Synthesis and Characterization of Silver Nanoparticle-Polydimethylsiloxane (Ag-NP-PDMS) Stretchable Conductive Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
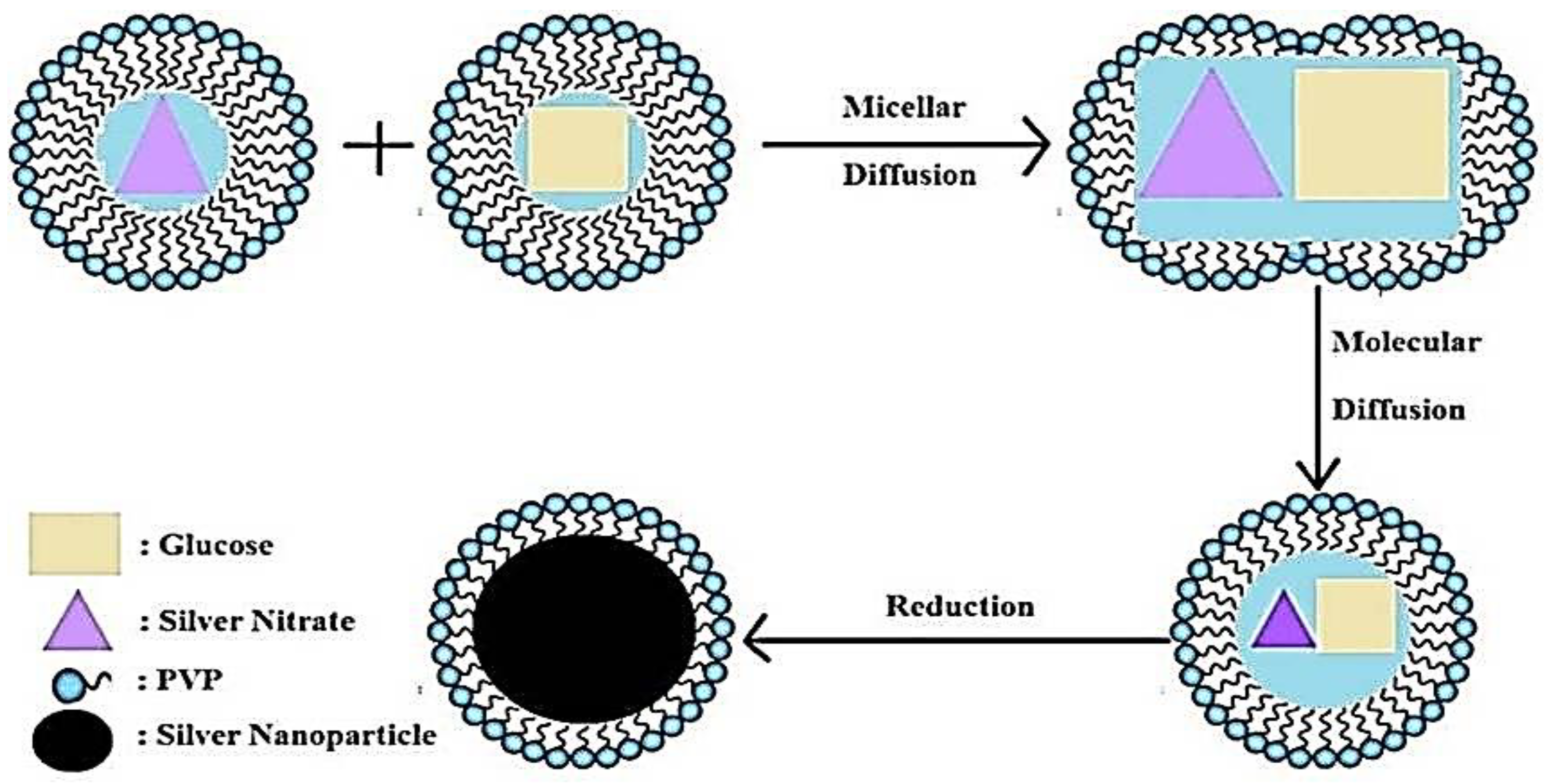
2.1. Synthesis of Ag-NPs
2.2. Preparation of Ag-NPs/Ethylene Glycol Ink
2.3. Fabrication of Ag-NP Thin-Film
2.4. Characterization of the Ag-NPs and the Ag-NP-PDMS Thin-Film
3. Results and Discussion
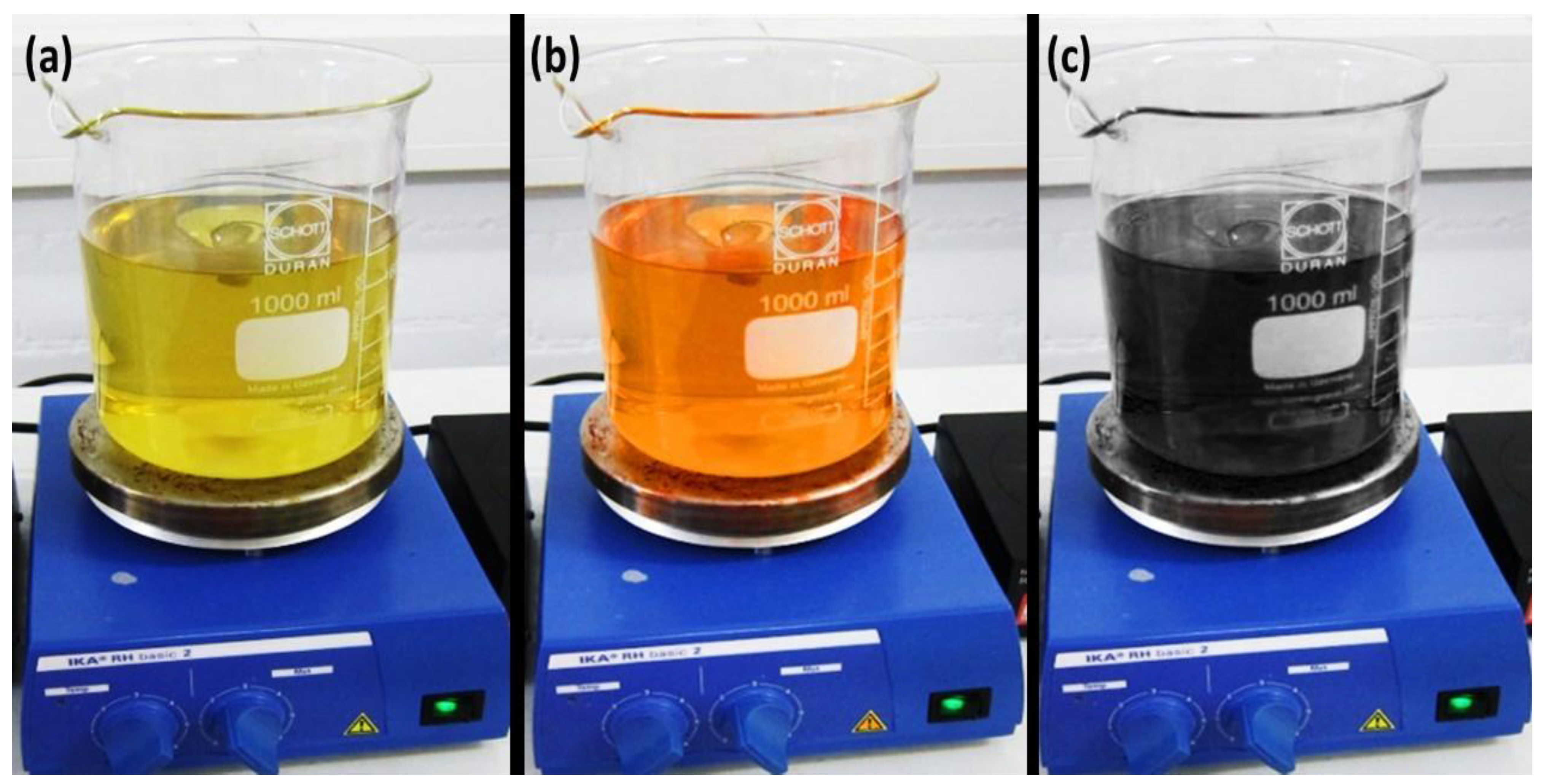
3.1. Ag-NPs’ Characterization
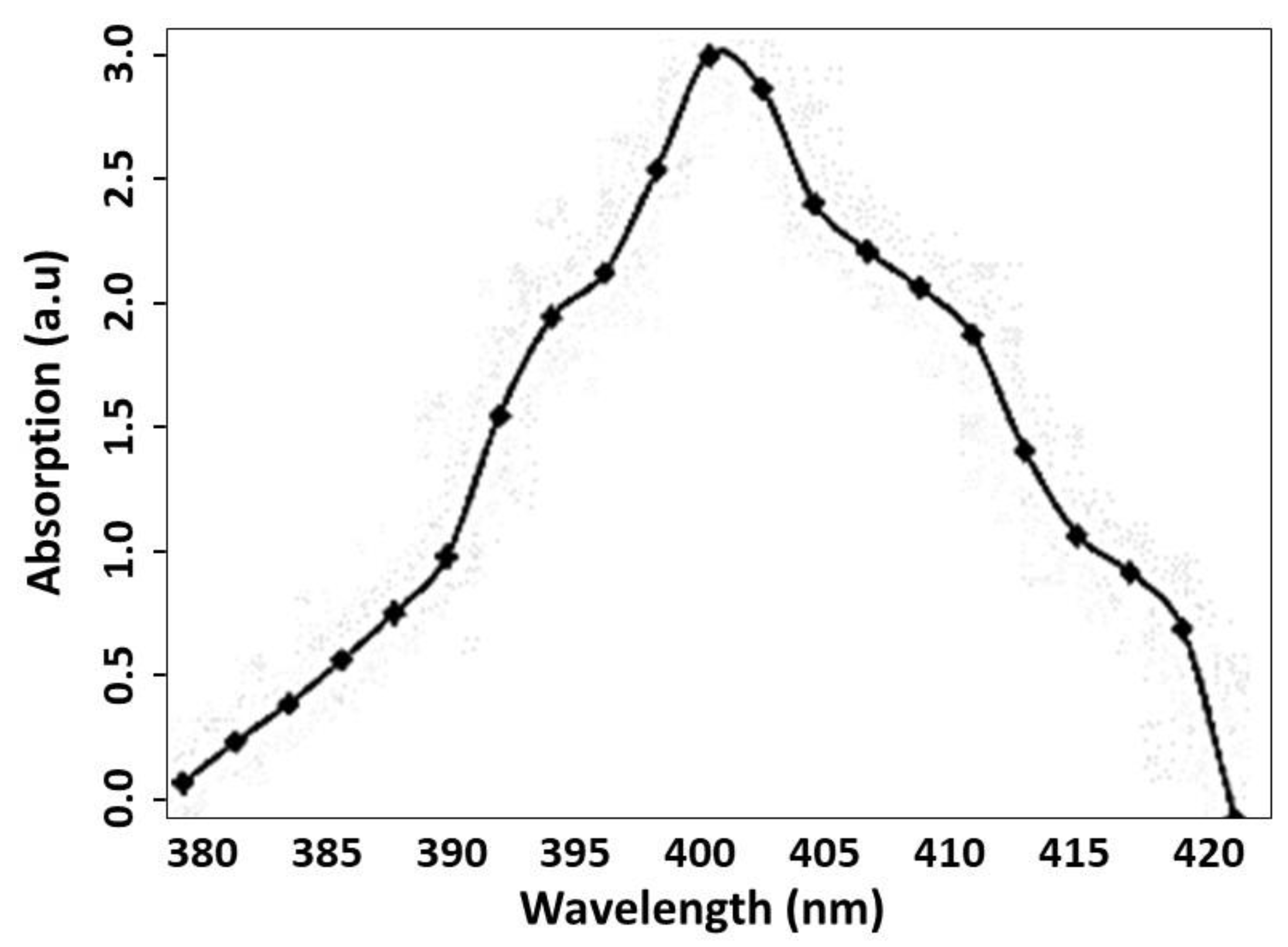
3.2. UV Absorption Spectrum of the Ag-NPs
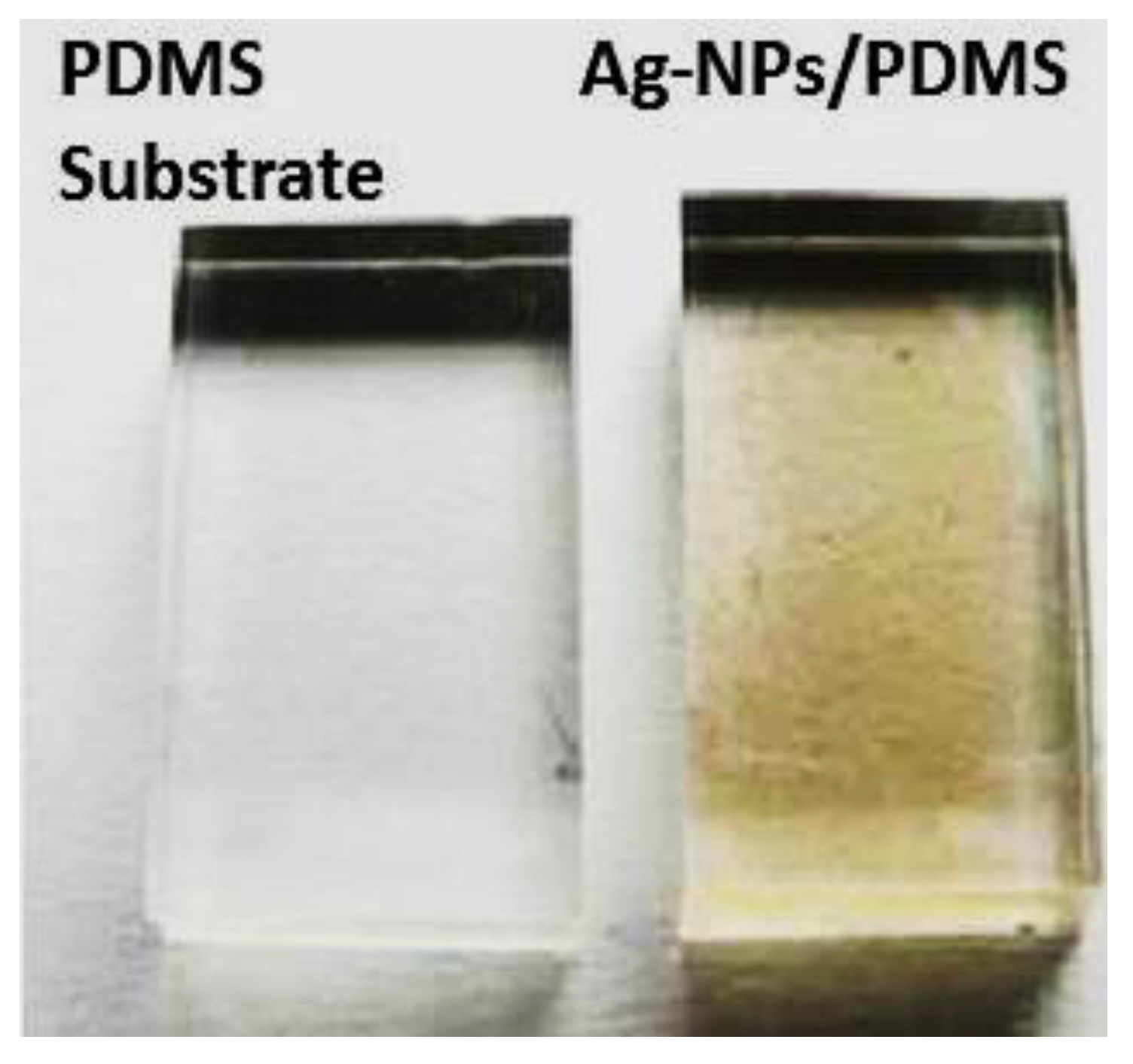
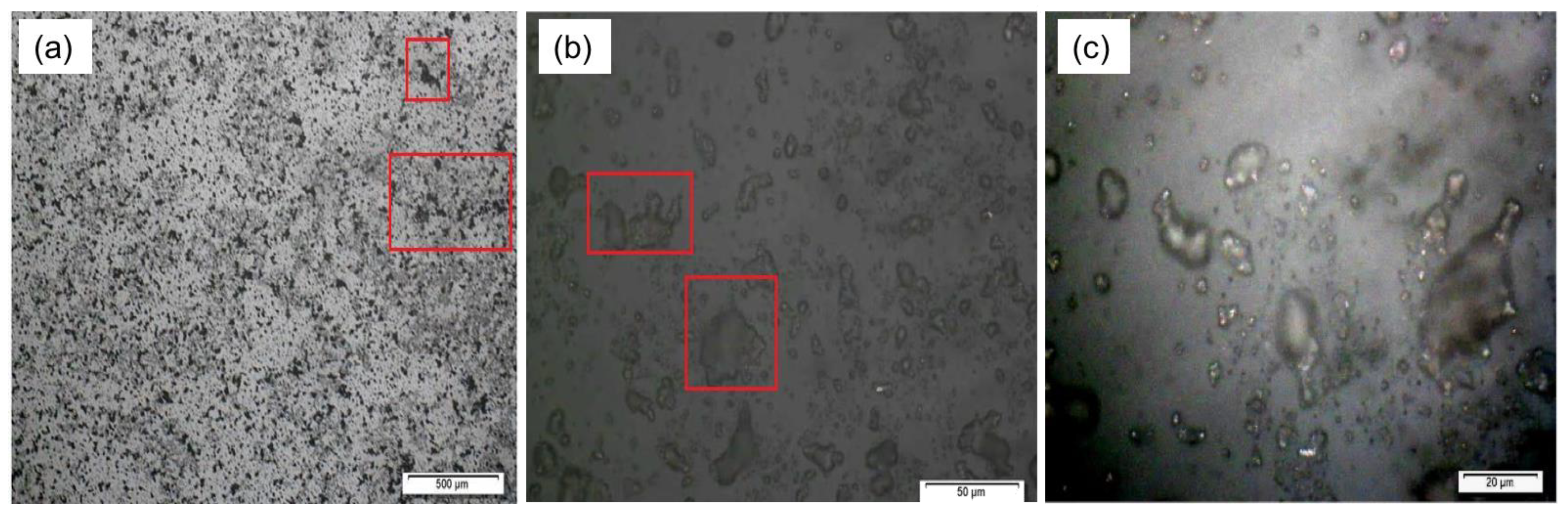
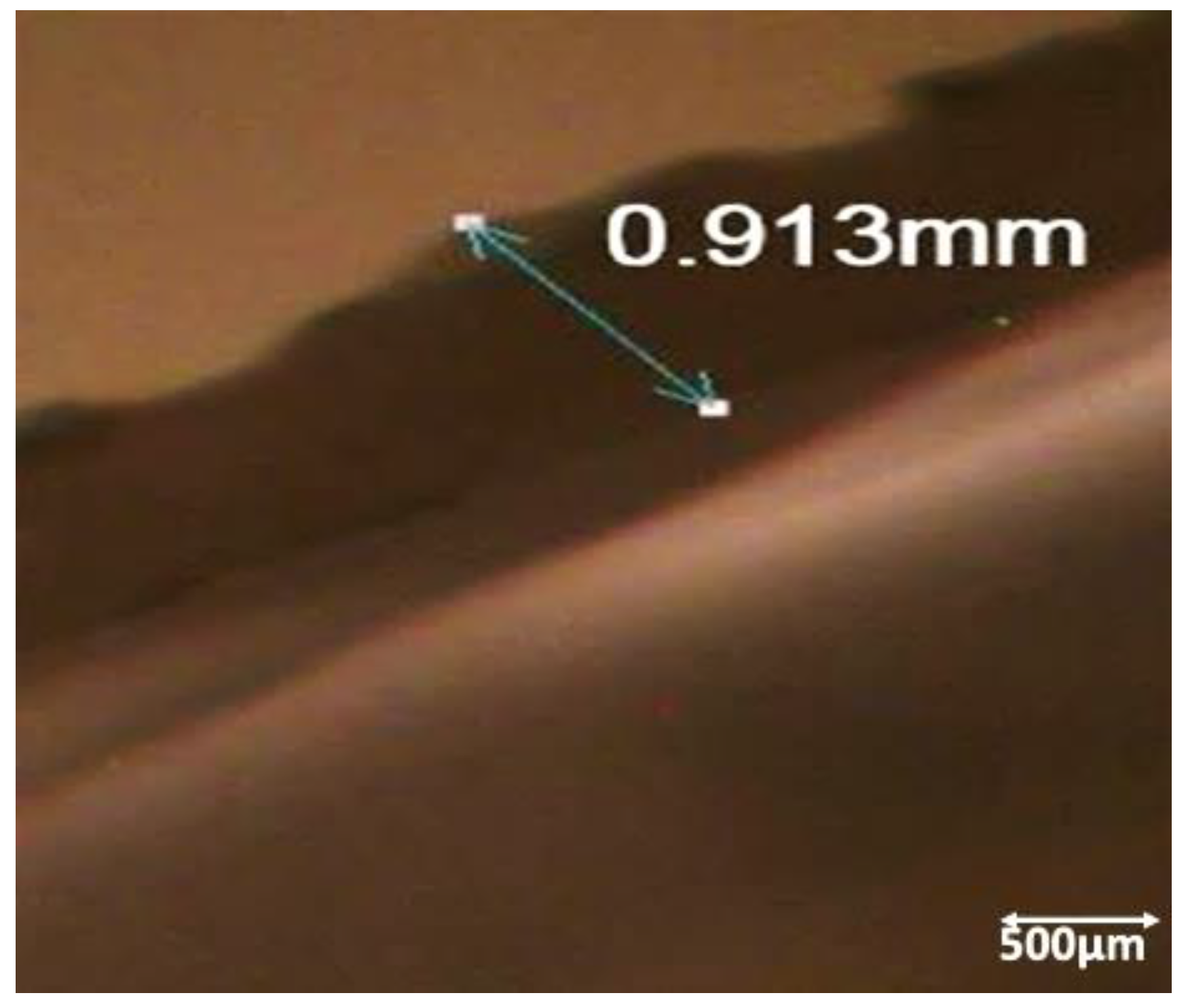
3.3. Surface Characterization of Ag-NP-PDMS Thin-Film
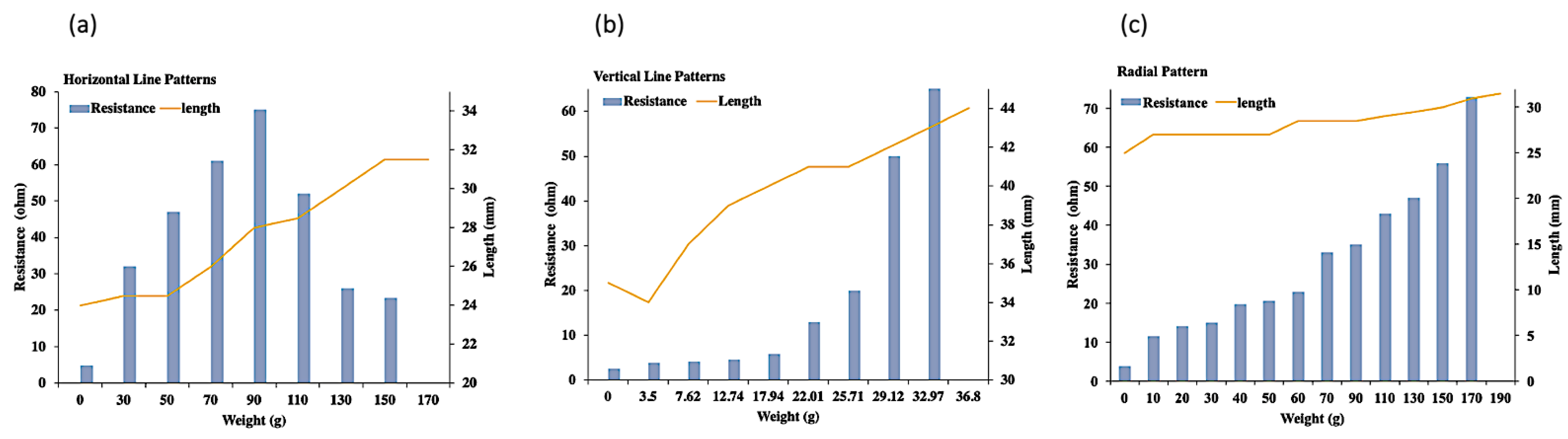
3.4. Electrical Conductivity of the Sintered Ag-NP-PDMS Thin-Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Khalaf, J.M.; Al-Ghussain, L.; Al-Halhouli, A.A. Fabrication of stretchable circuits on polydimethylsiloxane (PDMS) pre-stretched substrates by inkjet printing silver nanoparticles. Materials 2018, 11, 2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Ji, H.; Zhang, L.; Luo, X.; Leng, X.; He, P.; Zhao, W. Highly stretchable patternable conductive circuits and wearable strain sensors based on polydimethylsiloxane and silver nanoparticles. Nanotechnology 2019, 30, 185501. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.M.; Abd Manaf, A.; Matsuda, A.; Jaafar, M. Development and fabrication of highly flexible, stretchable, and sensitive strain sensor for long durability based on silver nanoparticles–polydimethylsiloxane composite. J. Mater. Sci. Mater. Electron. 2020, 31, 11897–11910. [Google Scholar] [CrossRef]
- Min, S.H.; Lee, G.Y.; Ahn, S.H. Direct printing of highly sensitive, stretchable, and durable strain sensor based on silver nanoparticles/multi-walled carbon nanotubes composites. Composites Part B Eng. 2019, 161, 395–401. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, J.; Li, C.; Li, Z.; Pan, J.; Liu, A.; Zhang, C. SERS substrate based on the flexible hybrid of polydimethylsiloxane and silver colloid decorated with silver nanoparticles. Opt. Express 2018, 26, 21784–21796. [Google Scholar] [CrossRef]
- Al-Halhouli, A.A.; Al-Ghussain, L.; El Bouri, S.; Liu, H.; Zheng, D. Fabrication and evaluation of a novel non-invasive stretchable and wearable respiratory rate sensor based on silver nanoparticles using inkjet printing technology. Polymers 2019, 11, 1518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, H.; Yao, G.; Liao, F.; Gao, M.; Huang, Z.; Lin, Y. Highly stretchable, sensitive, and flexible strain sensors based on silver nanoparticles/carbon nanotubes composites. J. Alloys Compd. 2015, 652, 48–54. [Google Scholar] [CrossRef]
- Soe, H.M.; Abd Manaf, A.; Matsuda, A.; Jaafar, M. Performance of a silver nanoparticles-based polydimethylsiloxane composite strain sensor produced using different fabrication methods. Sens. Actuators A Phys. 2021, 329, 112793. [Google Scholar] [CrossRef]
- Choi, Y.I.; Hwang, B.U.; Meeseepong, M.; Hanif, A.; Ramasundaram, S.; Trung, T.Q.; Lee, N.E. Stretchable and transparent nanofiber-networked electrodes based on nanocomposites of polyurethane/reduced graphene oxide/silver nanoparticles with high dispersion and fused junctions. Nanoscale 2019, 11, 3916–3924. [Google Scholar] [CrossRef]
- Tavakoli, M.; Malakooti, M.H.; Paisana, H.; Ohm, Y.; Green Marques, D.; Alhais Lopes, P.; Majidi, C.E. GaIn-Assisted Room-Temperature Sintering of Silver Nanoparticles for Stretchable, Inkjet-Printed, Thin-Film Electronics. Adv. Mater. 2018, 30, 1801852. [Google Scholar] [CrossRef]
