Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Nanostructured ZnO Array
2.3. Characterization of Physic and Chemical Properties
2.4. Functionalization of Nanostructured ZnO Array with Hemoglobin
2.5. Evaluation of CO2 Capture
3. Results and Discussion
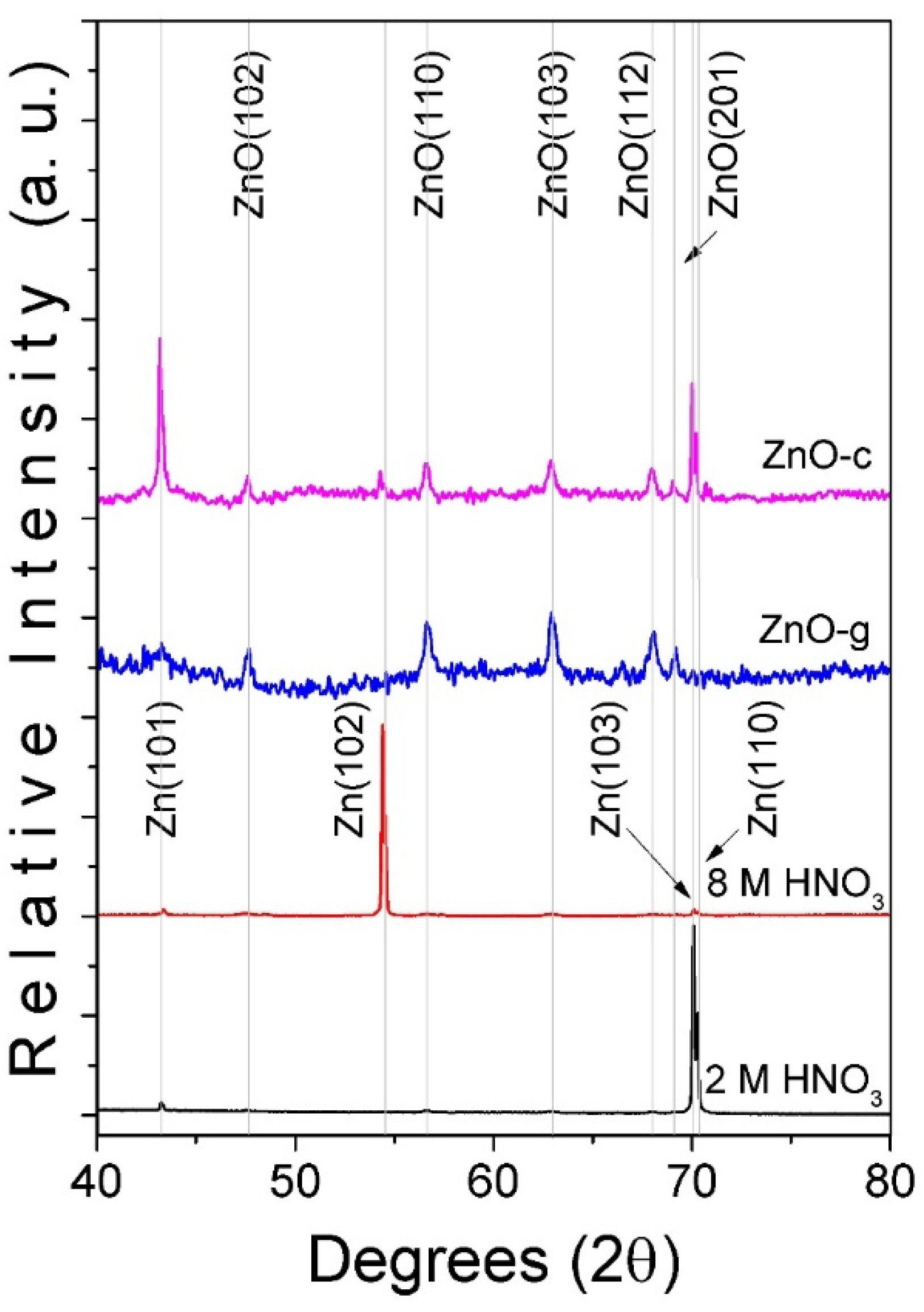
3.1. Morphological and Structural Characterization of ZnO Arrays
3.2. Growing Path of ZnO Arrays
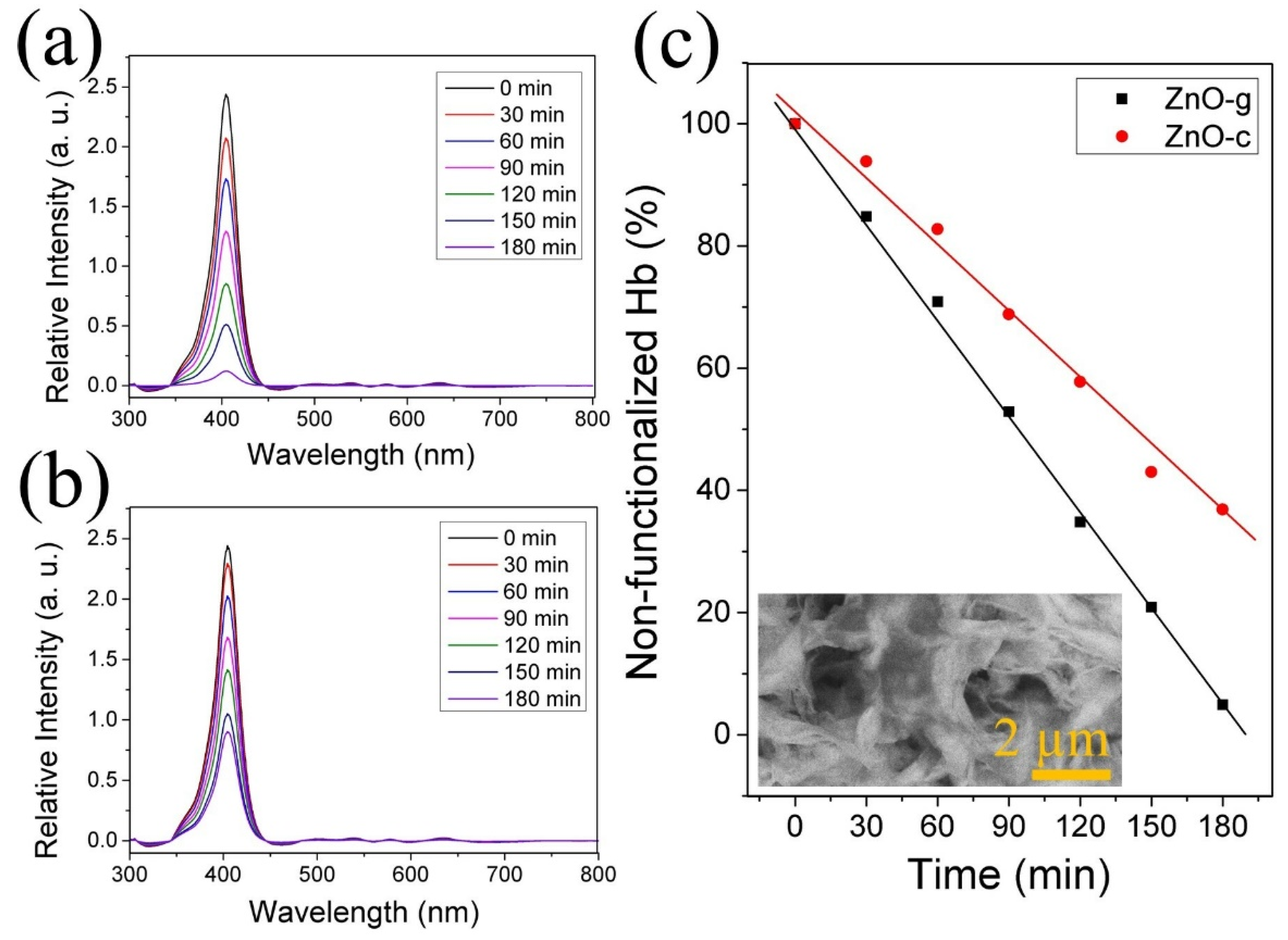
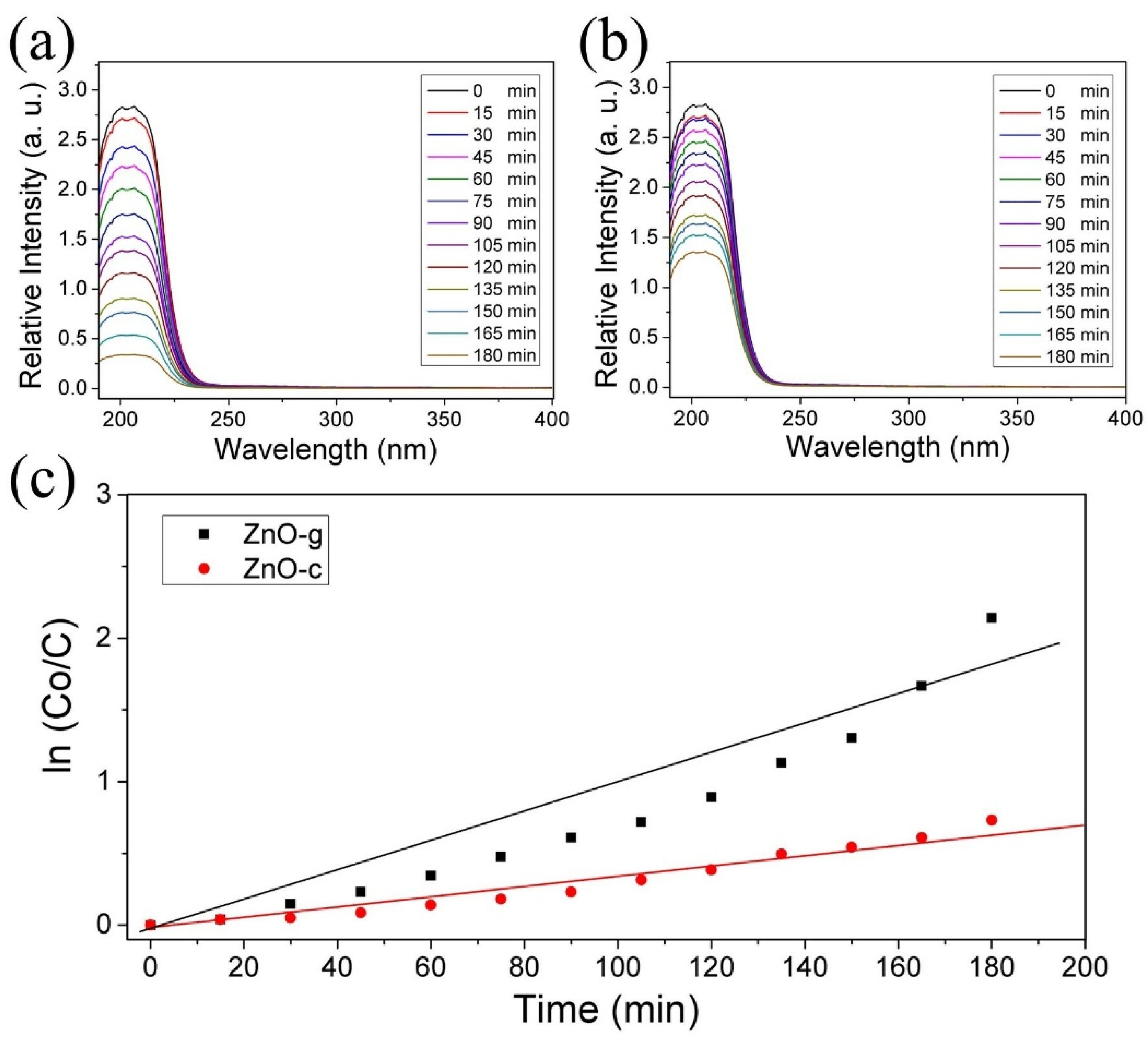
3.3. Hb Functionalization of ZnO Nanostructures and CO2 Capture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigoya, M.F.; Mendoza, J.G.; Abril, S.O. International Energy Transition: A Review of its Status on Several Continents. Int. J. Energy Econ. Policy 2020, 10, 216–224. [Google Scholar] [CrossRef]
- Castells-Quintana, D.; Dienesch, E.; Krause, M. Air pollution in an urban world: A global view on density, cities and emissions. Ecol. Econ. 2021, 189, 107153. [Google Scholar] [CrossRef]
- Kim, J.S.; Kug, J.S.; Jeong, S.; Zeng, N.; Hong, J.; Jeong, J.-H.; Zhao, Y.; Chen, X.; Williams, M.; Ichii, K.; et al. Arctic warming-induced cold damage to East Asian terrestrial ecosystems. Commun. Earth Environ. 2022, 3, 16. [Google Scholar] [CrossRef]
- Beusch, L.; Nauels, A.; Gudmundsson, L.; Gütschow, J.; Schleussner, C.-F.; Seneviratne, S.I. Responsibility of major emitters for country-level warming and extreme hot years. Commun. Earth Environ. 2022, 3, 7. [Google Scholar] [CrossRef]
