Effect of Ce-Doping on Microstructure and Adsorption- Photodegradation Behaviors of the Hydrothermally-Synthesized TiO2 Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ce-Doped TNPs
2.2. Preparation of Ce-Doped TNTs
2.3. Adsorption and Photochemical Tests
2.3.1. Adsorption Test
2.3.2. Photocatalytic Test
2.3.3. Photodegradation Test
2.4. Characterizations
3. Results and Discussion
3.1. Morphology and Phase Composition of the TNTs
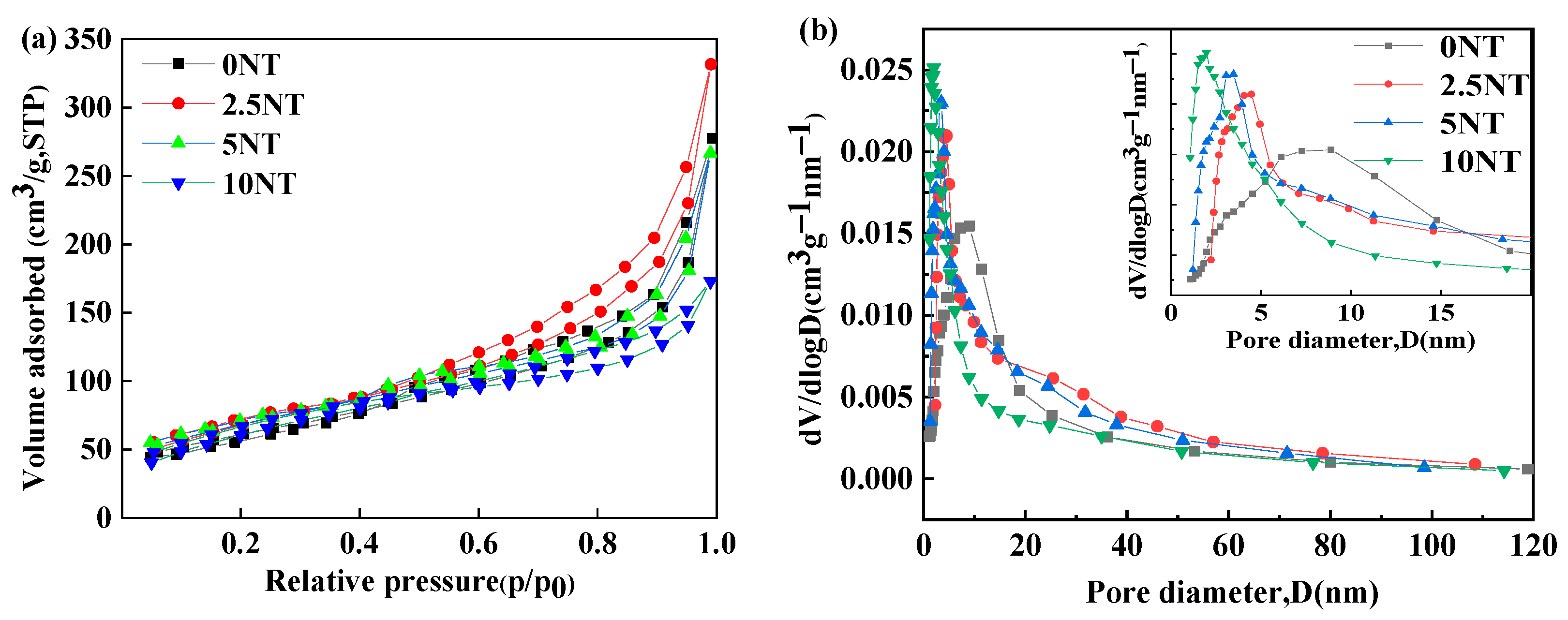
3.2. Specific Surface Analysis
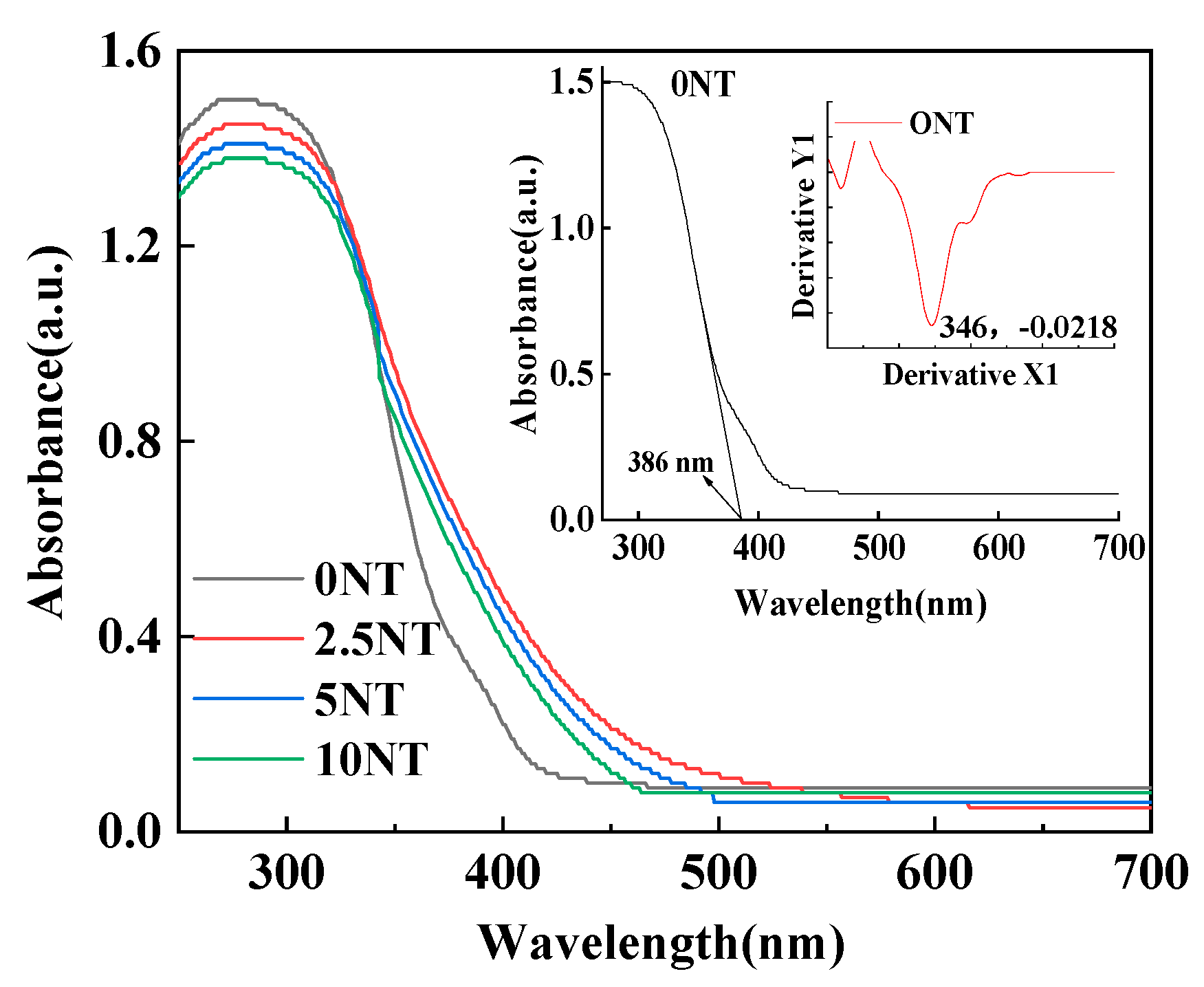
3.3. Photoabsorption of the TNT Samples
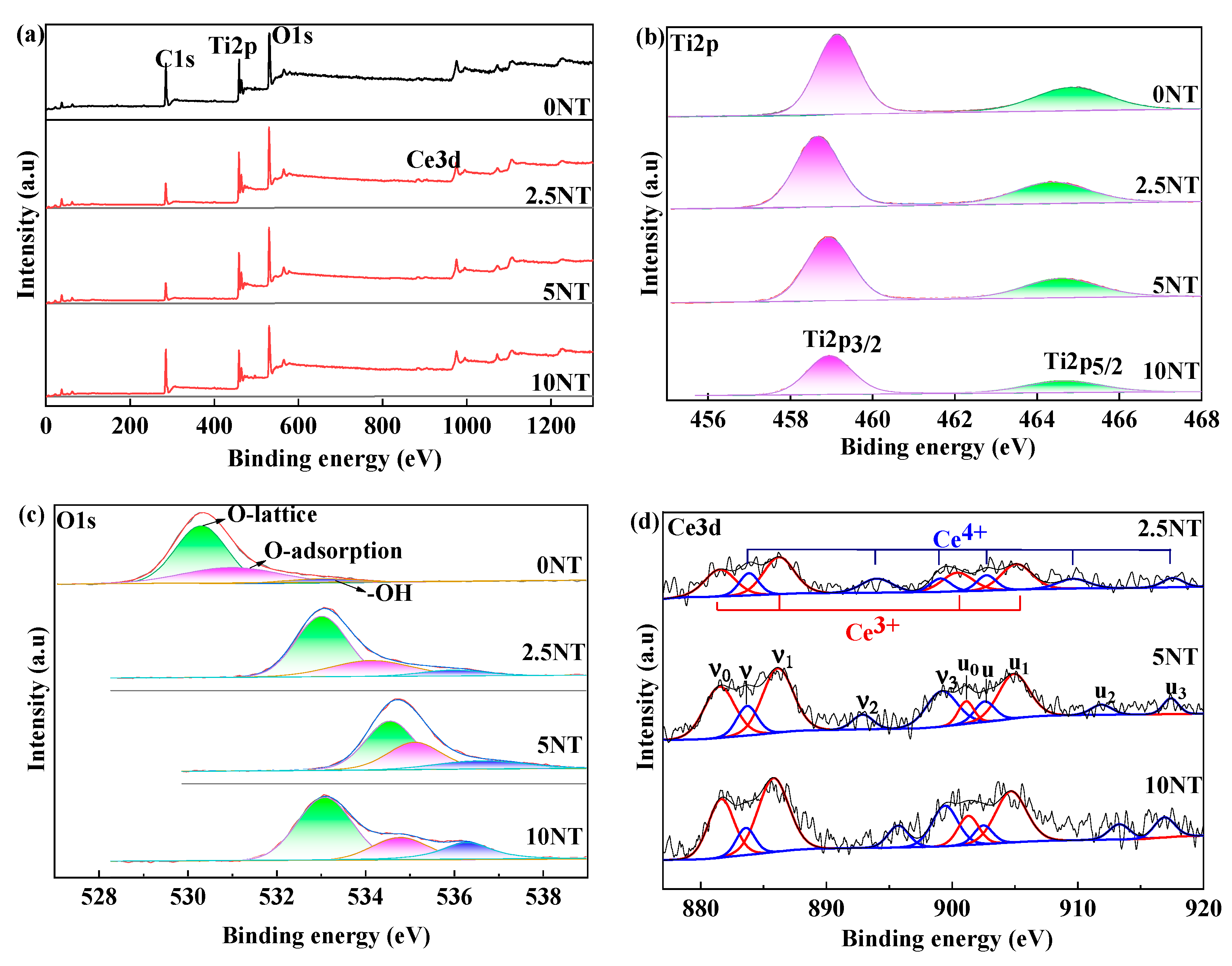
3.4. Valance of Ti and Ce Cations and the Occupation of O2−in Ce-Doped TNTs
3.5. Adsorption and Photocatalytic Behaviors of the Samples
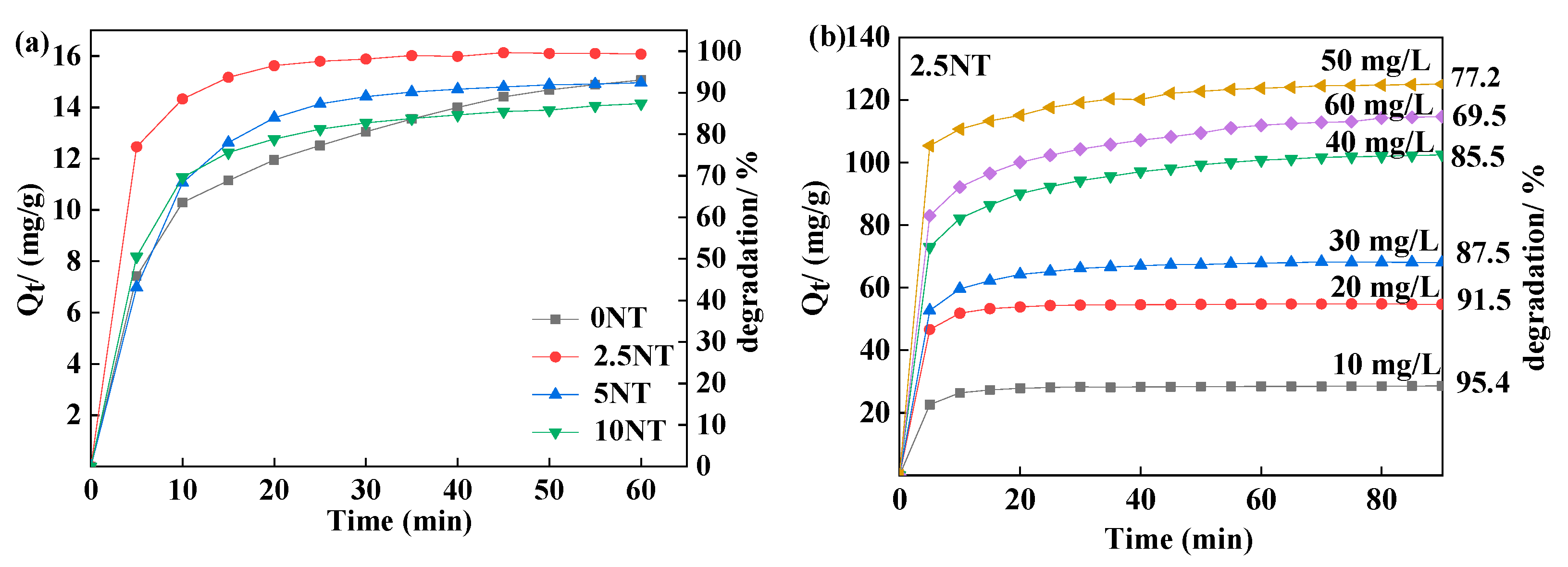
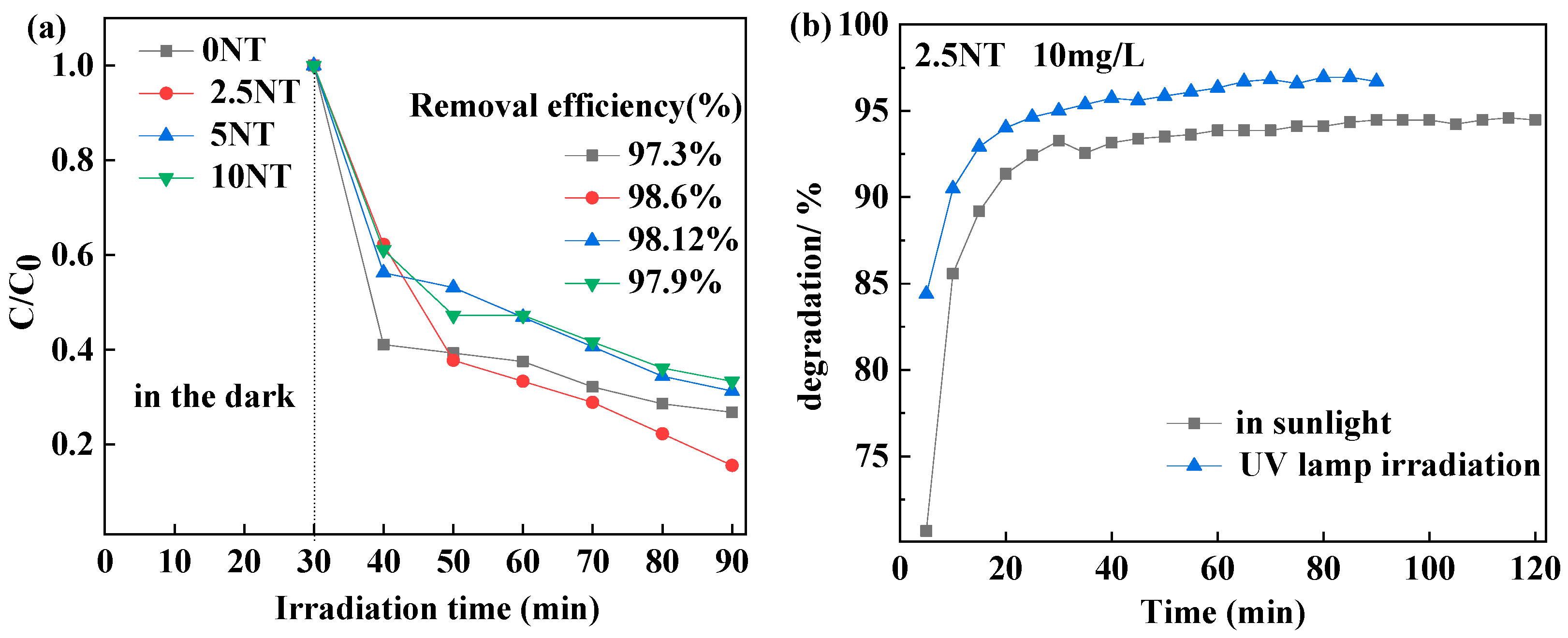
3.5.1. Adsorption
3.5.2. Photocatalytic Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic Regeneration. J. Colloid Inter. Sci. 2014, 433, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Gerislioglu, B.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.; et al. Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Melki, R.; Laoufi, N.A.; Mouheb, A. Effects of iron ions, doping methods and nanotubular morphology on TiO2 solar photocatalytic performance. Water Sci. Tech. 2021, 83, 12. [Google Scholar] [CrossRef]
