Evolution of Microstructure and Mechanical Properties of the CoFeNiMnMox High-Entropy Alloys
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
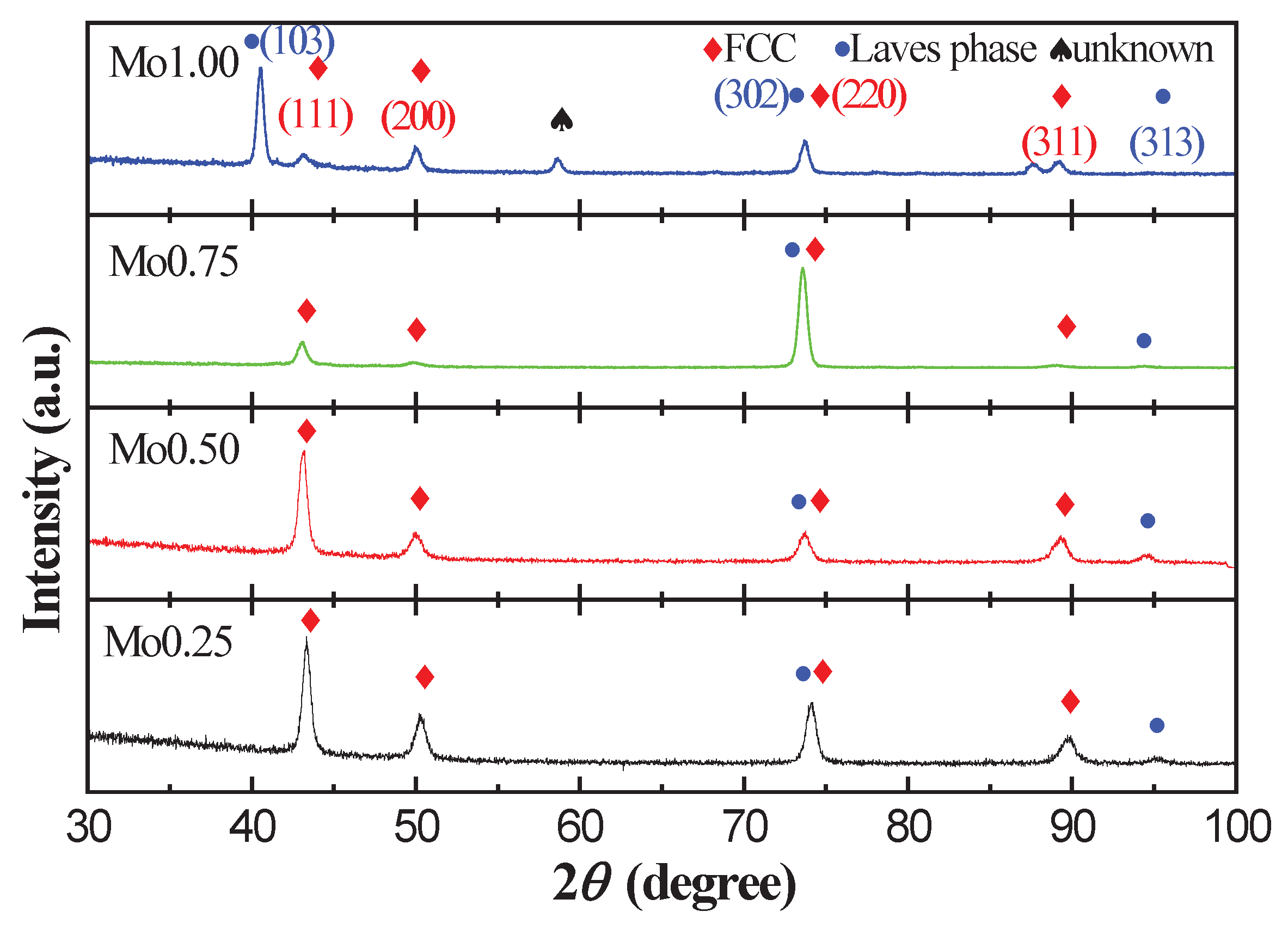
