1. Introduction
One of the most challenging problems in pharmaceutical science is the bioavailability limitations of drugs with poor solubility. About 40% of the drugs currently on the market are struggling with poor aqueous solubility [
1,
2] and approximately 90% of drugs in development are classified as poorly soluble drugs [
3] based on the definition of the biopharmaceutical classification system (BCS). In particular, BCS II drugs with low solubility and high permeability (
Figure 1a) account for approximately 70% [
4]. The Developability Classification System (DCS) for oral administered drugs was proposed based on the BCS classification system. According to the DCS, DCS II drugs can be divided into two categories, one is dissolving rate limiting DCS IIa, which is insoluble in water and organic phases, and the other is solubility limiting DCS IIb, which is usually soluble in at least some lipids (
Figure 1b) [
5,
6]. Until now, several techniques have been proposed to solve the problems of drug insolubility, mainly involving two approaches: (i) modification of morphological properties of raw drug particles, i.e., improving the surface area to volume ratio by preparing a fine powder or promoting the porosity; and (ii) modification of some physicochemical and structural properties of insoluble active pharmaceutical ingredients (APIs), such as preparation of polymorphic forms, cocrystal, solid dispersions, etc. [
7,
8,
9]. However, the second approach usually requires large screening efforts (e.g., the selection of solvents and ligands) when it comes to DCS IIa drugs. Therefore, reducing drug particle size is the best option for DCS IIa drugs [
10].
Nanocrystal technology brings a new dawn for improving the solubility and bioavailability of insoluble drugs [
11]. Nanocrystals (usually 1–1000 nm) are pure drug particles stabilized by suitable surfactants/polymers [
2,
11,
12]. Nanocrystals have the following features. (i) The surface area of nanocrystals increase with decreasing particle size. According to the Noyes-Whitney equation [
13], the dissolution rate of nanocrystals increase with improving the surface area. (ii) According to the Ostwald-Freundlich equation [
14], downsizing the size to the nanometer range (
Figure 1c) significantly enhances the solubility of a drug [
15]. (iii) A mucus layer with a porous structure is present on the surface of the gastrointestinal tract. The nanograined size is small, which can rapidly permeate into the pore channels of the mucus layer and tightly adhere to them [
2,
16,
17], so it can prolong the effective range and time of drugs in the gastrointestinal tract (
Figure 1d). All of these properties contribute to enhancing the absorption and bioavailability of drugs [
17]. In fact, nanocrystals were originally invented to improve the oral bioavailability of insoluble drugs [
18]. With advanced research in drug nanocrystals, other advantages have also been explored, such as loading high active pharmaceutical ingredients (APIs) [
4,
16], improving the metabolism behavior of drugs [
19], reducing toxic and side effects, promoting patient compliance [
20], and so on. Consequently, the superior properties of drug nanocrystals have attracted more and more attention from pharmaceutical enterprises.
Figure 1.
(
a) description of BCS, (
b) description of DCS, (
c) size boundaries of nanoparticles, (
d) features of nanocrystals: increased saturation solubility (upper), increased dissolution velocity (middle), and increased adhesiveness of nanomaterial, for surface: calculations were performed as cubes.
Figure 1a was reprinted from ref. [
4] with permission from Elsevier, Copyright
® 2019.
Figure 1b was reprinted from ref. [
5] with permission from Elsevier, Copyright
® 2010.
Figure 1d was reprinted from ref. [
21] with permission from Elsevier, Copyright
® 2011.
Figure 1.
(
a) description of BCS, (
b) description of DCS, (
c) size boundaries of nanoparticles, (
d) features of nanocrystals: increased saturation solubility (upper), increased dissolution velocity (middle), and increased adhesiveness of nanomaterial, for surface: calculations were performed as cubes.
Figure 1a was reprinted from ref. [
4] with permission from Elsevier, Copyright
® 2019.
Figure 1b was reprinted from ref. [
5] with permission from Elsevier, Copyright
® 2010.
Figure 1d was reprinted from ref. [
21] with permission from Elsevier, Copyright
® 2011.
In this review, the latest applications of combinative drug nanocrystal preparation technology are highlighted. Firstly, the current technologies for the fabrication of drug nanocrystals are introduced. Subsequently, the characterization and evaluation methods are summarized. Finally, applications and future prospects are briefly mentioned.
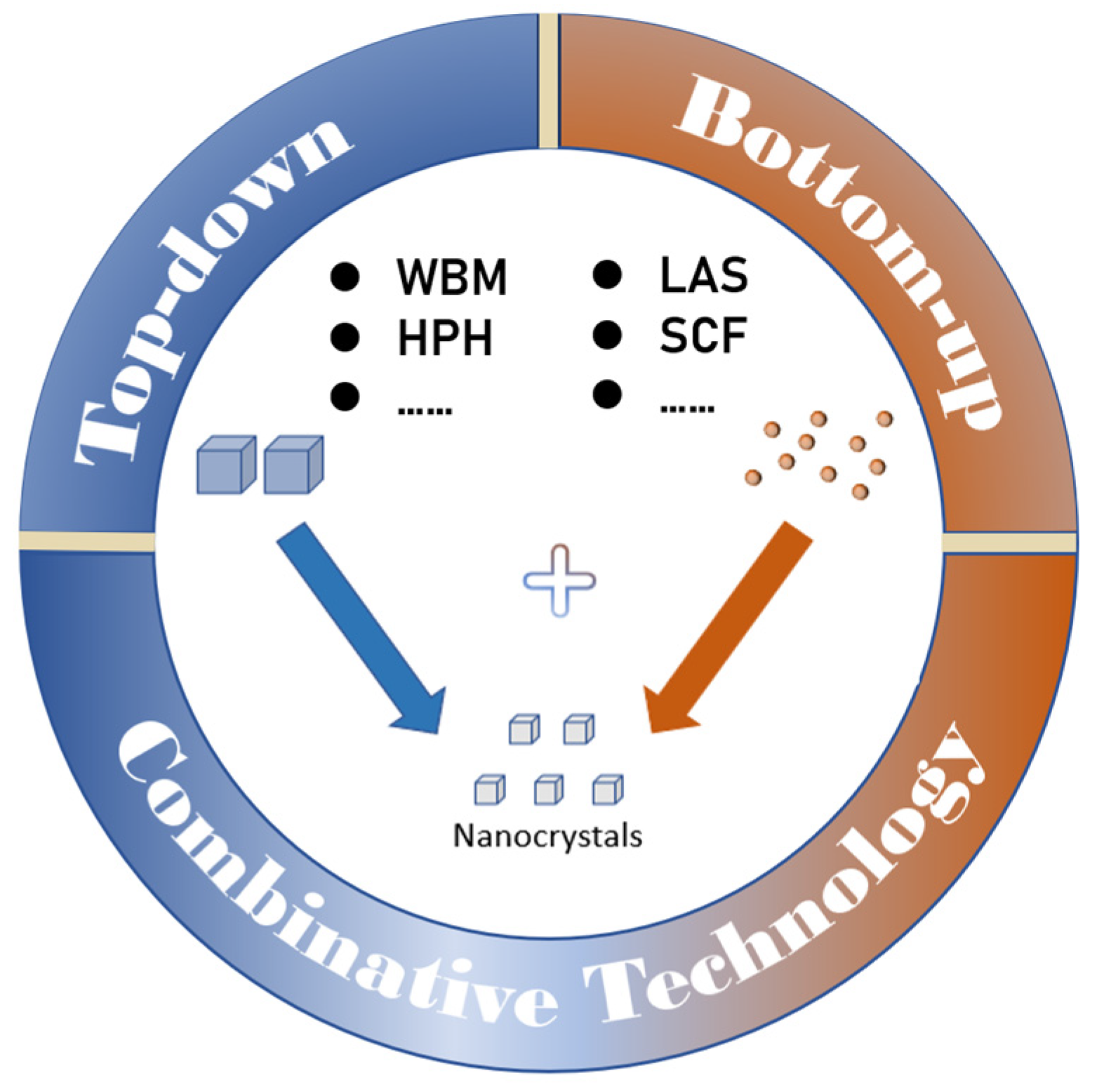
2. Preparation Technology
Nanocrystals are generated by reducing particle size (top-down technology), growing particles in the nanometer size range (bottom-up technology), and combining these two technologies [
22].