- Méndez-Galván, M.; Celaya, C.A.; Jaramillo-Quintero, O.A.; Muñiz, J.; Díaz, G.; Lara-García, H.A. Tuning the band gap of M-doped titanate nanotubes (Mn, Fe, Co, Ni, and Cu): An experimental and theoretical study. Nanoscale Adv. 2021, 3, 1382–1391. [Google Scholar] [CrossRef]
- Emran, K.M.; Alanazi, H.E. Double layer lanthanide Pt/TiO2 nanotube arrays electrode as a cost-highly efficient electrocatalyst for hydrogen evolution in acid media. J. Exp. Nanosci. 2021, 16, 81–100. [Google Scholar] [CrossRef]
- Bae, H.-S.; Mahadik, M.A.; Seo, Y.-S.; Chae, W.S.; Chung, H.-S.; Ryu, H.I.; Cho, M.; Shea, P.J.; Choi, S.H.; Jang, J.S. Palladium metal oxide/hydroxide clustered cobalt oxide co-loading on acid treated TiO2 nanorods for degradation of organic pollutants and Salmonella typhimurium inactivation under simulated solar light. Chem. Eng. J. 2021, 408, 127260. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Yu, H.; Liu, Y.; Gao, J.; Yang, L.; Zhang, M.; He, G.; Sun, Z. Effect of TiO2 arrays on surface enhanced Raman scattering (SERS) performance for Ag/TiO2 substrates. Nanotech 2021, 32, 075708. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.S.; Barkul, R.P.; Patil, M.K. Sunlight assisted photocatalytic degradation of different organic pollutants and simultaneous degradation of cationic and anionic dyes using titanium and zinc based nanocomposites. J. Mol. Liqu. 2021, 340, 117191. [Google Scholar] [CrossRef]