3.1. Crystal Structure Characterization of the CoFeNiMnMox Alloys
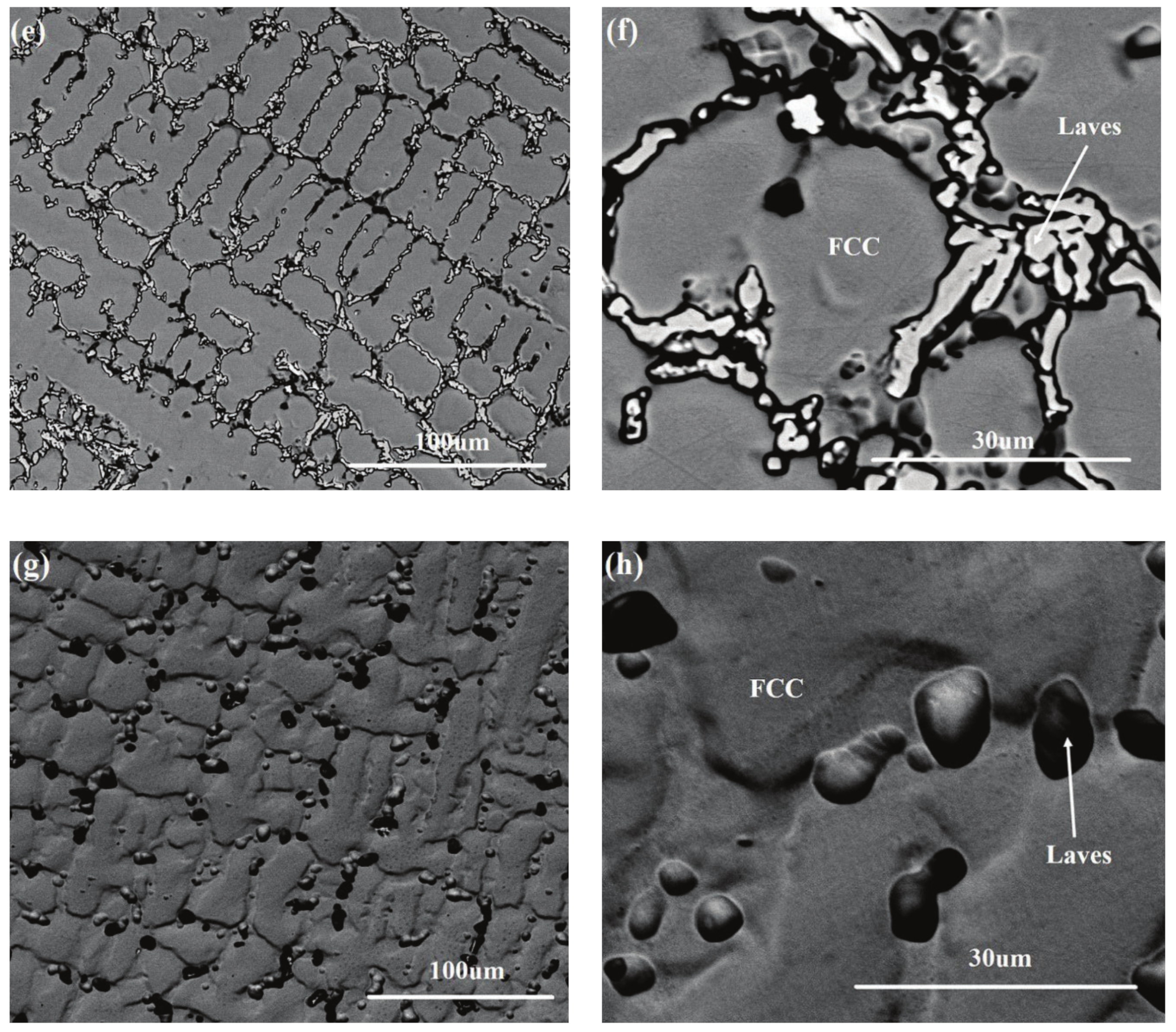
3.2. Microstructure Observation in the CoFeNiMnMox Alloys
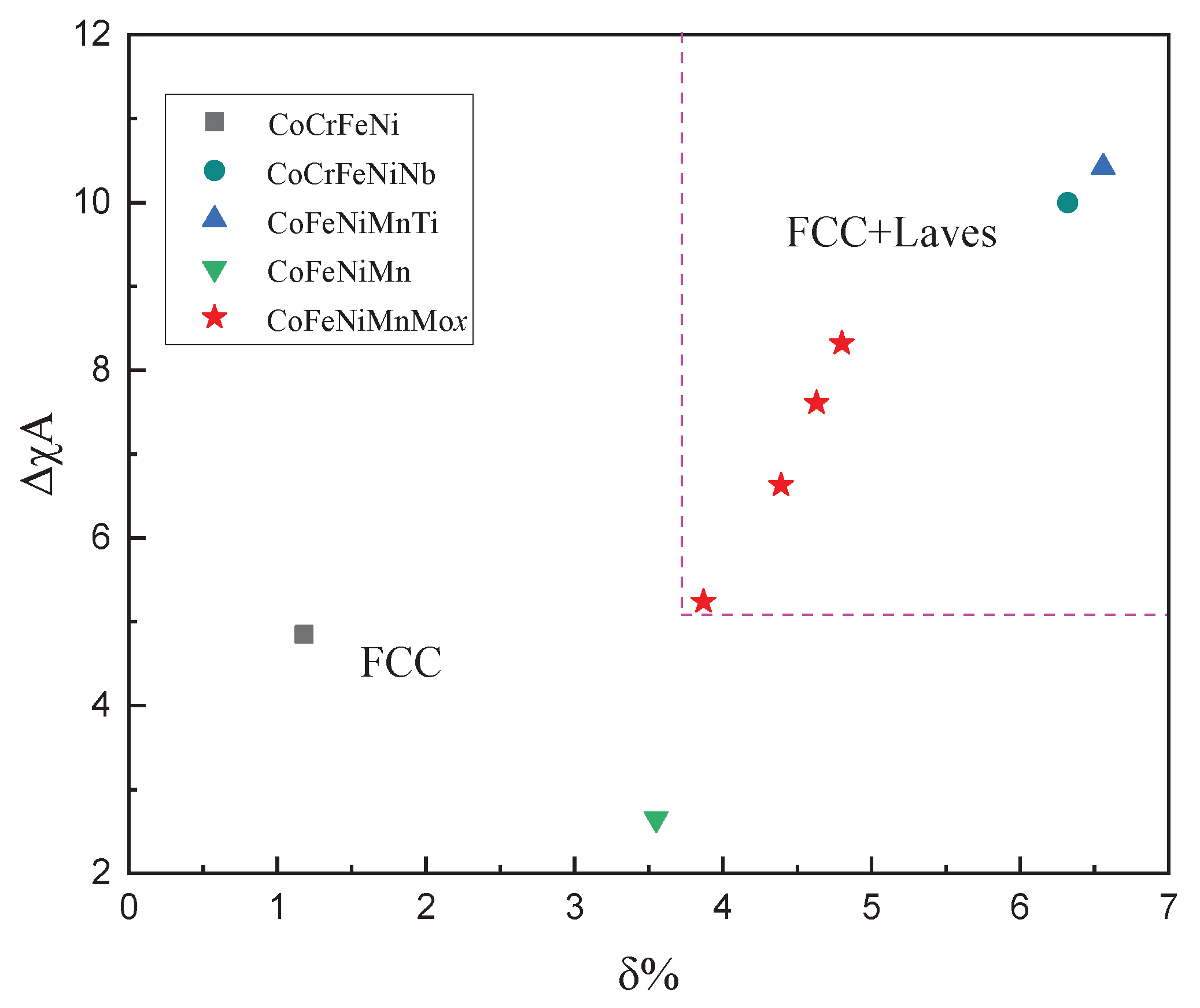
3.3. Phase Stability of CoFeNiMnMox Alloys