2.1. Top-Down Technology
Top-down technology mainly includes wet bead milling and high pressure homogenization [
23], which is easily industrialized. Drug particles decrease by mechanically generated shear and collision forces [
24], accompanied by the fragmentation of crystalline species and the appearance of secondary nucleation nuclei. The formation rate of top-down technology is independent of supersaturation. Most previous reported anticancer drugs have been prepared by this technology because they do not require organic solvents and are relatively easy to scale up production [
25]. In summary, top-down technology can be used for drugs that are insoluble in both the aqueous and organic phases. It processes quickly and is widely used for marketed drug nanocrystals.
2.1.1. Wet Bead Milling
Wet bead milling involves crushing the drug itself into nanoparticles by high intensity mechanical force with stabilizers and water [
26]. The particle size of nanocrystals is mainly relevant to the size of the milling beads [
27] (usually 0.1–20 nm), the property parameters of the drug, and the setting parameters [
24]. Since the temperature can be controlled in the preparation process, wet bead milling is especially suitable for preparing thermally unstable drug nanocrystals [
16]. It operates easily to obtain a uniform product. However, stabilizers and wetting agents still need to be added, and several cycles are required to reach the specific particle size range. Meanwhile, the obtained product has disadvantages in the contamination caused by grinding beads [
28] and poor physical storage stability due to agglomeration. Funahashi et al. [
29] found that ice beads melted after the milling process, which could avoid contamination. Most of the drug nanocrystals that have been successfully translated industrially are prepared using milling, including the earliest marketed pentoxifylline capsule Verelan
®PM, a fenofibrate tablet for the treatment of hypercholesterolemia Tricor
®, and an anti-inflammatory drug Naprelan
® [
30].
2.1.2. High Pressure Homogenization
High pressure homogenization (HPH) utilizes violent shearing, collision, and cavitation generated in a high pressure homogenization chamber to break down drug particles. Depending on the instrumentation and solution used, it can be divided into microfluidization, IDD-P™, Dissocubes
®, and Nanopure
®. Microfluidization has the ‘Z’ or ‘Y’ type chamber based on the jet stream principle. IDD-P™ uses a jet homogenizer for the homogenization of suspensions. Dissocubes
® uses a piston gap homogenizer for homogenization in aqueous media. Nanopure
® is suitable for the production of easily hydrolyzed drugs in reduced/non-aqueous media [
31]. In general, the setting parameters of the homogenization process and the hardness of the drug mainly affect the properties of the product [
32]. Through these efficient methods, the obtained product has a small particle size with narrow distribution and is not contaminated by the grinding medium. Most importantly, the method can be better combined with other methods to reduce the cycle number of homogenizations and the requirement of homogenization pressure. However, expensive equipment and demanding techniques hinder the transferability to larger scales [
33]. In addition, high pressure may unintentionally lead to crystal structure changes, increase the content of amorphous states, and affect the stability of some amorphous nanosuspensions [
32]. The currently marketed paliperidone palmitate intramuscular suspension Invega Sustenna, fenofibrate tablets Triglide
®, and Luteolin nanocrystals [
34] are prepared by the HPH technique [
30].
2.1.3. Laser Ablation
Laser ablation is a new technique developed in recent years for nanocrystal preparation. During laser ablation, the solid target is irradiated and the ejected material forms nanoparticles in the surrounding liquid. Then, stirred suspensions of microparticles are broken into nanoparticles by laser-mediated fragmentation [
35]. According to the laser processing time, it is divided into nanosecond, picosecond, and femtosecond laser irradiation, among which more nanoscale particles can be produced [
36]. The parameters affecting the particle size include the laser intensity, scanning speed, and the properties of the suspension, etc. In this process, no organic solvent is involved, but a small fraction of the drug may undergo oxidative degradation and crystal state changes due to excessive power. This method has been successfully used to prepare paclitaxel, megestrol acetate, and curcumin nanosuspensions [
37].
2.1.4. Ultrasound
Ultrasound is an efficient method to break drug particles into smaller particles through the vibration of acoustic waves. Ultrasound has been shown to enhance nucleation by creating acoustic cavitation in solution and rapidly dispersing the drug solution [
38]. Because it is easily operated in the laboratory and is highly reproducible, it is also usually combined with other techniques [
39]. Ultrasound-assisted precipitation of nanoparticles mainly alters the mixing process, nucleation, growth, and agglomeration [
40]. The size of the nanocrystal depends on the intensity of the ultrasound treatment, the horn length, the horn immersion depth, and the cavitation depth [
12].