- Ho, Y.-R.; Chang, Y.-H.; Lin, C.-H.; Ou, S.-L.; Huang, J.-J. Al2O3-passivated TiO2 nanorods for solid–liquid heterojunction ultraviolet photodetectors. J. Mater. Sci. 2021, 56, 6052–6063. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Hou, M. Photocatalytic degradation of 2-mercaptobenzothiazole in aqueous La3+_TiO2 suspension for odor control. Appl. Catal. B Environ. 2004, 48, 185–194. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, W.; Bayar, X. Study of crystallization behavior of Ce4+-modified titania gels. Scr. Mater 2004, 50, 885–889. [Google Scholar] [CrossRef]
- Barkul, R.P.; Shaikh, F.-N.A.; Delekar, S.D.; Patil, M.K. Visible light active Ce-doped TiO2 nanoparticles for photocatalytic degradation of methylene blue. Curr. Nanosci. 2017, 13, 110–116. [Google Scholar] [CrossRef]
- Rong, X.; Yan, J.; Yi, J.; Du, J.; Kang, K.; Gan, G. Analysis of chemical states of Ce-doped TiO2. J. Synth. Cryst. 2015, 44, 1383–1388. [Google Scholar]
- Asemi, M.; Ghanaatshoar, M. Hydrothermal growth of one-dimensional Ce-doped TiO2 nanostructures for solid-state DSSCs comprising Mg-doped CuCrO2. J. Mater. Sci. 2017, 52, 489–503. [Google Scholar] [CrossRef]
- Touati, A.; Hammedi, T.; Najjar, W.; Ksibi, Z.; Sayadi, S. Photocatalytic degradation of textile wastewater in presence of hydrogen peroxide: Effect of cerium doping titania. J. Ind. Eng. Chem. 2016, 35, 36–44. [Google Scholar] [CrossRef]
