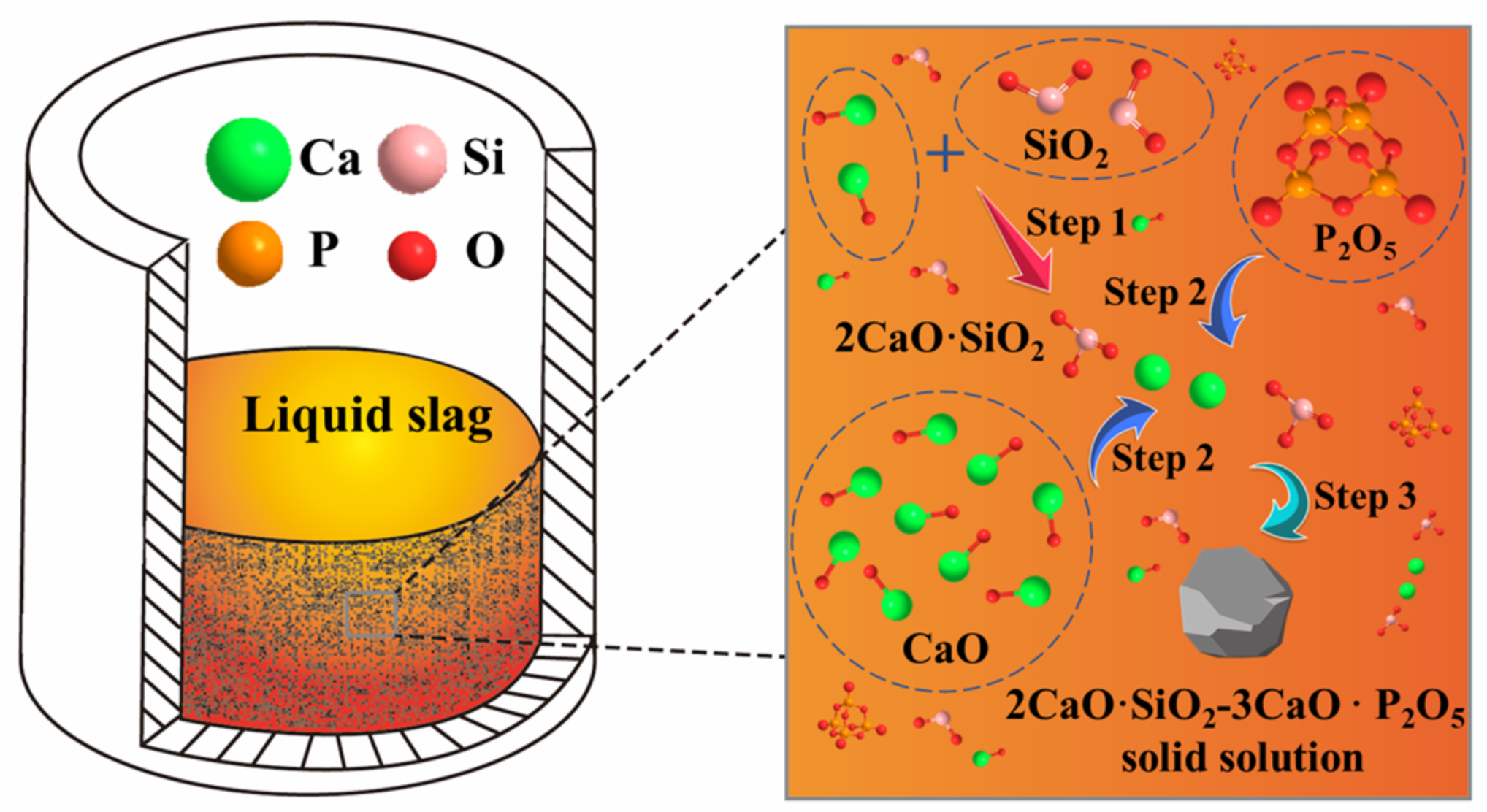
3.3. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Zhang, Q.; Han, Y.; Gao, P.; Li, G. Comprehensive utilization of iron and phosphorus from high-phosphorus refractory iron ore. JOM 2018, 70, 144–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Q.; Wang, G.; Wang, J. Phosphorus-containing mineral evolution and thermodynamics of phosphorus vaporization during carbothermal reduction of high-phosphorus iron ore. Metals 2018, 8, 451. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, Y.; Han, Y.; Li, Y. Migration behaviors and kinetics of phosphorus during coal-based reduction of high-phosphorus oolitic iron ore. Int. J. Miner. Metall. Mater. 2019, 26, 938–945. [Google Scholar] [CrossRef]
- Wu, J.; Wen, Z.; Cen, M. Development of technologies for high phosphorus oolitic hematite utilization. Steel Res. Int. 2011, 82, 494–500. [Google Scholar]
- Yang, W.; Yang, J.; Shi, Y.; Yang, Z.; Gao, F.; Zhang, R.; Ye, G. Effect of basicity on dephosphorization of hot metal with a low basicity slag at 1653 K. Ironmak. Steelmak. 2021, 48, 69–77. [Google Scholar] [CrossRef]
- Zhou, W.; Han, Y.; Sun, Y.; Li, Y. Strengthening iron enrichment and dephosphorization of high-phosphorus oolitic hematite using high-temperature pretreatment. Int. J. Miner. Metall. Mater. 2020, 27, 443–453. [Google Scholar] [CrossRef]
- Enzian, G. Some effects of phosphorus and nitrogen on the properties of low carbon steel. JOM 1950, 2, 346–353. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Perez-Garcia, F.; Parron-Rubio, M.E.; Garcia-Manrique, J.M.; Rubio-Cintas, M.D. Study of the suitability of different types of slag and its influence on the quality of green grouts obtained by partial replacement of cement. Materials 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, J.; Yuan, T.; Yoon, Y.; Mitchell, D. Comparing properties of concrete containing electric arc furnace slag and granulated blast furnace slag. Materials 2019, 12, 1371. [Google Scholar] [CrossRef] [Green Version]
- Yüksel, İ. A review of steel slag usage in construction industry for sustainable development. Environ. Dev. Sustain. 2017, 19, 369–384. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.; Wang, Y.; Zeng, Y. Sustainable utilization of steel slag from traditional industry and agriculture to catalysis. Sustainability 2020, 12, 9295. [Google Scholar] [CrossRef]
- Varanasi, S.S.; More, V.M.R.; Rao, M.B.V.; Alli, S.R.; Tangudu, A.K.; Santanu, D. Recycling ladle furnace slag as flux in steelmaking: A review. J. Sustain. Metall. 2019, 5, 449–462. [Google Scholar] [CrossRef]