2.2. Bottom-Up Technology
Bottom-up technology is mainly based on precipitation and evaporation [
33]. The basic principle is to obtain drug nanocrystals from the supersaturated state of drugs and subsequently control the size distribution of the nanoparticles by appropriate methods [
41]. Nucleation is especially important for the formation of small and homogeneous nanocrystals. Controlling the crystal growth is the best way to precisely control the particle size of drug particles. Many physical methods have been used to control the crystal growth, such as high gravity controlled precipitation. Compared with top-down methods, these methods provide better control of particle properties [
42]. In conclusion, bottom-up technology is simple in principle and operation, but difficult to scale up due to poor reproducibility. The process may also use organic solvents.
2.2.1. Liquid Antisolvent Precipitation
Liquid antisolvent (LAS) precipitation is the preparation of nanocrystals by mixing a solution stream (organic phase) dissolved with an insoluble drug with an aqueous antisolvent. The solution–antisolvent method of nanoprecipitation is the most commonly reported. Since this method only contains nucleation and growth steps, it is simple and cost-effective [
12]. The optimized nanocrystals can be prepared through two steps. However, unstable crystal particles are also recrystallized in the process, leading to the aggregation and precipitation of nanocrystals [
16]. Additionally, the use of organic solvents in the preparation process leads to the problem of solvent residues, and it is unsuitable for drugs that are neither soluble in aqueous nor insoluble in non-aqueous solvents. Currently, some studies have used this method to obtain suspensions of hydrochlorothiazide [
39], budesonide [
43], etc.
2.2.2. Precipitation Assisted by Acid-Base Method
The carbon dioxide-assisted precipitation method using acid-base reactions usually involves dissolving the drug in a weak acid solution as the acid phase, and weak base in a solution containing stabilizer as the base phase. The acid phase is slowly added to the base phase to produce carbon dioxide, then the drug nanocrystals are precipitated by vapor effervescence [
44]. This method avoids the addition of organic solvents, which is more friendly to the environment. However, it is only applicable to insoluble drugs whose solubility is related to pH and is stable to acids-bases [
19]. Wang et al. [
45] prepared tacrolimus nanocrystal suspensions using this method.
In vivo pharmacokinetic results indicated that tacrolimus nanosuspensions significantly increased the oral bioavailability compared with commercial hard capsules.
2.2.3. High Gravity Controlled Precipitation
High gravity controlled precipitation (HGCP) is [
46] the improvement of the precipitation method using gravity control to obtain more uniform and smaller drug nanocrystals. Reactant concentration, rotational speed, and volumetric flow rate are effective factors influencing particle size. In this process, the drug suspension in the device can be circulated for long-term mixing and reaction. However, local oversaturation of the feed stream at the turbulent edge during mixing leads to continuous nucleation, thus limiting the industrial application of this method [
25]. To date, this method has been successfully used on the laboratory scale to prepare salbutamol sulfate [
47] and sorafenib [
48].
2.2.4. Supercritical Fluid Method
The supercritical fluid (SCF) method means drugs dissolve in a supercritical fluid (e.g., CO
2) and precipitate nanocrystals with the rapid vaporization of the supercritical fluid as the fluid is atomized under reduced pressure through a nozzle with a tiny aperture [
11]. According to the function of supercritical fluid in the crystallization process, supercritical fluid methods include rapid expansion of supercritical solution (RESS) and supercritical antisolvent (SAS). According to the different nozzle positions, the rapid expansion of a supercritical fluid method was improved to yield the rapid expansion of a supercritical solution into a liquid solvent (RESOLV) method, in which the former places the nozzle in the air while the latter places the nozzle in aqueous solution. The state parameters of the supercritical fluid in the process, the morphology of the nozzle, and the concentration of the drug will influence the particle size of the nanocrystals [
7]. This process does not require organic solvent to produce high purity products. However, the consumption of supercritical fluids is large and it is only suitable for drugs dissolved in supercritical fluids [
16]. Zhang et al. [
49] prepared apigenin (AP) nanocrystals using the SAS method. No substantial changes in the crystal structure were observed in the nanocrystals, but decreased particle size and smooth spherical surface were noticed. The AP nanocrystals exhibited faster dissolution profiles than the original AP in dissolution media.
2.2.5. Emulsion Polymerization Method
API is dissolved in volatile organic solvents or solvents partially mixed with aqueous as the dispersed phase, then the organic solvent is emulsified dropwise into the aqueous phase (usually including stabilizers) to form an O/W emulsion [
11]. The emulsion droplet size is easy to control. After that, the emulsions are evaporated, stirred, and extracted to obtain drug nanocrystals. Factors such as emulsifier, stirring speed, evaporation rate, temperature gradient, and pH value have significant effects on product quality. Because this emulsion polymerization method requires the assistance of homogenization or ultrasound, it is suitable for laboratory operations but not for large-scale pilot production [
11]. Chen [
50] obtained florfenicol nanocrystals with a mean particle size of 226.1 ± 11.3 nm using the emulsification solvent volatilization method. Compared with the original powder, the solubility and dissolution of florfenicol nanocrystals were remarkably improved.
2.3. Combinative Technology
There are always many limitations to obtain the desired nanocrystals using a single preparation technology due to the properties of various drugs and characteristics of the instruments. When top-down technology is selectively united with bottom-up technology, forming combinative technology, the disadvantages of a single preparation technique can be overcome and the efficiency of particle size reduction can be improved. Combinative technology is divided into the Nanoedge
® technology developed by Baxter [
51] and the SmartCrystal
® technology (Abbott/Soliqs, Ludwigshafen/Germany). Combinative technology (
Figure 2) combines pre-treatment and a particle size reduction step. It can eliminate the drawbacks of instrument clogging [
52] and improve the stability of nanocrystals. Combinative technology can take full advantage of various technologies and are also suitable for a wide range of insoluble drugs. However, there are limitations in terms of economics and process complexity.
2.3.1. Nanoedge Technology
Nanoedge technology was the first combined method for particle size reduction developed for nanodrug production. It utilizes the HPH method assisted by the precipitation method. Initial crystal particles are obtained by precipitation, reducing the high pressure homogenizer slit blockage and improving the efficiency of reducing particle size during the homogenization process [
53]. Subsequently, the homogenization process from the HPH method is used to further crush the particles, preventing secondary growth and overcoming the problem of uneven particle size distribution and Oswald ripening in the precipitation method, which increases the physical stability of the nanocrystal particles. Furthermore, other alternative techniques such as ultrasound or microfluidization can be used for the high-energy process [
33].