- Lyam, P.T.; Duque-Lazo, J.; Hauenschild, F.; Schnitzler, J.; Muellner-Riehl, A.N.; Greve, M.; Ndangalasi, H.; Myburgh, A.; Durka, W. Climate change will disproportionally affect the most genetically diverse lineages of a widespread African tree species. Sci. Rep. 2022, 12, 7035. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Catalytic Solvent Regeneration for Energy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 18755–18788. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.-S.; Jin, Z.; Shokouhimehr, M.; Jang, H.W.; Hu, C.; Singh, P.; Raizada, P.; Peng, W.; Lam, S.S.; et al. Towards artificial photosynthesis: Sustainable hydrogen utilization for photocatalytic reduction of CO2 to high-value renewable fuels. Chem. Eng. J. 2020, 402, 126184. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Sheng, J.; Tang, F.; Zeng, K.; Wang, L.; Liang, K.; Hu, H.; Liu, Y.-N. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2. Appl. Surf. Sci. 2019, 498, 143899. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO nanosheets assembled microspheres for high performance trimethylamine gas sensing. J. Appl. Phys. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Park, J.; Ghosh, R.; Song, M.S.; Hwang, Y.; Tchoe, Y.; Saroj, R.K.; Ali, A.; Guha, P.; Kim, B.; Kim, S.-W.; et al. Individually addressable and flexible pressure sensor matrixes with ZnO nanotube arrays on graphene. NPG Asia Mater. 2022, 14, 40. [Google Scholar] [CrossRef]