- Ye, G.; Yang, J.; Zhang, R.; Yang, W.; Sun, H. Behavior of phosphorus enrichment in dephosphorization slag at low temperature and low basicity. Int. J. Miner. Metall. Mater. 2021, 28, 66–75. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Y.; Wang, D.; Wang, M. Effective removal of phosphorus from high phosphorus steel slag using carbonized rice husk. J. Environ. Sci. 2023, 124, 156–164. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, D.; Wang, S.; Li, C.; Guo, R. Phosphorus vaporization behaviour from converter slag. Ironmak. Steelmak. 2020, 47, 892–898. [Google Scholar] [CrossRef]
- Lin, L.; Bao, Y.; Wang, M.; Zhou, H. Influence of Al2O3 modification on phosphorus enrichment in P bearing steelmaking slag. Ironmak. Steelmak. 2014, 41, 193–198. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z. Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metall. Mater. Trans. B 2015, 46, 758–765. [Google Scholar] [CrossRef]
- Xie, S.; Wang, W.; Pan, Z.; Li, H.; Huang, D.; Du, y. Effect of Al2O3 on the melting, viscosity, and phosphorus distribution of CaO–SiO2–Fe2O3–P2O5 slag system. Steel Res. Int. 2018, 89, 1700516. [Google Scholar] [CrossRef]
- Yan, W.; Hao, Z.; Chen, W.; Li, J. Mixing effect of slag compositions and additives on crystallization of mold fluxes for Ti-bearing steels. Steel Res. Int. 2021, 10, 882–894. [Google Scholar] [CrossRef]
- Teratoko, T.; Maruoka, N.; Shibata, H.; Kitamura, S. Dissolution behavior of dicalcium silicate and tricalcium phosphate solid solution and other phases of steelmaking slag in an aqueous solution. High Temp. Mater. Process. 2012, 31, 329–338. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Kitamura, S. Measures to decrease and utilize steelmaking slag. J. Sustain. Metall. 2019, 5, 141–153. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. Separation and recovery of phosphorus from steelmaking slag via a selective leaching–chemical precipitation process. Hydrometallurgy 2019, 189, 105109. [Google Scholar] [CrossRef]
- Lang, Y.; Matsuura, H.; Tsukihashi, F. Long-term dissolution behavior of steelmaking slag and its composite materials in seawater. J. Sustain. Metall. 2017, 3, 729–736. [Google Scholar] [CrossRef]
- Matsuura, H.; Zhang, X.; Zang, L.; Zhang, G.; Tsukihashi, F. Dissolution mechanisms of steelmaking slags in sea water. Miner. Process Extr. Metall. 2017, 126, 11–21. [Google Scholar] [CrossRef]
- Zhang, X.; Atsumi, H.; Matsuura, H.; Tsukihashi, F. Influence of gluconic acid on dissolution of Si, P and Fe from steelmaking slag with different composition into seawater. ISIJ Int. 2014, 54, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Diao, J.; Xie, B.; Wang, Y.; Guo, X. Recovery of phosphorus from dephosphorization slag produced by duplex high phosphorus hot metal refining. ISIJ Int. 2012, 52, 955–959. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Gao, X.; Kim, S.; Ueda, S.; Kitamura, S. Effects of acid and Na2SiO3 modification on the dissolution behavior of 2CaO·SiO2-3CaO·P2O5 solid solution in aqueous solutions. ISIJ Int. 2016, 56, 1436–1444. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.; Liu, Y. Effects of basicity, MgO and MnO on mineralogical phases of CaO–FeOx–SiO2–P2O5 slag. Ironmak. Steelmak. 2018, 45, 363–370. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guo, M.; Yang, X. Phosphate enrichment mechanism in CaO–SiO2–FeO–Fe2O3–P2O5 steelmaking slags with lower binary basicity. Int. J. Miner. Metall. Mater. 2016, 23, 520–533. [Google Scholar] [CrossRef]