2.3.2. SmartCrystal Technology
SmartCrystal
® technology mainly combines a pre-treatment step followed by a high pressure homogenization step. The pre-treatment step can be, for example, wet bead milling, spray drying, freeze drying, or precipitation, followed by HPH [
54]. SmartCrystal technology is recognized as a second-generation nanocrystal preparation method. Specifically, the joint method in this technology takes the form of collection, which is a toolbox of technology optimization for the preparation of drug nanocrystals [
55]. It includes H69, H42, H96, and combination technology (CT) techniques.
H69 is similar to NanoEdge technology, combining nanoprecipitation and HPH methods. The formation of nanocrystals occurs in the high pressure homogeneous cavitation region, which contributes to ultra-small and homogeneous particle size. Li et al. [
56] tried to prepare ursodeoxycholic acid nanosuspension by homogenization technology and high pressure precipitation tandem homogenization technology. The dissolution rate of ursodeoxycholic acid nanocrystalline powder was quicker than that of the raw and physical mixture powder.
- 2.
H42 and H96
H42 and H96 are combined with spray drying, freeze drying, and HPH techniques, respectively. The solution of insoluble drug and stabilizer is pretreated by spray/freeze drying, uniformly dispersed in the stabilizer skeleton, then redispersed into the water by HPH to prepare drug nanocrystals. The combination reduces particle aggregation and improves processing efficiency. It is suitable for large-scale production. Möschwitzer et al. [
57] used poloxamer 188 as a stabilizer to prepare hydrocortisone acetate powder by H42. More uniform nanosuspensions exhibiting good long-term storage stability were obtained. Yu [
58] prepared meloxicam and naproxen drug nanoamorphous using the H96 technique.
- 3.
CT
CT is a combination of top-down technology. The two most common types of wet bead milling methods used are rotor-stator and mills [
59]. Taking the former as an example, artcrystals is a combined rotor-stator high-speed shear and HPH technology. Firstly, the drug suspension is pretreated by shearing in a rotor-stator high-speed shear, then the nanocrystals are homogenized at high pressure to obtain stable and homogeneous suspensions. Wadhawan et al. [
60] obtained crystalline acyclovir nanocrystals with a mean particle size of 400–500 nm using high pressure homogenizer and hydroxypropyl cellulose-LF as a stabilizer followed by wet bead milling. The saturation solubility of the nanocrystals was 1.6 times higher than that of micronized acyclovir. Martena et al. [
61] prepared nicergoline nanocrystals in aqueous solutions of polysorbate 80. Four different techniques, HPH, bead milling (BM), and combined techniques (HPH + BM, BM + HPH) were explored in his work. The combined technique was found to be superior, but HPH + BM produced nanocrystals with a smaller mean particle size than BM + HPH. Particle solubility increased for all nanocrystals, especially for HPH and the combination technique, which obtained nanocrystals showing a higher dissolution rate.
2.3.3. Other New Combinative Technology
PLH is a combination of precipitation-lyophilization-homogenization method. Morakul et al. [
62] obtained clarithromycin nanocrystals by this method, using poloxamer 407 and sodium dodecyl sulfate (SDS) as co-stabilizers. The obtained clarithromycin nanocrystals were cubic particles, about 400 nm, in a crystalline or partially amorphous state. It had high solubility and permeability.
- 2.
High gravity antisolvent precipitation process (HGAP)
HGCP technology is merged with antisolvent precipitation process to form HGAP. The benefits of the HGCP are retained while the disadvantages of impurities in the product are eliminated [
12]. Zhao et al. [
63] prepared danazol nanocrystals with uniform size distribution by the HGAP process. The average particle size was 190 nm. The molecular state and crystalline form of Danazol nanoparticles were maintained. The nanoparticles were highly evaluated by the industry for its high recovery rate and continuous production capacity.
- 3.
Microjet reactor technology (MRT)
MRT is similar to HPH. The drug solution is mixed in the high pressure chamber through the micro-hole of the nozzle to form a high-speed fluid sprayed into the reaction chamber, and convective shear in the reaction chamber to form turbulence. At the same time, there is cavitation, impact, and shear effect to reduce the product particle size. The influencing factors of MRT include the mixing ratio of solution and antisolvent, jet strength, stabilizer dosage, temperature, etc. This method can realize continuous large-scale production. However, the energy consumption and path clogging [
37] cannot be ignored. Chen et al. [
64] prepared albendazole nanocrystals by MRT with a mean particle size of 367.34 ± 0.68 nm under the optimal preparation process. The nanocrystals can significantly improve the dissolution performance of albendazole and facilitate the improvement of oral absorption of the drug.
- 4.
Evaporative precipitation into aqueous solution (EPAS)
The EPAS method dissolves the API in the low-boiling-point solvent and heats above its boiling point. Thereafter, the heated solution is sprayed into heated aqueous solutions containing stabilizers [
12]. Chen et al. [
65] produced amorphous nanoparticle suspensions of cyclosporine A by EPAS. Due to the low crystallinity, small particle size of nanoparticles, and hydrophilic stabilizers, it has shown a high dissolution rate.
- 5.
Antisolvent precipitation-high pressure homogenization method
Huang et al. [
66] combined the antisolvent precipitation method and HPH method to prepare celecoxib nanocrystal suspensions with a particle size of 283.67 ± 20.84 nm, using polyvinylpyrrolidone K30 (PVP K30) and SDS as crystal stabilizers. The solubility of celecoxib nanocrystals was obviously higher than that of the raw celecoxib and the physical mixture. The product remained quite stable under high temperature and high moisture conditions for 10 days of storage.
- 6.
Ultrasound probe-high pressure homogenization method
Jin et al. [
67] used an ultrasound probe combined with HPH and fluidized drying process to prepare baicalin nanocrystals with an average particle size of 248 ± 6 nm and PDI 0.181 ± 0.065 by selecting mixed surfactant poloxamer 188 as a steric stabilizer and SDS as an electrostatic stabilizer. The results of pharmacokinetic experiments in rats showed that the drug bioavailability
in vivo was significantly improved.
- 7.
Rotary evaporation method-high pressure homogenization method
Zuo [
68] prepared curcumin-artemisinin cocrystal nanomedicine by the rotary evaporation-HPH method. The particle size of nanomedicine was 234.6 nm after optimization. Curcumin-artemisinin cocrystal nanomedicine showed obvious solubility advantages and excellent stability compared with that of raw curcumin, curcumin-artemisinin cocrystals, and curcumin nanocrystals. Yu [
58] also obtained quercetin drug nanoamorphous using the rotary evaporation method assisted by the HPH method.