- Chung, L.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Effect of Ce-doping on the photocatalytic performance of TiO2 thin films. Mater. Chem. Phys. 2017, 197, 236–239. [Google Scholar] [CrossRef]
- Lutic, D.; Petrovschi, D.; Ignat, M.; Creţescu, I.; Bulai, G. Mesoporous cerium-doped titania for the photocatalytic removal of persistent dyes. Catal. Today 2018, 306, 300–309. [Google Scholar] [CrossRef]
- Shi, J.; Zou, Y.; Ma, D. Ce-doped titania nanoparticles: The effects of doped amount and calcination temperature on photocatalytic activity. IOP Conf. Series: Mater. Sci. Eng. 2017, 167, 012039. [Google Scholar] [CrossRef] [Green Version]
- Suhaili, S.Z.; Jais, U.S. Improved visible light photocatalytic activity of TiO2 nano powder by Ce3+ doping. In Proceedings of the 2012 IEEE Colloquium on Humanities, Science and Engineering (CHUSER), Kota Kinabalu, Malaysia, 3–4 December 2012. [Google Scholar] [CrossRef]
- Reli, M.; Ambrožová, N.; Šihor, M.; Matějová, L.; Čapek, L.; Obalová, L.; Matěj, Z.; Kotarba, A.; Kočí, K. Novel cerium doped titania catalysts for photocatalytic decomposition of ammonia. Appl. Catal. B Environ. 2015, 178, 108–116. [Google Scholar] [CrossRef]
- Alberoni, C.; Barroso-Martín, I.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Talon, A.; Zhao, H.; You, S.; Vomiero, A.; Moretti, E. Ceria doping boosts methylene blue photodegradation in titania nanostructures. Mater. Chem. Front. 2021, 5, 4138–4152. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, G.; Xu, X.; Yang, X.; Liu, C.; Xu, Y. Preparation of titania nanotubes doped with cerium and their photocatalytic activity for glyphosate. Chem. Eng. J. 2011, 167, 397–402. [Google Scholar] [CrossRef]
- Qu, X.; Xie, D.; Gao, L.; Du, F. Synthesis and photocatalytic activity of TiO2/CeO2 core–shell nanotubes. Mater. Sci. Semicond. Process. 2014, 26, 26–657. [Google Scholar] [CrossRef]
- Eleburuike, N.A.; Bakar, W.A.W.A.; Ali, R.; Omar, M.F. Photocatalytic degradation of paraquat dichloride over CeO2-modified TiO2 nanotubes and the optimization of parameters by response surface methodology. RSC Adv. 2016, 6, 104082–104093. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Song, K.; Wang, X.; Yu, K.; Liang, C. Enhanced photocatalytic activities of Nd-doped TiO2 under visible light using a facile sol-gel method. J. Rare Earths 2020, 38, 38–148. [Google Scholar] [CrossRef]
- Parnicka, P.; Mazierski, P.; Lisowski, W.; Klimczuk, T.; Nadolna, J.; Zaleska-Medynska, A. A new simple approach to prepare rare-earth metals-modified TiO2 nanotube arrays photoactive under visible light: Surface properties and mechanism investigation. Results Phys. 2019, 12, 12–412. [Google Scholar] [CrossRef]
- Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Škapin, A.S. Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.H.; Juang, R.-S. Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using platinum-doped titanate nanomaterials. J. Taiwan Inst. Chem. Eng. 2019, 99, 166–179. [Google Scholar] [CrossRef]