3.4. Mechanical Properties
4. Conclusions
- (1)
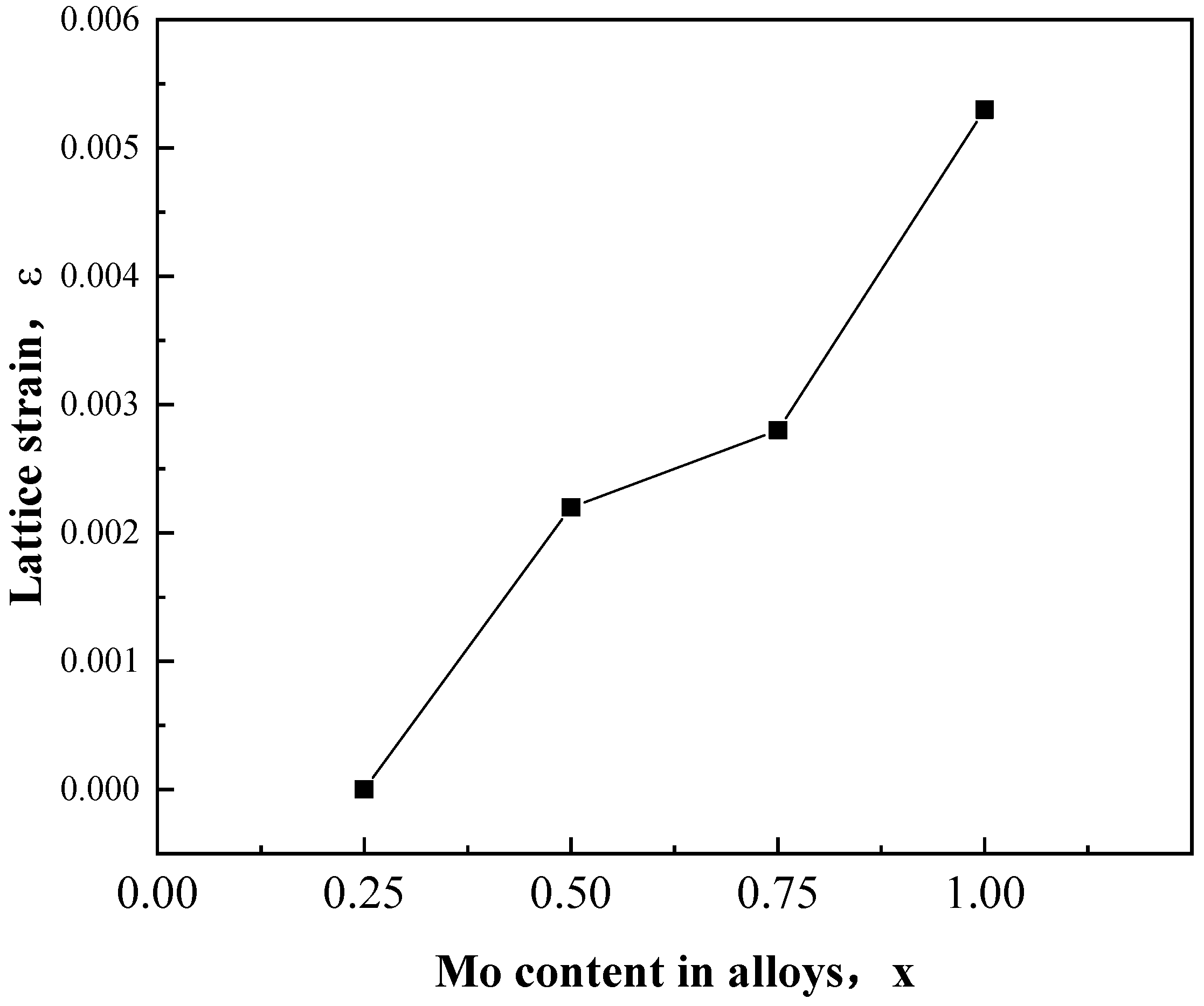
- With the increase in Mo content, the atomic size difference δ increased and the VEC decreased, indicating that the addition of the Mo element leads to the severe lattice distortion and structural instability of CoFeNiMnMox alloys.XRD and SEM analysis indicate that the microstructures of CoFeNiMnMox alloys are composed of FCC and a Laves phase.