- 8.
Melt quench-high pressure homogenization method
Yu [
58] also used the combined melt quench-high pressure homogenization technique to prepare nanoamorphous indomethacin. The particle size of the prepared suspension was 245 nm. The solubility of the nanosuspensions was significantly enhanced. However, the stability of the nanoamorphous was poor. The particle size started to increase significantly within 7 days and even reached 890 nm after 30 days due to the presence of water and the occurrence of recrystallization.
- 9.
Antisolvent precipitation-ultrasound method
Zhang et al. [
69] obtained fenofibrate nanocrystals using the ultrasound probe-precipitation method. However, one of the disadvantages of ultrasonic probes is that they can leave metal particles and thus are not suitable for industrial production. Liu et al. [
70] used alpha tocopherol succinate as an auxiliary stabilizer in the organic phase to prepare carvedilol nanosuspensions by this method. The mean particle size of the nanoparticles was 212 nm and it was stable at 25 °C for 1 week. The dissolution rate of the nanosuspension was significantly increased.
In vivo tests indicated that the nanosuspensions showed approximately two-fold increase in each index compared with commercial tablets. Additionally, the method is fast, inexpensive, and easy to control. Paclitaxel nanocrystals [
71], zaleplon nanocrystals [
72], and nintedanib nanocrystals [
73] are also prepared using this method.
3. Characterization and Evaluation
After obtaining nanocrystal products, characterization and performance evaluation are crucial to the further application of the products. On the one hand, the purpose of characterization is to understand the properties of the sample such as size, morphology, and crystalline form. Based on this, the performance of the product can be quantitatively controlled on different quality characteristics, and then the quality controllability of the product can be achieved [
19]. On the other hand, evaluation is to obtain the nanocrystal performance index (stability, cytotoxicity, dissolution rate) to explore more effective drug delivery strategies.
3.1. Characterization
3.1.1. Particle Size and Distribution
The particle size range of drug nanocrystals has a certain size requirement, which is generally less than 1 μm. Therefore, it is very important for drug nanocrystals to precisely control the particle size of the drug to obtain narrow and uniform particle size distribution. The particle size not only affects the drug loading and release behavior of API, but also is closely related to pharmacokinetics, biodistribution, and even the delivery mechanism of drug nanocrystals. To summarize, particle size and distribution are considered to be the most important index parameters of nanocrystals, which is one of the key elements in the development and control quality of nanocrystals [
41].
The particle size and distribution can be evaluated with the help of offline or online evaluation tools. The measurement is usually performed by dynamic light scattering (DLS), also known as photon correlation spectroscopy (PCS). It observes Brownian motion using laser irradiation of particles and relates Brownian motion to particle size by analyzing the light intensity fluctuations of the scattered light. The measurement result is the hydrodynamic particle size, and the particle size distribution is generally expressed using PDI. In general, a PDI value of 0.1–0.25 represents a narrow particle size distribution and also indicates that the nanocrystal system is stable [
74]. DLS can obtain accurate and statistical particle size distributions, but it is necessary to make nanocrystals into well-dispersed suspensions. From the variation of Z-average and PDI values, small increases in drug nanocrystal size can also be assessed by DLS. Therefore, DLS is considered effective for measuring the particle size of submicron and nanoparticles.
Laser Diffraction (LD) analyzers are designed based on the phenomenon of light diffraction, which occurs when light passes through particles and the angle of the diffracted light is inversely proportional to the size of the particles. Typical characterization parameters of LD are 50%, 90%, and 99% of the diameter, expressed as D50, D90, and D99, respectively (i.e., D50 means that 50% of the particle volume is below a given size). The measurement range of LD and PCS varies, where LD is generally used to measure particle sizes larger than 0.05 μm, while PCS usually gives the practical size in the range from 3 nm to 3 μm [
75]. Furthermore, it is important to note that the data of particle size acquired by LD and PCS for nanosuspensions are different because the data from LD is volume-based, while PCS gives the average particle size based on light intensity-weighted particle size [
2].
Small-angle X-ray scattering (SAXS) uses the X-ray small-angle scattering effect to measure the particle size distribution of nanocrystals. This is a simple method with high accuracy, but it is also relatively expensive. Typically, it can measure the size distribution of particles in the range of 1–300 nm.
3.1.2. Morphological Characterization
Both the size and morphological state of nanoparticles can influence the structural properties of nanocrystals. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) are frequently applied to characterize the morphological appearance of drug nanocrystals. It should be mentioned that some people use electron microscopy images supplemented with statistics to obtain the mean value of the particle size of nanocrystals, which is the actual particle size. In contrast, the previous section obtained by DLS or LD is only the hydrodynamic size of the nanoparticles.
SEM uses narrow-focused beams of high energy electrons to scan the sample for the purpose of characterizing the microscopic morphology of the substance. The imaging stereo effect is good, and the resolution can reach 1 nm. Therefore, it is now widely used to observe nanomaterials. In particular, when formulated nanosuspensions are converted into dry powders, SEM analysis is critical to monitor changes in particle shape and size. SEM images of different nanocrystals obtained in the literature are as follows (
Figure 3).
TEM uses an electron beam as the light source to project accelerated and aggregated electron beams onto the very thin sample. In order for the electron beam to penetrate, the thickness of the sample should be less than 100 nm. TEM requires an appropriate concentration of the wet sample. The current resolution is up to 0.2 nm. TEM images of different nanocrystals obtained in the literature are as follows (
Figure 4).
AFM is a new generation of scanning probe microscopy. It is capable of imaging in any environment (including liquids), and the low force of the needle tip on the sample surface prevents damage to the sample. It does not require the sample to be electrically conductive. The sample can be directly observed at the nanoscale without special treatment. A three-dimensional image of the sample surface can be obtained by collecting feedback signals from the force applied to the sample by the probe [
80]. AFM can also provide information on the shape and structure of nanocrystals that cannot be accessed by other methods [
81]. Overall, AFM has become an important tool for conducting real-time observations at the nanoscale.
3.1.3. Structural Characterization
The crystalline form is also a quality factor of nanocrystal that needs to be noted. During the process of nanosizing, the crystalline state of drug particles may change, such as from crystalline state to amorphous state, which has a significant difference in physical properties, such as density, hardness, solubility, and stability. Therefore, the structure characterizations of nanocrystal need to be carefully considered.