- Oh, H.; Tchoe, Y.; Kim, H.; Yun, J.; Park, M.; Kim, S.; Lim, Y.-S.; Kim, H.; Jang, W.; Hwang, J.; et al. Large-scale, single-oriented ZnO nanostructure on h-BN films for flexible inorganic UV sensors. J. Appl. Phys. 2021, 130, 223105. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.M.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O.E. Needle- and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Polyakov, B.; Butanovs, E.; Popov, A.A.; Sokolov, M.; Bocharov, D.; Piskunov, S. Excited States Calculations of MoS2@ZnO and WS2@ZnO Two-Dimensional Nanocomposites for Water-Splitting Applications. Energies 2022, 15, 150. [Google Scholar] [CrossRef]
- Uklein, A.V.; Multian, V.V.; Kuz’micheva, G.M.; Linnik, R.P.; Lisnyak, V.V.; Popov, A.I.; Gayvoronsky, V.Y. Nonlinear optical response of bulk ZnO crystals with different content of intrinsic defects. Opt. Mater. 2018, 84, 738–747. [Google Scholar] [CrossRef]
- Khaliullin, S.M.; Zhuravlev, V.D.; Ermakova, L.V.; Buldakova, L.Y.; Yanchenko, M.Y.; Porotnikova, N.M. Solution Combustion Synthesis of ZnO Using Binary Fuel (Glycine + Citric Acid). Int. J. Self-Propag. High-Temp. Synth. 2019, 28, 226–232. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; He, S.; Wang, R.; Zhu, J.; Guo, X.; Liu, N.; Guo, R.; Mo, Z. A superhydrophobic polyphenylene sulfide composite coating with anti-corrosion and self-cleaning properties for metal protection. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 648, 129152. [Google Scholar] [CrossRef]