- (2)
- The microstructure formation can be explained by atomic size difference δ and parameter ΔχA. δ ≥ 3.87% and ΔχA ≥ 5.24 criterions are more reliable parameters for correctly predicting the (FCC + Laves) coexistence region of CoFeNiMnMox alloys.
- (3)
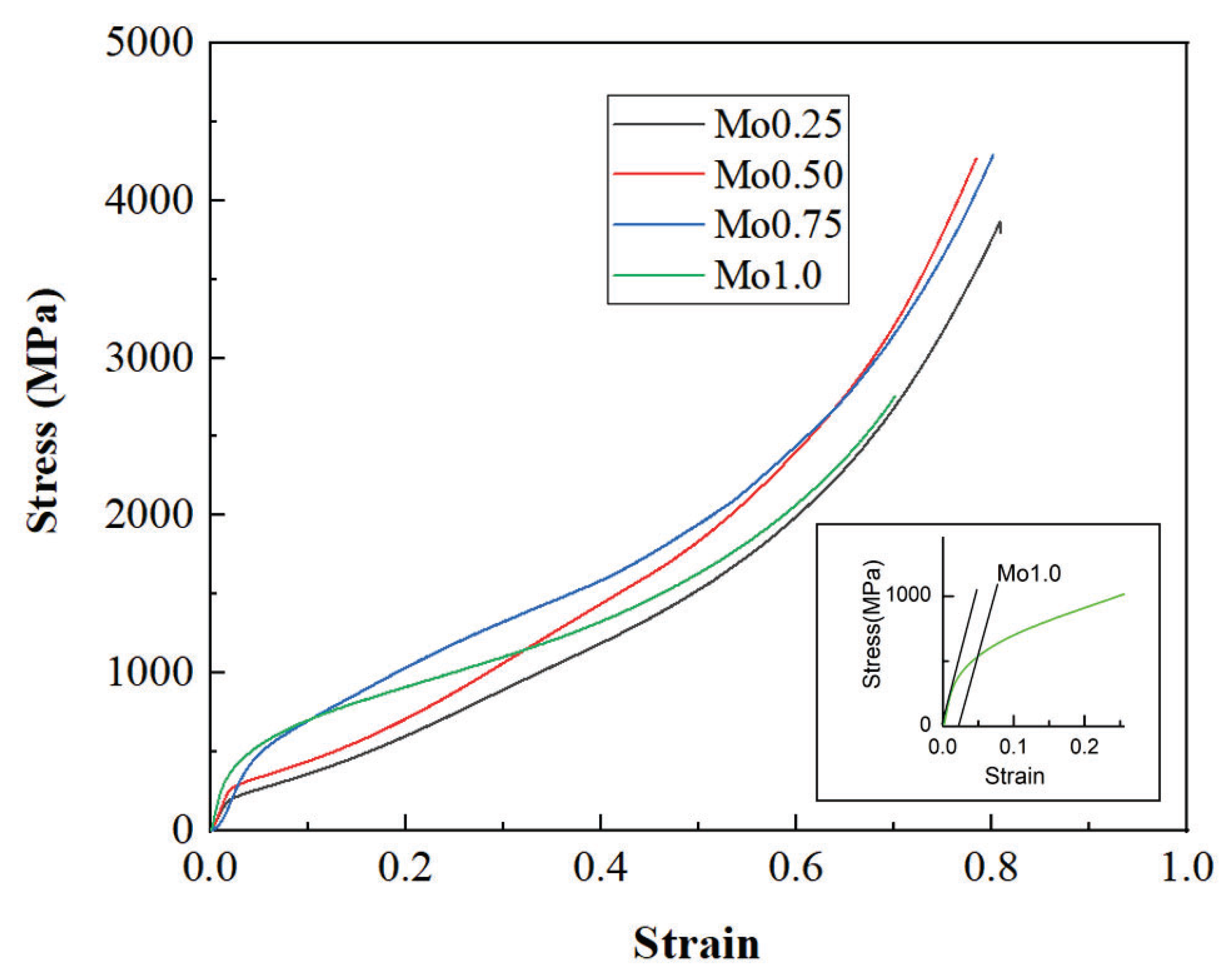
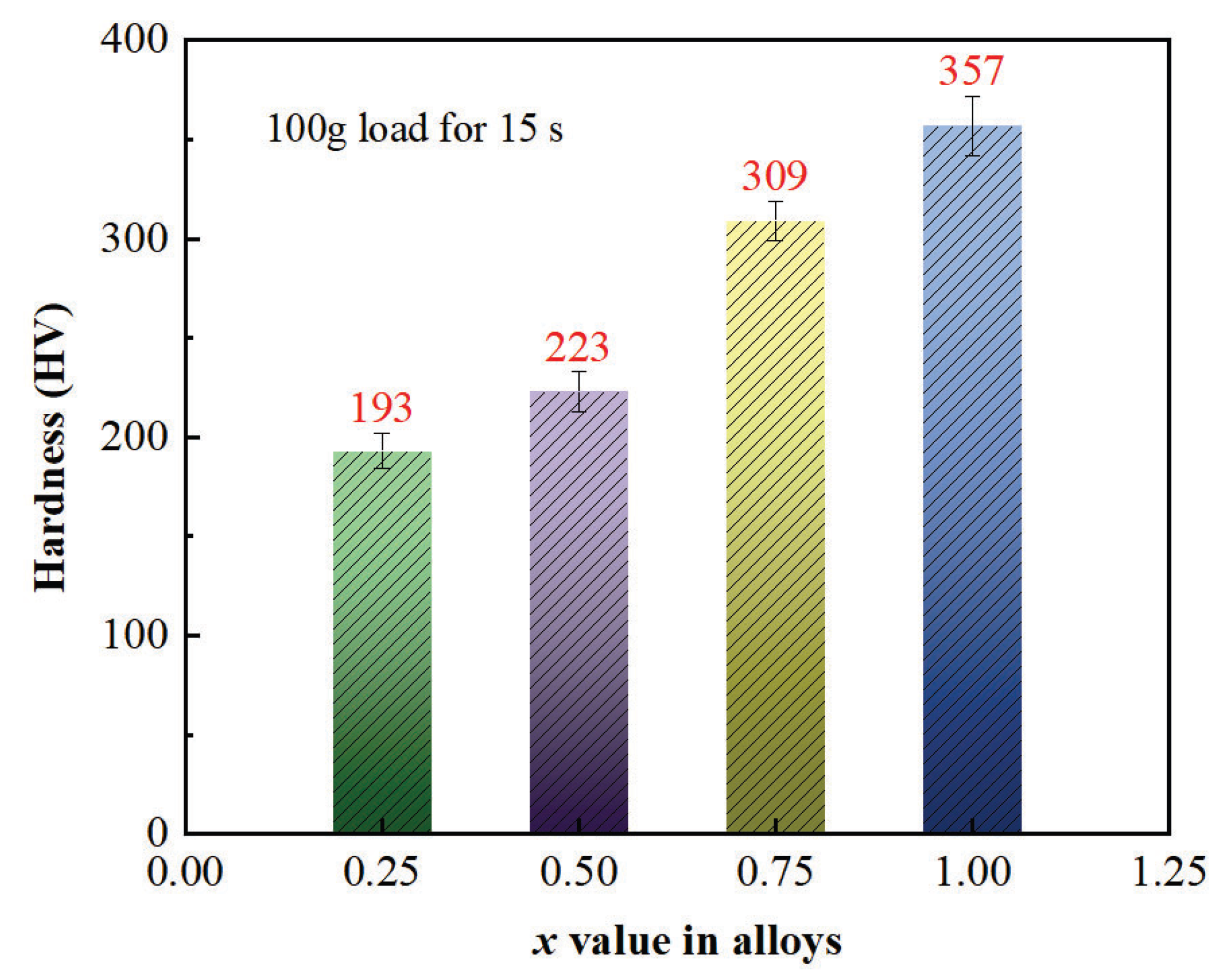
- With the increase in Mo content, the hardness and yield strength of CoFeNiMnMox alloys increased from 193 to 357 and from 187 MPa to 537 MPa, respectively, but there was no compression fracture which occurred when the plastic deformation reached 80%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Hong, K.J.; Bei, B. Physical Properties of High Entropy Alloys. Encycl. Mater. Met. Alloys 2022, 2, 474–483. [Google Scholar]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kulagin, R.; Baretzky, B.; Anisimova, N.Y.; Kiselevskiy, M.V.; Klinger, L.; Straumal, P.B.; Kogtenkova, O.A.; Valiev, R.Z. Severe Plastic Deformation and Phase Transformations in High Entropy Alloys: A Review. Crystals 2022, 12, 54. [Google Scholar] [CrossRef]
- Antão, F.; Martins, R.; Correia, J.B.; da Silva, R.C.; Gonçalves, A.P.; Tejado, E.; Pastor, J.Y.; Alves, E.; Dias, M. Improvement of Mechanical Properties with Non-Equimolar CrNbTaVW High Entropy Alloy. Crystals 2022, 12, 219. [Google Scholar] [CrossRef]
- Srikanth, M.; Annamalai, A.R.; Muthuchamy, A.; Jen, C.-P. A Review of the Latest Developments in the Field of Refractory High-Entropy Alloys. Crystals 2021, 11, 612. [Google Scholar] [CrossRef]