- Lin, L.; Bao, Y.; Wang, M.; Zhou, H.; Zhang, L. Influence of SiO2 modification on phosphorus enrichment in P bearing steelmaking slag. Ironmak. Steelmak. 2013, 40, 521–527. [Google Scholar] [CrossRef]
- Lin, L.; Bao, Y.; Wang, M.; Jiang, W.; Zhou, H. Separation and recovery of phosphorus from P-bearing steelmaking slag. J. Iron Steel Res. Int. 2014, 21, 496–502. [Google Scholar] [CrossRef]
- Matsubae-Yokoyama, K.; Kubo, H.; Nagasaka, T. Recycling effects of residual slag after magnetic separation for phosphorus recovery from hot metal dephosphorization slag. ISIJ Int. 2010, 50, 65–70. [Google Scholar] [CrossRef] [Green Version]
| NO. | CaO | SiO2 | Fe2O3 | P2O5 | MnO | MgO | R | Temperature/°C | Time/min |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36.67 | 18.33 | 25 | 10 | 5 | 5 | 2 | 1400 | 30 |
| 2 | 36.67 | 18.33 | 25 | 10 | 5 | 5 | 2 | 1375 | 30 |
| 3 | 36.67 | 18.33 | 25 | 10 | 5 | 5 | 2 | 1350 | 30 |
| 4 | 36.67 | 18.33 | 25 | 10 | 5 | 5 | 2 | 1300 | 30 |
| 5 | 35.36 | 19.64 | 25 | 10 | 5 | 5 | 1.8 | 1350 | 30 |
| 6 | 33 | 22 | 25 | 10 | 5 | 5 | 1.5 | 1350 | 30 |
| 7 | 27.5 | 27.5 | 25 | 10 | 5 | 5 | 1 | 1350 | 30 |
| 8 | 35.36 | 19.64 | 25 | 10 | 5 | 5 | 1.8 | 1350 | 15 |
| 9 | 35.36 | 19.64 | 25 | 10 | 5 | 5 | 1.8 | 1350 | 45 |
| 10 | 35.36 | 19.64 | 25 | 10 | 5 | 5 | 1.8 | 1350 | 60 |
| Area | CaO | SiO2 | Fe2O3 | MgO | MnO | P2O5 |
|---|---|---|---|---|---|---|
| P-rich phase | 50.0 | 25.6 | 0 | 5.1 | 0 | 19.3 |
| 52.1 | 21.2 | 0 | 4.2 | 0 | 22.5 | |
| 50.7 | 22.3 | 0 | 4.6 | 0 | 22.4 | |
| Fe-rich phase | 4.4 | 3.6 | 75.2 | 14.3 | 0 | 2.6 |
| 4.6 | 4.1 | 75.4 | 13.5 | 0 | 2.4 | |
| 8.6 | 6.0 | 66.9 | 14.2 | 0 | 4.3 | |
| Matrix phase | 34.4 | 38.3 | 9.5 | 12.0 | 0 | 5.8 |
| 34.1 | 36.7 | 9.3 | 12.7 | 0 | 7.3 | |
| 33.8 | 37.8 | 9.2 | 13.0 | 0 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Bao, Y.; Wang, D.; Gu, C.; Wang, M. Study on the Effect of Different Factors on the Change of the Phosphorus-Rich Phase in High Phosphorus Steel Slag. Crystals 2022, 12, 1030. https://doi.org/10.3390/cryst12081030
Wang Z, Bao Y, Wang D, Gu C, Wang M. Study on the Effect of Different Factors on the Change of the Phosphorus-Rich Phase in High Phosphorus Steel Slag. Crystals. 2022; 12(8):1030. https://doi.org/10.3390/cryst12081030
Chicago/Turabian StyleWang, Zhongliang, Yanping Bao, Dazhi Wang, Chao Gu, and Min Wang. 2022. "Study on the Effect of Different Factors on the Change of the Phosphorus-Rich Phase in High Phosphorus Steel Slag" Crystals 12, no. 8: 1030. https://doi.org/10.3390/cryst12081030