- Wang, L.N. Study of Hydrothermal Synthesis Process and Photocatalytic Properties of TiO2 Nanotubes. Master’s Thesis, Inner Mongolia University of Technology, Hohhot, China, 2017. [Google Scholar]
- Tuyen, L.T.T.; Quang, D.A.; Toan, T.T.T.; Tung, T.Q.; Hoa, T.T.; Mau, T.X.; Khieu, D.Q. Synthesis of CeO2/TiO2 nanotubes and heterogeneous photocatalytic degradation of methylene blue. J. Environ. Chem. Eng. 2018, 6, 599–601. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, R.M.; Jayavel, R. Facile synthesis of heterostructure CeO2-TiO2 nanocomposites for enhanced electrochemical sensor and solar cell applications. J. Alloy. Compd. 2019, 773, 449–461. [Google Scholar] [CrossRef]
- Vieira, G.B.; José, H.J.; Peterson, M.; Baldissarelli, V.Z.; Alvarez, P.; Moreira, R.D.F.P.M. CeO2/TiO2 nanostructures enhance adsorption and photocatalytic degradation of organic compounds in aqueous suspension. J. Photochem. Photobiol. A Chem. 2018, 353, 325–336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, C.; Cui, L.; Kang, S.; Li, X.; Zhou, L. Ordered mesoporous CeO2-TiO2 composites: Highly efficient photocatalysts for the reduction of CO2 with H2O under simulated solar irradiation. Appl. Catal. B Environ. 2013, 130–131, 277–284. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Xiu, Z.; Xing, Z.; Li, Z.; Wu, X.; Yan, X.; Hu, M.; Cao, Y.; Yang, S.; Zhou, W. Ti3+-TiO2/Ce3+-CeO2 Nanosheet heterojunctions as efficient visible-light-driven photocatalysts. Mater. Res. Bull. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Sahu, M.; Biswas, P. Single-step processing of copper-doped titania nanomaterials in a flame aerosol reactor. Nanoscale Res. Lett. 2011, 6, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for adsorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Trapalis, C. Effects of calcination temperature on the microstructures and photocatalytic activity of titanate nanotubes. J. Mol. Catal. A Chem. 2006, 249, 135–142. [Google Scholar] [CrossRef]
- Rapsomanikis, A.; Apostolopoulou, A.; Stathatos, E.; Lianos, P. Cerium-modified TiO2 nanocrystalline films for visible light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2014, 280, 46–53. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Zhang, N.; Zhao, Q.; He, X.; Feng, J. Photocatalytic mechanism of TiO2–CeO2 films prepared by magnetron sputtering under UV and visible light. Surf. Sci. 2005, 595, 203–211. [Google Scholar] [CrossRef]
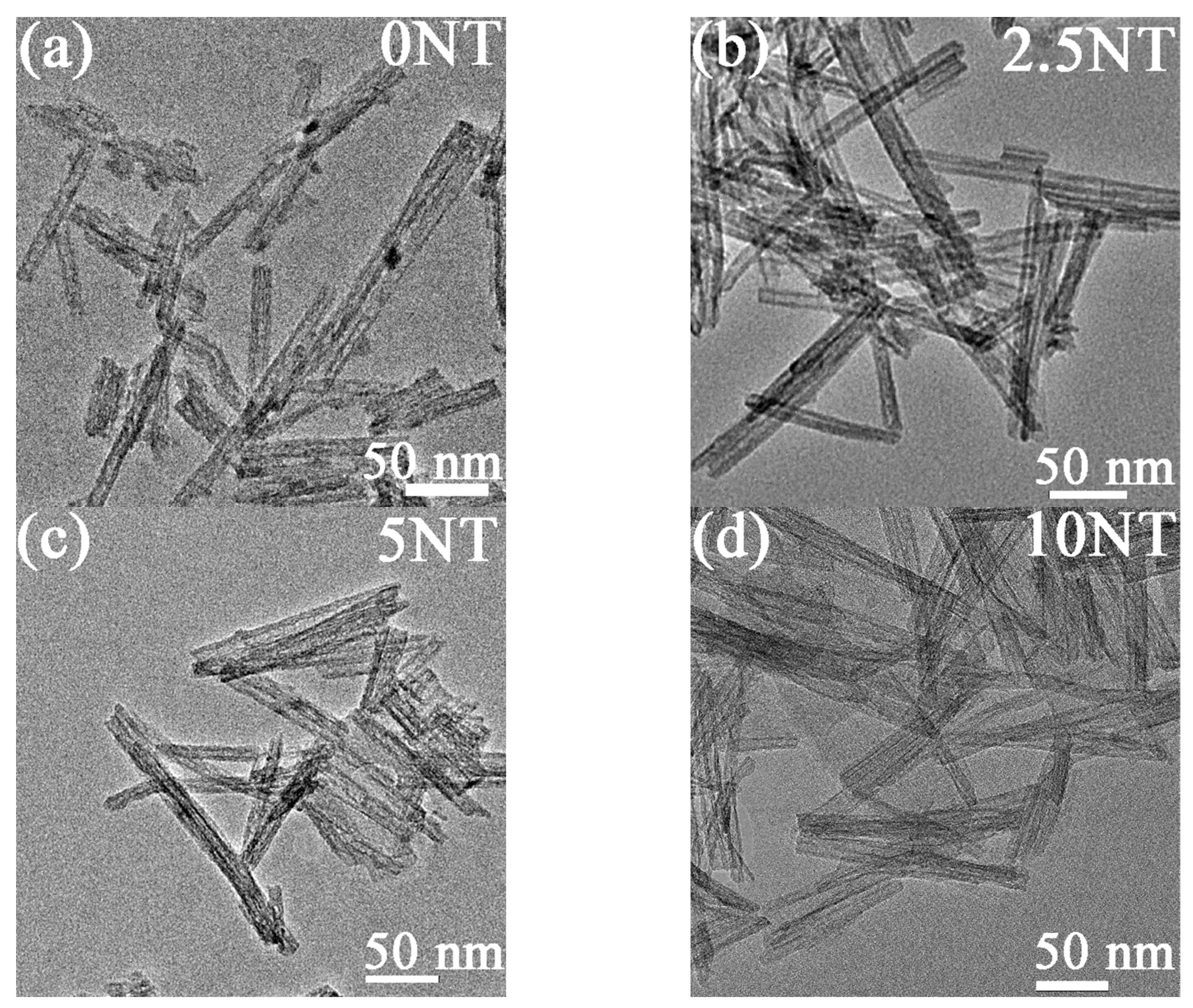
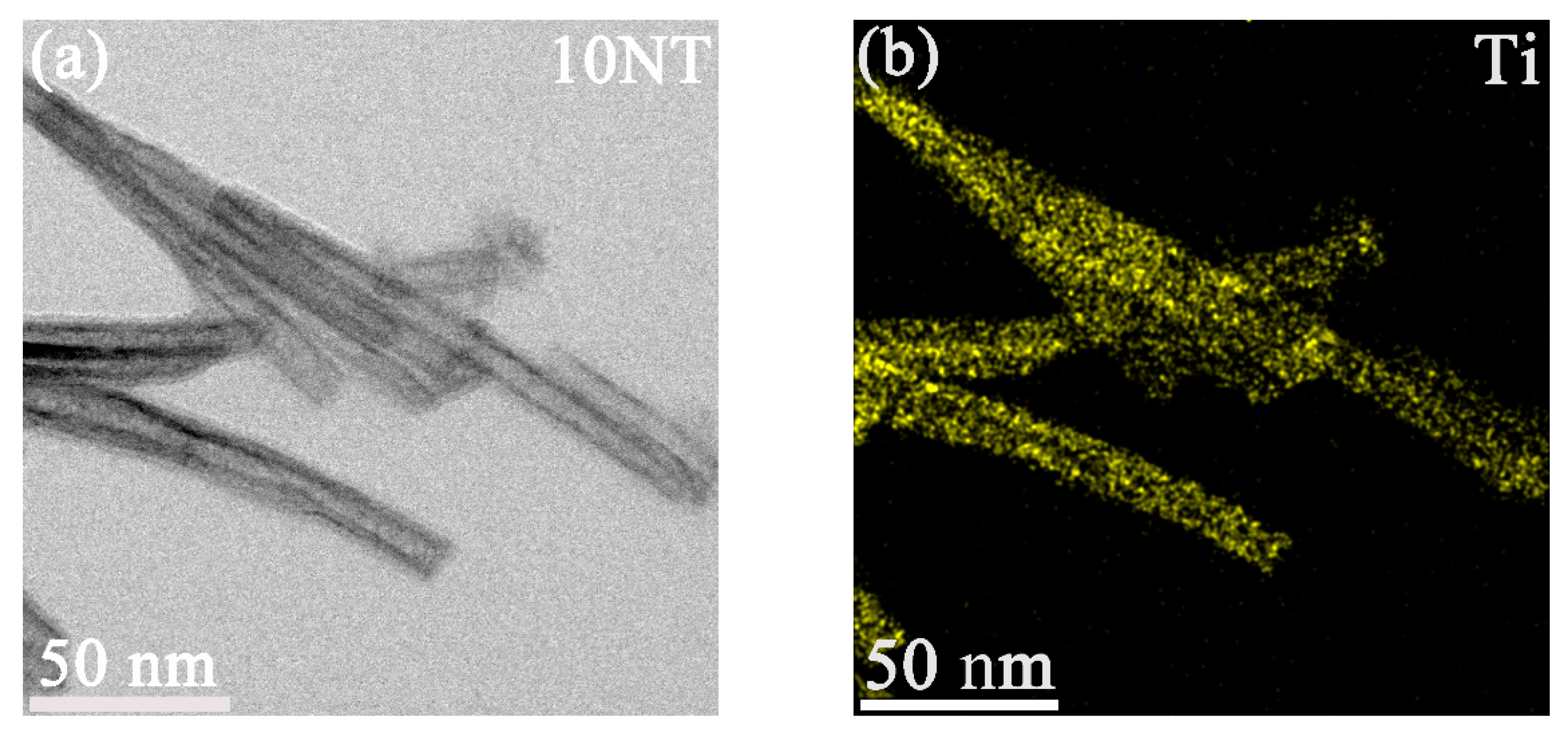
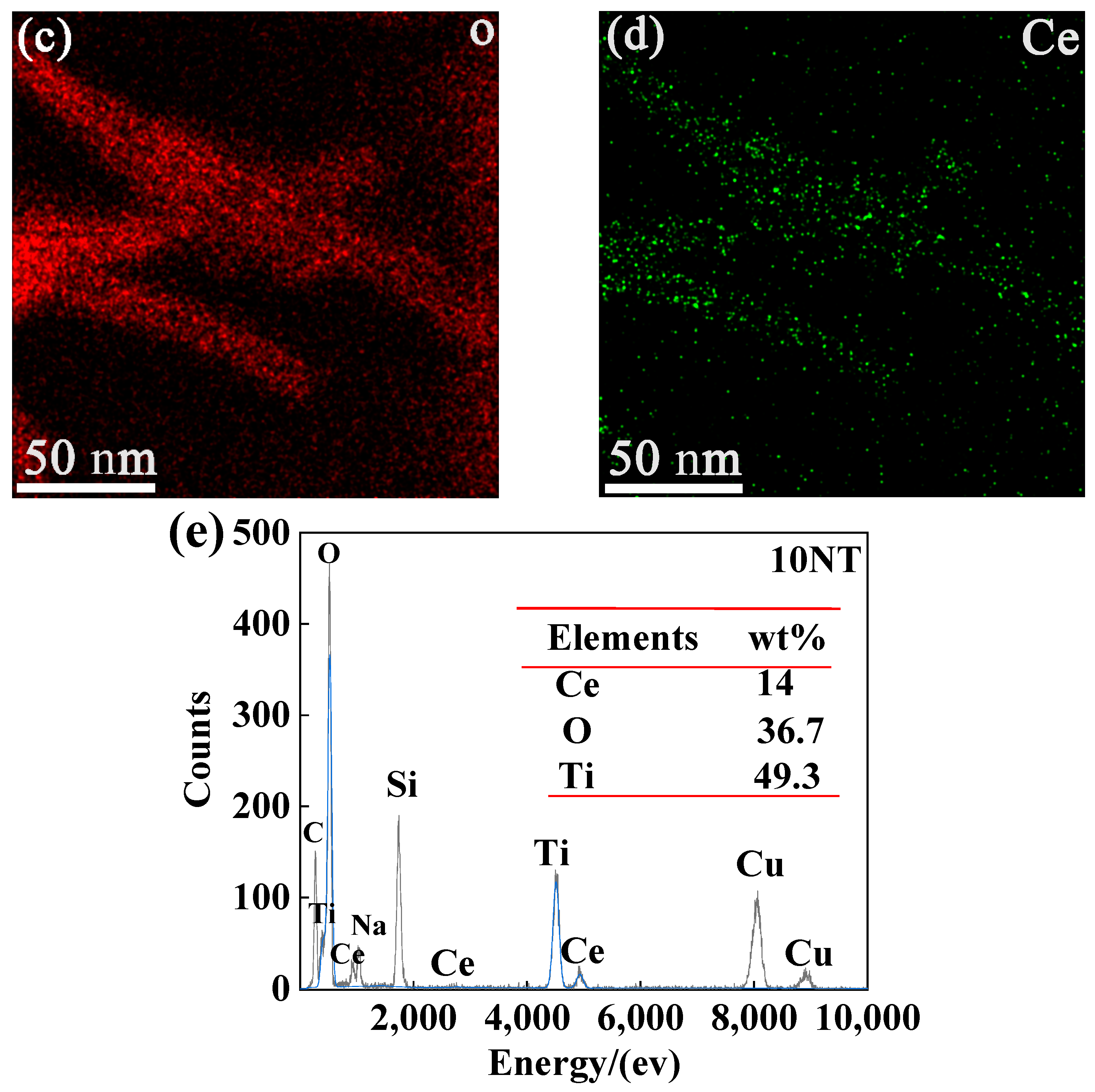
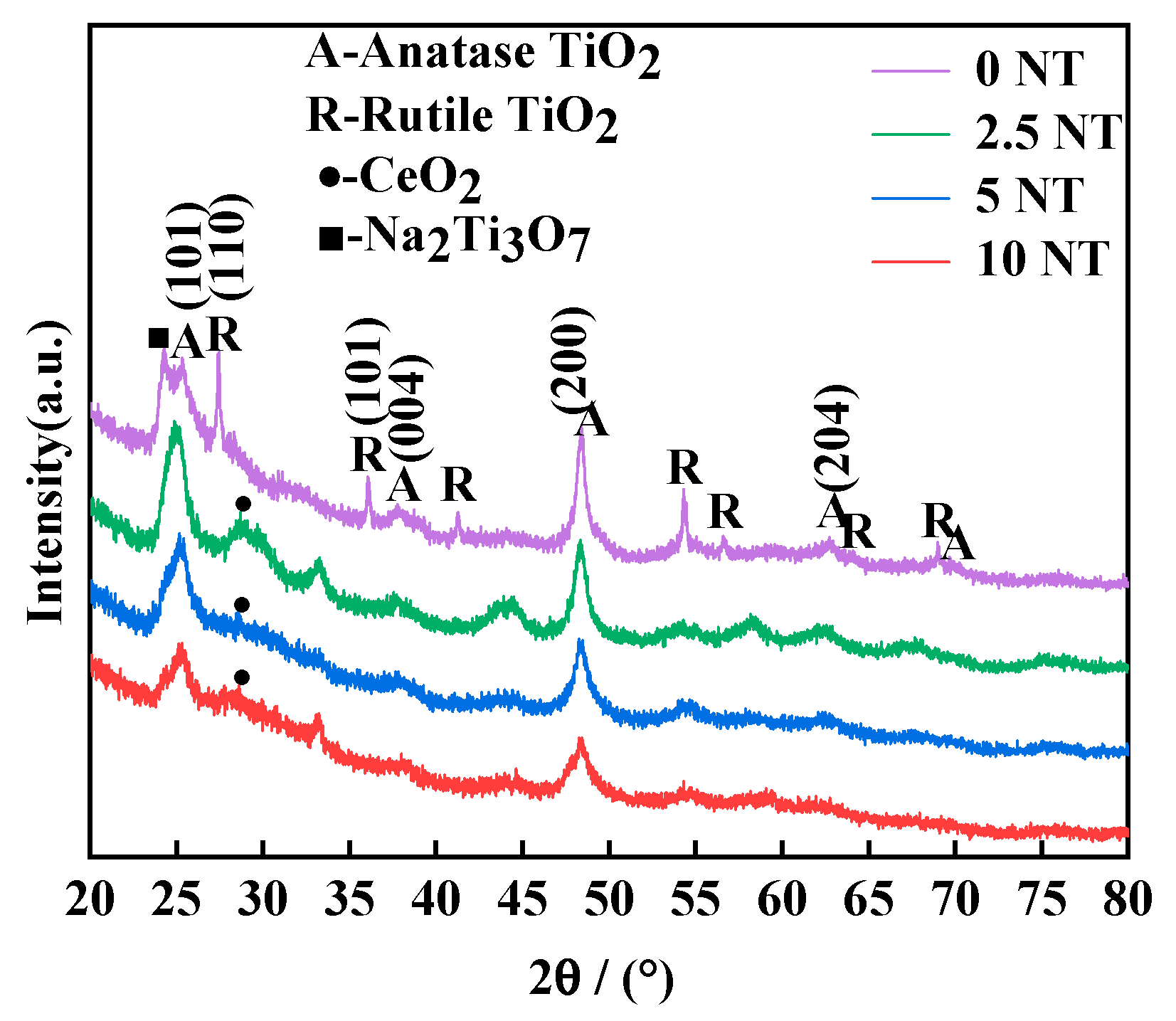

| Sample | Specific Surface Area (m2/g) | Zeta | Pore Volume (cm3/g) | Peak Pore Diameter (nm) |