- Usseinov, B.; Akilbekov, A.T.; Kotomin, E.A.; Popov, A.I.; Seitov, D.D.; Nekrasov, K.A.; Giniyatova, S.G.; Karipbayev, Z.T. The first principles calculations of CO2 adsorption on (10⎯10) ZnO surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar] [CrossRef]
- Tang, C.; Sun, F.; Chen, Z.; Chen, D.; Liu, Z. Improved thermal oxidation growth of non-flaking CuO nanorod arrays on Si substrate from Cu film and their nanoscale electrical properties for electronic devices. Ceram. Int. 2019, 45, 14562–14567. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Cruz-Martínez, H.; Montejo-Alvaro, F.; Farías, R.; Hernández-Rodríguez, Y.M.; Guillen-Cervantes, A.; Ávila-García, A.; Cayetano-Castro, N.; Medina, D.I.; Cigarroa-Mayorga, O.E. The formation of ZnO structures using thermal oxidation: How a previous chemical etching favors either needle-like or cross-linked structures. Mater. Sci. Semicond. Process. 2020, 108, 104888. [Google Scholar] [CrossRef]
- Gibson, J.S.; Cossins, A.R.; Ellory, J.C. Oxygen-sensitive membrane transporters in vertebrate red cells. J. Exp. Biol. 2000, 203 Pt 9, 1395–1407. [Google Scholar] [CrossRef]
- Chu, H.; McKenna, M.M.; Krump, N.A.; Zheng, S.; Mendelsohn, L.; Thein, S.L.; Garrett, L.J.; Bodine, D.M.; Low, P.S. Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 2016, 128, 2708–2716. [Google Scholar] [CrossRef]
- Gibson, J.S. Mechanism of O2-sensitive red cell properties. Blood 2016, 128, 2593–2595. [Google Scholar] [CrossRef] [Green Version]