- Vaidya, M.; Guruvidyathri, K.; Murty, B.S. Phase formation and thermal stability of CoCrFeNi and CoCrFeMnNi equiatomic high entropy alloys. J. Alloys Compd. 2019, 774, 856–864. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. A 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Fu, W.; Zheng, W.; Huang, Y. Cryogenic mechanical behaviors of CrMnFeCoNi high-entropy alloy. Mater. Sci. Eng. A 2020, 789, 139579. [Google Scholar] [CrossRef]
- Abdukadir, A.-M.; Xiang, S.; Le, G.-M. Microstructure and low temperature mechanical properties of CrMnFeCoNi high-entropy alloys deposited by laser melting. Trans. Mater. Heat Treat. 2020, 41, 70–75. [Google Scholar]
- Zuo, T.T. Microstructure and Properties of Co-Fe-Ni Based Magnetic High Entropy Alloys. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Cui, P.; Ma, Y.; Zhang, L.; Zhang, M.; Fan, J.; Dong, W.; Yu, P.; Li, G. Microstructure and mechanical behaviors of CoFeNiMnTixAl1−x high entropy alloys. Mater. Sci. Eng. A 2018, 731, 124–130. [Google Scholar] [CrossRef]
- Zhu, J.M.; Zhang, H.F.; Fu, H.M.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrCuFeNiMox alloys. J. Alloys Compd. 2010, 497, 52–56. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. A 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Quan, H.; Chen, Y.; Wang, S.; Zhang, S. Effect of Mo addition on structures and properties of FeCoNiCrMn high entropy alloy film by direct current magnetron sputtering. J. Alloys Compd. 2022, 895, 162709. [Google Scholar] [CrossRef]