|---|---|---|---|---|
| 0TNT | 171.38 | −1.67 | 0.38 | 8.91 |
| 2.5TNT | 196.01 | −3.64 | 0.49 | 6.62 |
| 5TNT | 184.22 | −2.97 | 0.34 | 5.66 |
| 10TNT | 180.78 | −3.47 | 0.15 | 3.87 |
| Samples | Thresholds | |
|---|---|---|
| nm | ev | |
| 0TNT | 386.13 | 3.21 |
| 2.5TNT | 447.96 | 2.53 |
| 5TNT | 438.14 | 2.83 |
| 10TNT | 417.51 | 2.91 |
| Sample | O Species | Ce Species | ||||
|---|---|---|---|---|---|---|
| OT | OL | OC | OS | Ce3+ | Ce4+ | |
| 0TNT | 44.5 | 69.1 | 26.3 | 4.6 | - | - |
| 2.5TNT | 50.2 | 60.5 | 27.7 | 11.8 | 63.3 | 36.7 |
| 5TNT | 50.8 | 58.8 | 27.9 | 13.3 | 73.4 | 26.6 |
| 10TNT | 54.6 | 64.5 | 24.4 | 9.1 | 72.1 | 279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Shi, Z.; Wang, X.; Wang, J. Effect of Ce-Doping on Microstructure and Adsorption- Photodegradation Behaviors of the Hydrothermally-Synthesized TiO2 Nanotubes. Crystals 2022, 12, 1094. https://doi.org/10.3390/cryst12081094
Wu K, Shi Z, Wang X, Wang J. Effect of Ce-Doping on Microstructure and Adsorption- Photodegradation Behaviors of the Hydrothermally-Synthesized TiO2 Nanotubes. Crystals. 2022; 12(8):1094. https://doi.org/10.3390/cryst12081094
Chicago/Turabian StyleWu, Kaili, Zhiming Shi, Xiaohuan Wang, and Jun Wang. 2022. "Effect of Ce-Doping on Microstructure and Adsorption- Photodegradation Behaviors of the Hydrothermally-Synthesized TiO2 Nanotubes" Crystals 12, no. 8: 1094. https://doi.org/10.3390/cryst12081094