- Rojas, L.M.G.; Huerta-Aguilar, C.A.; Orta-Ledesma, M.T.; Sosa-Echeverria, R.; Thangarasu, P. Zinc oxide nanoparticles coated with benzimidazole based ionic liquid performing as an efficient CO2 capture: Experimental and Theoretical studies. J. Mol. Struct. 2022, 1265, 133466. [Google Scholar] [CrossRef]
- Anuar, S.A.; Ahmad, K.N.; Al-Amiery, A.; Masdar, M.S.; Isahak, W.N.R.W. Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio. Catalysts 2021, 11, 1253. [Google Scholar] [CrossRef]
- Jena, K.K.; Panda, A.P.; Verma, S.; Mani, G.K.; Swain, S.K.; Alhassan, S.M. MWCNTs-ZnO-SiO2 mesoporous nano-hybrid materials for CO2 capture. J. Alloys Compd. 2019, 800, 279–285. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Screening Study of Different Amine-Based Solutions as Sorbents for Direct CO2 Capture from Air. ACS Sustain. Chem. Eng. 2020, 8, 14013–14021. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, R.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Degradation of ZnO Nanostructures Interface by Effect of Photocatalytic Activity in Methylene Blue. In Proceedings of the 2021 IEEE International Summer Power Meeting/International Meeting on Communications and Computing (RVP-AI/ROC&C), Acapulco, Mexico, 14–18 November 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Tan, W.K.; Razak, K.A.; Ibrahim, K.; Lockman, Z. Oxidation of etched Zn Foil for the formation of ZnO nanostructure. J. Alloys Compd. 