- Shankar, A.; Salcedo, E.; Berndt, A.; Choi, D.; Ryu, J.E. Pulsed light sintering of silver nanoparticles for large deformation of printed stretchable electronics. Adv. Compos. Hybrid Mater. 2018, 1, 193–198. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Gao, Q.; Zhang, J.; Zhang, J.; Omisore, O.M.; Li, H. Polydimethylsiloxane (PDMS)-based flexible resistive strain sensors for wearable applications. Appl. Sci. 2018, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Al-Milaji, K.N.; Zhao, H. Inkjet printing of silver nanowires for stretchable heaters. ACS Appl. Nano Mater. 2018, 1, 4528–4536. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Hidayah, I.N.; Mariatti, M. Performance of inkjet-printed strain sensor based on graphene/silver nanoparticles hybrid conductive inks on polyvinyl alcohol substrate. J. Mater. Sci. Mater. Electron. 2020, 31, 15361–15371. [Google Scholar] [CrossRef]
- Wang, T.; Wang, R.; Cheng, Y.; Sun, J. Quasi in situ polymerization to fabricate copper nanowire-based stretchable conductor and its applications. ACS Appl. Mater. Interfaces 2016, 8, 9297–9304. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khalaf, J.; Al-Ghussain, L.; Nadi, A.; Saraireh, R.; Rabayah, A.; Altarazi, S.; Al-Halhouli, A.A. Optimization of geometry parameters of inkjet-printed silver nanoparticle traces on pdms substrates using response surface methodology. Materials 2019, 12, 3329. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.; Liu, Y.; Li, X.; Liu, S.; Fang, Y.; Deng, Y.; Li, L. Conductive grid patterns prepared by microcontact printing silver nanoparticles ink. Mater. Res. Express 2017, 4, 015021. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, D.; Park, J. Fabrication of silver nanowire-based stretchable electrodes using spray coating. Thin Solid Film. 2016, 608, 34–43. [Google Scholar] [CrossRef]
- Duan, S.; Yang, K.; Wang, Z.; Chen, M.; Zhang, L.; Zhang, H.; Li, C. Fabrication of highly stretchable conductors based on 3D printed porous poly (dimethylsiloxane) and conductive carbon nanotubes/graphene network. ACS Appl. Mater. Interfaces 2016, 8, 2187–2192. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Chen, J.; Xiong, T.; Jiang, B.; Huang, Y. Self-healing and stretchable PDMS-based bifunctional sensor enabled by synergistic dynamic interactions. Chem. Eng. J. 2021, 412, 128734. [Google Scholar] [CrossRef]
- Martinez, V.; Stauffer, F.; Adagunodo, M.O.; Forro, C.; Vörös, J.; Larmagnac, A. Stretchable silver nanowire–elastomer composite microelectrodes with tailored electrical properties. ACS Appl. Mater. Interfaces 2015, 7, 13467–13475. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhong, M.; Zhao, W. Stretchable multifunctional dielectric nanocomposites based on polydimethylsiloxane mixed with metal nanoparticles. Mater. Res. Express 2019, 7, 015007. [Google Scholar] [CrossRef] [Green Version]
- Zou, Q.; He, K.; Ou-Yang, J.; Zhang, Y.; Shen, Y.; Jin, C. Highly Sensitive and Durable Sea-Urchin-Shaped Silver Nanoparticles Strain Sensors for Human-Activity Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 14479–14488. [Google Scholar] [CrossRef] [PubMed]
- Al-Milaji, K.N.; Huang, Q.; Li, Z.; Ng, T.N.; Zhao, H. Direct embedment and alignment of silver nanowires by inkjet printing for stretchable conductors. ACS Appl. Electron. Mater. 2020, 2, 3289–3298. [Google Scholar] [CrossRef]
- Duan, S.; Wang, Z.; Zhang, L.; Liu, J.; Li, C. Three-dimensional highly stretchable conductors from elastic fiber mat with conductive polymer coating. ACS Appl. Mater. Interfaces 2017, 9, 30772–30778. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Tian, Q.; Liu, L.; Yao, W.; Chi, C.; Wu, W. Highly conductive, flexible and stretchable conductors based on fractal silver nanostructures. J. Mater. Chem. C 2018, 6, 3999–4006. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, Y.; Bai, S.; Hu, A. Laser direct writing of waterproof sensors inside flexible substrates for wearable electronics. Opt. Laser Technol. 2021, 135, 106694. [Google Scholar] [CrossRef]