- Na, S.M.; Yoo, J.H.; Lambert, P.K.; Jones, N.J. Room-temperature ferromagnetic transitions and the temperature dependence of magnetic behaviors in FeCoNiCr-based high-entropy alloys. AIP Adv. 2018, 8, 056412. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2017, 33, 17–22. [Google Scholar] [CrossRef]
- Sheng, G.; Ng, C.; Jian, L.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 1035051. [Google Scholar]
- Chanda, B.; Das, J. Composition Dependence on the Evolution of Nanoeutectic in CoCrFeNiNbx (0.45 ≤ x ≤ 0.65) High Entropy Alloys. Adv. Eng. Mater. 2018, 20, 1700908. [Google Scholar] [CrossRef]

| Alloys | Region | Elements (at. %) | ||||
|---|---|---|---|---|---|---|
| Co | Fe | Ni | Mn | Mo | ||
| Mo0.25 | FCC | 22.09 | 19.08 | 23.44 | 23.67 | 11.73 |
| Laves phase | 19.87 | 18.78 | 24.72 | 24.99 | 11.97 | |
| Mo0.50 | FCC | 19.04 | 17.25 | 22.33 | 21.45 | 19.93 |
| Laves phase | 20.25 | 19.58 | 20.29 | 10.31 | 19.57 | |
| Mo0.75 | FCC | 20.87 | 19.79 | 20.38 | 17.72 | 21.25 |
| Laves phase | 20.53 | 20.65 | 18.53 | 18.80 | 21.51 | |
| Mo1.00 | FCC | 21.85 | 21.11 | 18.93 | 17.89 | 20.21 |
| Laves phase | 23.65 | 21.10 | 21.70 | 16.23 | 18.20 | |
| Elements (Melting Point, Atomic Radius) | Fe | Co | Ni | Mn | Mo |
|---|---|---|---|---|---|
| Fe (1811 K, 1.241 Å) | – | –1 | –2 | 0 | 0 |
| Co (1768 K, 1.251 Å) | – | 0 | –5 | –5 | |
| Ni (1728 K, 1.246 Å) | – | –8 | –8 | ||
| Mn (1519 K, 1.350 Å) | – | +5 | |||
| Mo (2883 K, 1.390 Å) | – |