2011, 509, 6806. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, C.; Cai, R.; Wang, Y.; Zhou, G. Temperature-dependent growth mechanism and microstructure of ZnO nanostructures grown from the thermal oxidation of zinc. J. Crystal Growth. 2014, 390, 101. [Google Scholar] [CrossRef]
- Xiang, K.; Chai, L.; Wang, Y.; Wang, H.; Guo, N.; Ma, Y.; Murty, K.L. Microstructural characteristics and hardness of CoNiTi medium-entropy alloy coating on pure Ti substrate prepared by pulsed laser cladding. J. Alloys Compd. 2020, 849, 156704. [Google Scholar] [CrossRef]
- Xing, Y.J.; Xi, Z.H.; Xue, Z.Q.; Zhang, X.D.; Song, J.H.; Wang, R.M.; Xu, J.; Song, Y.; Zhang, S.L.; Yu, D.P. Optical properties of the ZnO nanotubes synthesized via vapor phase growth. Appl. Phys. Lett. 2003, 83, 1689–1691. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Souissi, A.; Sartel, C.; Sayari, A.; Meftah, A.; Lusson, A.; Galtier, P.; Sallet, V.; Oueslati, M. Zn- and O-polar surface effects on Raman mode activation in homoepitaxial ZnO thin films. Solid State Commun. 2012, 152, 794–797. [Google Scholar] [CrossRef]
- Souissi, A.; Sartel, C.; Amiri, G.; Meftah, A.; Lusson, A.; Galtier, P.; Sallet, V.; Oueslati, M. Raman study of activated quasi-modes due to misorientation of ZnO nanowires. Solid State Commun. 2012, 152, 1729–1733. [Google Scholar] [CrossRef]
- Christiansen, J.; Douglas, C.G.; Haldane, J.S. The absorption and dissociation of carbon dioxide by human blood. J. Physiol. 1914, 48, 244–271. [Google Scholar] [CrossRef] [Green Version]