- Chou, N.; Kim, Y.; Kim, S. A method to pattern silver nanowires directly on wafer-scale PDMS substrate and its applications. ACS Appl. Mater. Interfaces 2016, 8, 6269–6276. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Q.; Luo, G.; Meng, W.; Cao, M.; Li, Y.; Lang, M.F. A novel flexible Ag/AgCl quasi-reference electrode based on silver nanowires toward ultracomfortable electrophysiology and sensitive electrochemical glucose detection. J. Mater. Res. Technol. 2020, 9, 13425–13433. [Google Scholar] [CrossRef]
- Hu, J.; Yu, J.; Li, Y.; Liao, X.; Yan, X.; Li, L. Nano carbon black-based high-performance wearable pressure sensors. Nanomaterials 2020, 10, 664. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Xia, Z.; Wang, X.; Nie, J.; Huang, P.; Zhao, S. 3D-printed highly stable flexible strain sensor based on silver-coated-glass fiber-filled conductive silicon rubber. Mater. Des. 2020, 193, 108788. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Kim, H.; Lee, B.; Kim, T.; Hong, Y. 2-D Strain Sensors Implemented on Asymmetrically Bi-Axially Pre-Strained PDMS for Selectively Switching Stretchable Light-Emitting Device Arrays. IEEE Sens. J. 2020, 20, 14655–14661. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, X.; Harkin-Jones, E.; Zhu, W.; Zhou, Z.; Shen, Y.; Wang, P. Synergistic effects of hybrid conductive nanofillers on the performance of 3D printed highly elastic strain sensors. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105730. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamali, A.R.; Bhatti, J.; Khan, W.; Akther, F.; Batool, M.; Batool, R.; Daoush, W.M. Synthesis and Characterization of Silver Nanoparticle-Polydimethylsiloxane (Ag-NP-PDMS) Stretchable Conductive Nanocomposites. Crystals 2022, 12, 1098. https://doi.org/10.3390/cryst12081098
Jamali AR, Bhatti J, Khan W, Akther F, Batool M, Batool R, Daoush WM. Synthesis and Characterization of Silver Nanoparticle-Polydimethylsiloxane (Ag-NP-PDMS) Stretchable Conductive Nanocomposites. Crystals. 2022; 12(8):1098. https://doi.org/10.3390/cryst12081098
Chicago/Turabian StyleJamali, Abdul Rauf, Jahanzeb Bhatti, Waseem Khan, Faheem Akther, Madiha Batool, Razia Batool, and Walid M. Daoush. 2022. "Synthesis and Characterization of Silver Nanoparticle-Polydimethylsiloxane (Ag-NP-PDMS) Stretchable Conductive Nanocomposites" Crystals 12, no. 8: 1098. https://doi.org/10.3390/cryst12081098