Differential scanning calorimetry (DSC) is a common method for thermal analysis of nanocrystals. DSC measures the crystallinity of drug nanoparticles by detecting the glass transition temperature, melting point, and associated enthalpy. DSC also can be used to determine the interactions between excipients and drugs in nanocrystals as well as numerous thermodynamic and kinetic parameters [
2]. The method has a wide temperature range and high resolution so that it has an indestructible place in structural characterization. In addition, thermal analysis can be investigated by thermogravimetric measurements or differential thermal analysis (DTA). Thermogravimetric analysis is a technique to study the thermal stability, heat flow, and structural deformation of particles in the inert environment [
82]. DTA can be used for phase changes and other thermal processes, such as the determination of melting points [
83]. In combination, thermogravimetric and differential thermal analysis (TG-DTA) can characterize multiple thermal properties of a nanocrystal sample at the same time. It is valuable for the investigation of thermal stability as well as for the determination of volatile content and other compositional analyses [
2]. X-ray powder diffraction (XPRD) analysis based on constructive interference between monochromatic X-rays and samples, is another tool for characterizing the crystallinity of nanocrystals [
52]. It can be used to determine the structural parameters and crystal structure of some crystalline substances, as well as amorphous substances.
Fourier transform infrared spectroscopy (FTIR) is a method of measuring interferograms and performing Fourier transform on the interferograms to measure infrared spectra with high resolution. FTIR can study the interaction between drugs and excipients in nanocrystal formulations at the molecular level. When the two components interact, FTIR spectroscopy shows the interaction by changing the vibrational frequency of the molecules [
84].
3.1.4. Surface Property
The particle surface charge situation is closely related to the stability of drug nanocrystals [
85], which is generally reflected by the zeta potential (ZP) values. Currently, the measurement methods include electrophoresis, electroosmosis, flow potential, and ultrasound methods, among which laser Doppler electrophoresis is commonly used for measurement. The higher the ZP values, the greater the electrostatic repulsion among the particles, and the more stable the system. Usually, when the particles have sufficient ZP values, it will provide effective charge repulsion to prevent particle aggregation. In general, an absolute ZP value above 30 mV provides great stability and about 20 mV provides only short-term stability. However, for larger molecular weights of APIs, the stabilizer acts mainly through steric stabilization. In this case, a ZP value of only 20 mV or even less can provide sufficient stabilization [
86].
3.2. Performance Evaluation
3.2.1. Stability
The stability of nanoparticles is essential for the generation of nanosized effects. The stability of nanocrystals includes chemical stability (degradation and spoilage) and physical stability (sedimentation, agglomeration, crystal growth, and crystalline state) [
87]. The large specific surface area of nanocrystals results in high free energy, which may result in physical instability, such as aggregation or agglomeration [
88]. Therefore, surfactants/polymers are necessary to stabilize the nanocrystals. The choices of stabilizers can also affect the performances of drug nanocrystals
in vivo and further formulations.
Stabilizers can mainly be divided into ionic and nonionic stabilizers (
Table 1). One mechanism by which stabilizers work is to reduce the surface tension at the interphase interface, where electrostatic repulsion from ionic surfactants stabilizes the nanosuspension. The other mechanism is steric stabilization, where polymers cover the particles to prevent particle-to-particle aggregation [
89]. The basic principles that must be followed in the selection of stabilizers are that the addition dose must be acceptable and safe to humans. For instance, stabilizers will not cause any allergic or immune response [
90]. The current screening of surfactants/stabilizers is primarily based on reported references and experience. Experience indicates that binary or ternary mixtures of electrostatic stabilizers typically generate enhanced stabilization [
2]. The stability is generally divided into long-term and short-term stability, which can be measured by the size, ZP values, and morphology of the nanocrystals [
91].
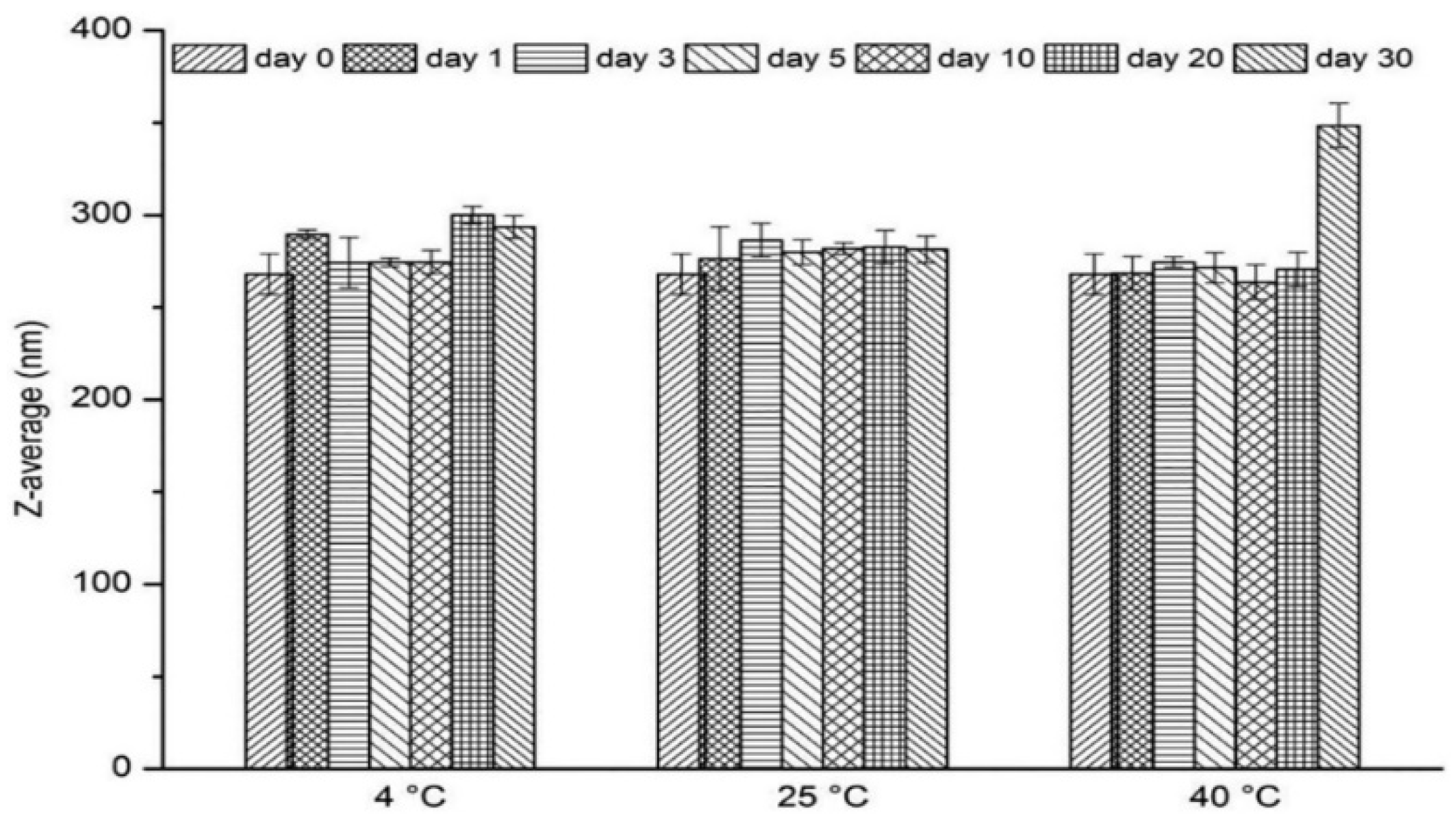
Guo et al. [
92] prepared nitrendipine nanocrystals (NTD-NCs) using a media milling method. Rectangular nanocrystals with 1.25% (
w/
v) HPMC-E5 and 0.4% (
w/
v) SDS as stabilizers were obtained with a particle size of 256.5 ± 6.6 nm. The size of the nanocrystals was monitored for 30 days (at 4 °C, 25 °C, and 40 °C). As shown in
Figure 5, there was no change in monitoring results after 30 days of storing at 25 ± 2 °C (