| Alloys | Phases | VEC | ΔHmix/kJ mol−1 | ΔSmix/J mol−1 K−1 | δ(%) | ΔχA (%) | Ω | ΔχP | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CoFeNiMnMo0.25 | FCC + Laves | 8.35 | −3.98 | 12.71 | 3.87 | 5.24 | 5.67 | 0.158 | This work |
| CoFeNiMnMo0.50 | FCC + Laves | 8.22 | −3.95 | 13.15 | 4.39 | 6.63 | 5.91 | 0.177 | This work |
| CoFeNiMnMo0.75 | FCC + Laves | 8.11 | −3.90 | 13.33 | 4.63 | 7.61 | 5.99 | 0.187 | This work |
| CoFeNiMnMo1.00 | FCC + Laves | 8.00 | −3.84 | 13.38 | 4.80 | 8.32 | 6.19 | 0.195 | This work |
| CoFeNiMn | FCC | 8.5 | −4.00 | 11.53 | 3.55 | 2.65 | 4.92 | 0.143 | [14] |
| Alloys | σ0.2 (MPa) | σf (MPa) | εf (%) | Reference |
|---|---|---|---|---|
| CoFeNiMnMo0.25 | 187 | – | >80 | This study |
| CoFeNiMnMo0.50 | 261 | – | >80 | This study |
| CoFeNiMnMo0.75 | 394 | – | >80 | This study |
| CoFeNiMnMo1.00 | 531 | – | >80 | This study |
| CoFeNiMn | 155.8 | – | >50 | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, M.; Yao, L.; Jian, Z. Evolution of Microstructure and Mechanical Properties of the CoFeNiMnMox High-Entropy Alloys. Crystals 2022, 12, 1124. https://doi.org/10.3390/cryst12081124
Liu Y, Zhu M, Yao L, Jian Z. Evolution of Microstructure and Mechanical Properties of the CoFeNiMnMox High-Entropy Alloys. Crystals. 2022; 12(8):1124. https://doi.org/10.3390/cryst12081124
Chicago/Turabian StyleLiu, Yongqin, Man Zhu, Lijuan Yao, and Zengyun Jian. 2022. "Evolution of Microstructure and Mechanical Properties of the CoFeNiMnMox High-Entropy Alloys" Crystals 12, no. 8: 1124. https://doi.org/10.3390/cryst12081124