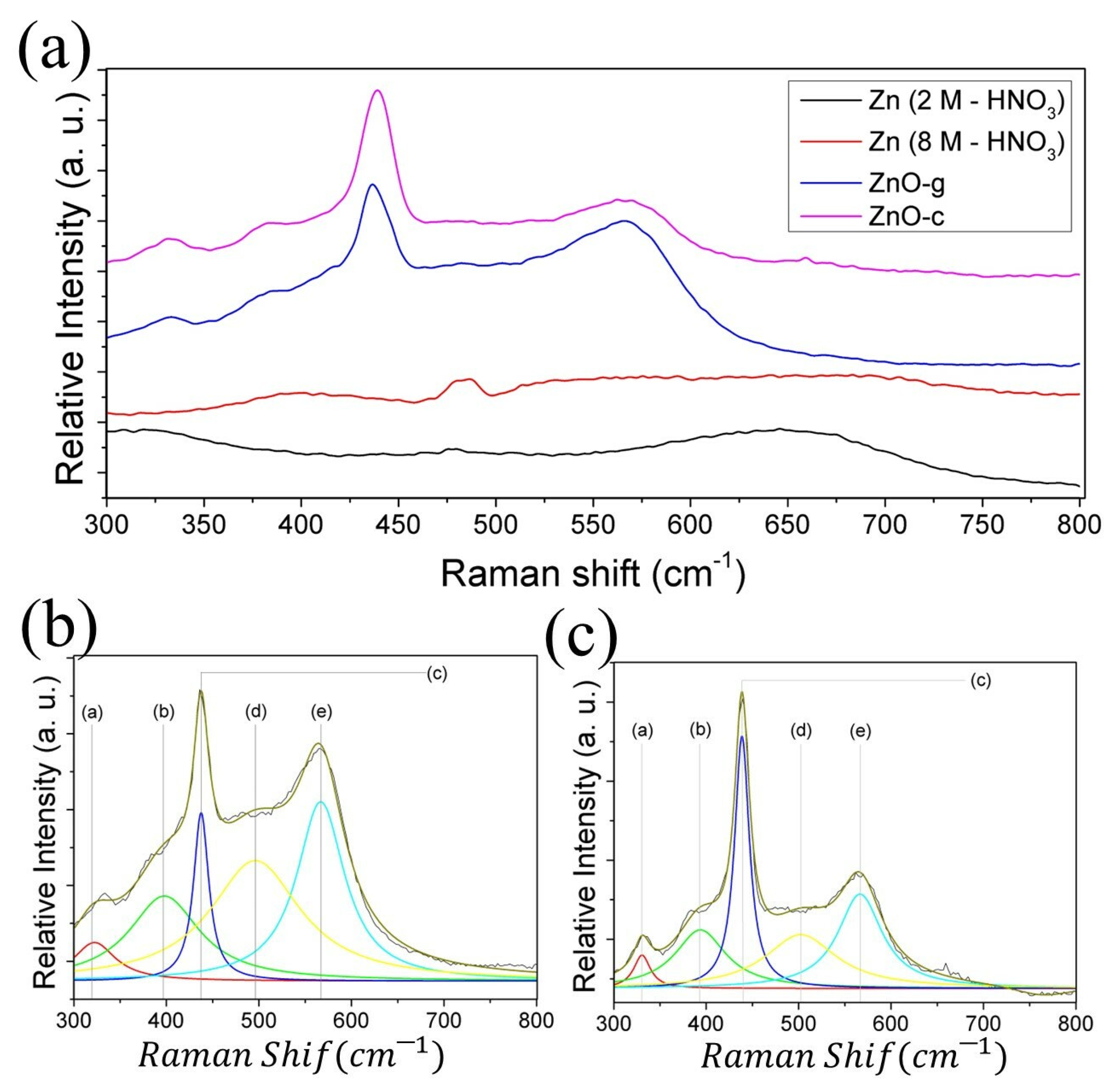
| Vibrational Mode | ZnO-g (cm−1) | ZnO-c (cm−1) | Label in Figure 5 | ZnO Bulk (cm−1) [33,34] | Nanotubes ZnO (cm−1) [32] |
|---|---|---|---|---|---|
| E2 (H)–E2 (L) | 321.97 | 331.03 | (a) | 321 | 331 |
| A1 (TO) | 387.51 | 392.98 | (b) | 384 | 383 |
| E2 (H) | 437.29 | 438.80 | (c) | 438 | 437 |
| * | 486.72 | 502.26 | (d) | - | - |
| E1 (LO) | 567.23 | 566.22 | (e) | 580 | 578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Sánchez, A.; Cano, F.J.; Hernández-Rodríguez, M.; Cigarroa-Mayorga, O. Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture. Crystals 2022, 12, 1086. https://doi.org/10.3390/cryst12081086
Mendoza-Sánchez A, Cano FJ, Hernández-Rodríguez M, Cigarroa-Mayorga O. Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture. Crystals. 2022; 12(8):1086. https://doi.org/10.3390/cryst12081086
Chicago/Turabian StyleMendoza-Sánchez, Alberto, Francisco J. Cano, Mariela Hernández-Rodríguez, and Oscar Cigarroa-Mayorga. 2022. "Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture" Crystals 12, no. 8: 1086. https://doi.org/10.3390/cryst12081086
APA StyleMendoza-Sánchez, A., Cano, F. J., Hernández-Rodríguez, M., & Cigarroa-Mayorga, O. (2022). Influence of ZnO Morphology on the Functionalization Efficiency of Nanostructured Arrays with Hemoglobin for CO2 Capture. Crystals, 12(8), 1086. https://doi.org/10.3390/cryst12081086