p > 0.05). When the product was stored at 4 ± 2 °C, it was noticed that there was a slight increase in particle size within 20 days. It is speculated to be due to recrystallization of free drug molecules from the existing larger crystal surfaces. When the product was stored at 4 ± 2 °C, a significant increase in particle size occurred. It is speculated that the Ostwald ripening phenomenon occurred. The particle sizes of the freshly prepared nanocrystals were monitored at 4 °C, 25 °C, and 40 °C for 30 days. As shown in
Figure 5, the particle size remained constant after storing at 25 ± 2 °C for 30 days (
p > 0.05). When stored at 4 ± 2 °C, a slight increase of particle size was observed from 268 nm to 300 nm for 20 days. This may be due to the recrystallization of free drug molecules on the surface of existing larger crystals. A significant increase in particle size was found at 40 ± 2 °C, where the Ostwald ripening phenomenon may have occurred. This indicates that the nanocrystals are unstable at higher temperatures. Therefore, appropriate temperature conditions are critical for the storage of NTD-NCs.
Al Shaal et al. [
93] prepared apigenin nanocrystals and assessed the long-term physical stability. The nanocrystals were stored under different conditions (refrigerated, room temperature, and 40 °C) for 6 months. According to PCS and LD data, neither significant increase in particle size nor change in particle size distribution can be found. No clearly visible aggregation was seen for the formulations on the day of production and after 6 months of storage at room temperature.
Luo et al. [
94] prepared silymarin nanocrystal micro pills with PVP K30 and SDS as stabilizers. The produced nanocrystal micro pills were placed in a weighing open bottle for 10 days under high temperature/humidity/light conditions. The samples were taken on days 0, 5, and 10, respectively. The results showed that no significant changes occurred but the samples should be stored in a dry environment due to slight moisture absorption under high humidity conditions.
3.2.2. Cytotoxicity
Safety is the primary concern for pharmaceuticals, so toxicity assessment is a priority for new drug registration [
90]. Cytotoxicity assays are assessed by measuring the number or growth of cells before and after exposure to the sample [
95]. It can be divided into leachate test, direct contact test, and indirect contact test. Cell viability is often used as an important evaluation indicator. Cytotoxicity experiments can provide the prerequisites for
in vivo animal experiments, so it plays a vital role in the evaluation of drugs. Additionally, embryo toxicity assessment is more rapid than whole organism methods (<96 h).
Sheng et al. [
96] prepared oridonin nanocrystals (ORI-NCs) and studied the
in vitro cytotoxicity of ORI-NCs on Madin-Darby canine kidney (MDCK) cells. Both ORI and ORI-NCs exhibited an effect on inhibiting MDCK cell proliferation. The decrease in cell viability with increasing concentration indicates that ORI-NCs and ORI drugs exert concentration-dependent effects on MDCK cells. With increasing concentration, the cell viability of ORI-NCs decreased more significantly than that of ORI.
Choi [
97] explored the cytotoxicity characteristics of cilostazol nanocrystals (CLT-NCs) in the Caco-2 cells. CLT-NC was successfully prepared by the ultrasonic probe method with a mean size of 500–600 nm. Cytotoxicity of CLT-NC and CLT was assessed in Caco-2 cells. The experimental results demonstrated that all CLT concentrations tested (0.02–20.0 µg/mL) did not exhibit toxicity (
Figure 6a). Therefore, CLT-NC is safe for cells at the related concentrations.
Liu et al. [
98] obtained ginkgolide B nanocrystals (GB-NCs), a potent anti-parkinsonism compound. The toxicity assays were done in zebrafish embryos. The zebrafish embryos showed no morphological changes following GB-NCs treatment (
Figure 6b), and no differences in hatching, heart rate, body length, or survival occurred at 96 h post-fertilization (hpf) in GB-NCs relative to control groups (
Figure 6c–f). These data confirmed that the GB-NCs are non-toxic to zebrafish.
3.2.3. In Vitro Dissolution
Dissolution or release of drugs is an important quality attribute of nanomedicines, which may have significant effects on drug absorption,
in vivo safety and efficacy.
In vitro dissolution or release can reflect the
in vivo behavior of nanomedicines to some extent [
41]. The flow cell method is often used to study the dissolution or release of drugs
in vitro. A constant-flow pump is used to pump the release medium at the desired temperature into contact with the sample at the lower end of the flow cell at a suitable flow rate, and the release medium is filtered through the upper end of the flow cell to measure the concentration of the drug in the release medium at set time intervals [
99]. Parameters such as dissolution rate and saturation solubility were determined to help predict the
in vivo performance of drug nanocrystals. To a large extent, the dissolution rate depends on the size and surface area of the drug particles. The most important evaluation indicator is the dissolution curve, which can distinguish differences in product bioequivalence.
Zhu et al. [
73] fabricated rod-shaped Nintedanib nanocrystals (BIBF-NCs) by the antisolvent precipitation-ultrasonic method with sodium carboxymethylcellulose as a stabilizer. The particle size was 325.30 ± 1.03 nm and the zeta potential was 32.70 ± 1.24 mV. Then, the
in vitro dissolution of BIBF powder and BIBF-NCs in pH 6.8 PBS during 120 min were explored. Compared with the dissolution rate of BIBF powder at 10 min ((6.10 ± 1.55)%,
p < 0.05), the BIBF-NCs was (73.74 ± 5.33)%. At 120 min both still exhibited large dissolution rate differences (
Figure 7a). This phenomenon is attributed to smaller particle size reducing the diffusion layer thickness and increasing the surface area available for dissolution. Meanwhile, BIBF-NCs (rod-shape) have a larger surface area, resulting in higher dissolution rates. Additionally, the cumulative release of the drug in the gastrointestinal tract was simulated. The results showed that the cumulative release of BIBF-NCs was significantly higher than that of BIBF crude powder (
Figure 7b).
3.2.4. In Vivo Dissolution
In order to observe the functional, metabolic, and morphological changes caused by drug nanocrystals, it is often necessary to deliver the drugs to animals. There are various ways of drug delivery with an emphasis on oral and injection. After the intervention with the drug, the corresponding tissues are taken for index testing. For example, the drug concentration in blood and certain organs is measured. Parameters such as dissolution rate, bioavailability, or relative bioavailability are calculated to study the in vivo effects of nanocrystals. Other qualitative and quantitative means such as detection of lesion sites can also be used. Further in vivo pharmacokinetic and pharmacodynamic investigations can be performed to calculate the key parameters of peak concentration, time to peak, and area under the curve (AUC).
Soroushnia et al. [
100] prepared midazolam nanosuspensions with a particle size of 197 ± 7 nm and zeta potential of 31 ± 4 mV using an ultrasound technique. Then,
in vivo tests were carried out in rabbits. Plasma concentration-time curves (
Figure 8) and pharmacokinetic parameters (
Table 2) were obtained for the midazolam nanosuspensions. In the
in vivo evaluation, a higher Cmax (111.90% higher), a higher AUC0-t (275.08%), and a shorter Tmax (15 min) were observed for the midazolam nanosuspension, indicating that the midazolam nanosuspension is more readily absorbed.
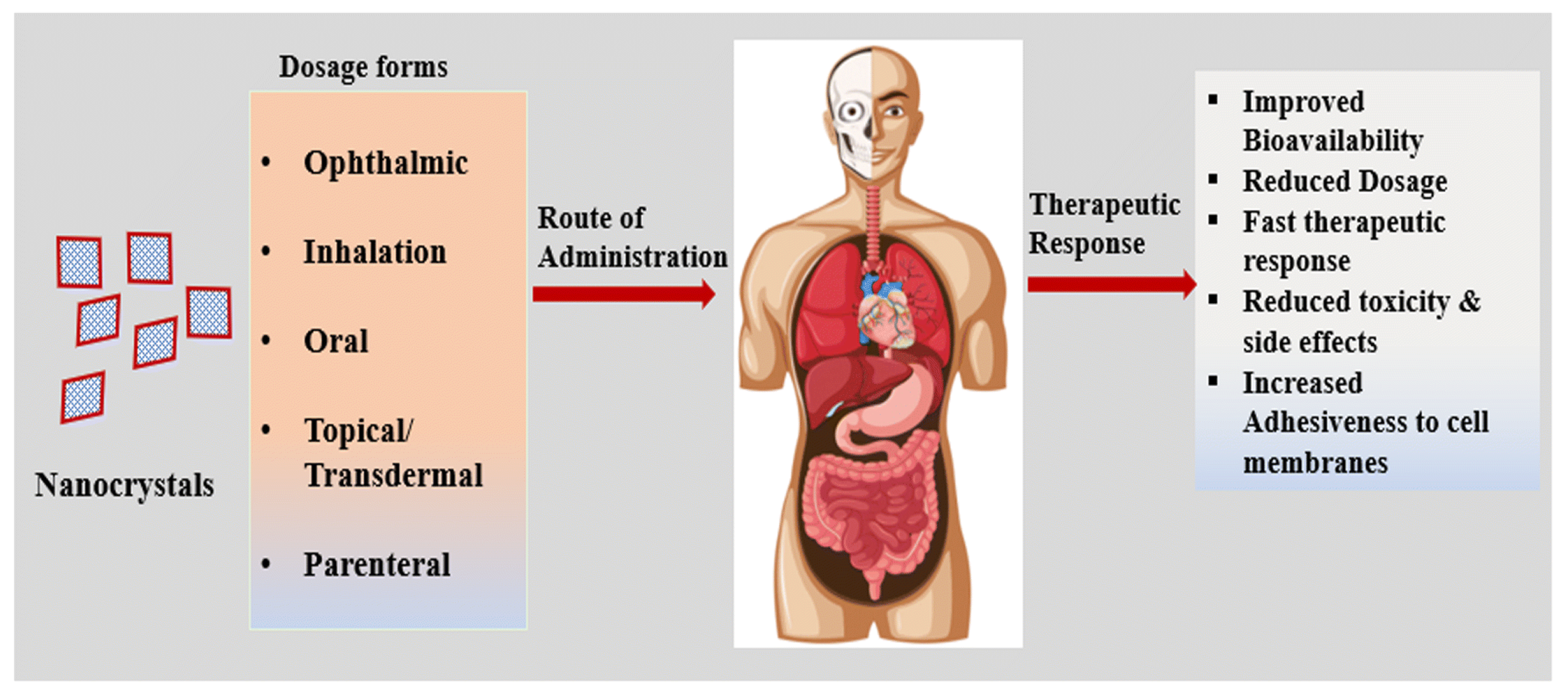
4. Applications
Nanocrystal formulations can be administered by various routes (
Figure 9), where oral routes of drug delivery account for more than 60% of current drug nanocrystal delivery routes [
101,
102]. Nanocrystal-based formulations (
Table 3) are widely used to treat cancer, inflammatory, cardiovascular, depression, and other diseases [
35].
The oral route is considered to be one of the most appropriate, safe, and preferred routes [
103]. During oral administration, dissolution of the nanocrystals begins in the intestine, where the drug particles are slightly absorbed by the cells. In contrast, cellular interactions and drug uptake play a key role in nanocrystals administered via parenteral routes. It can enhance the bioavailability, reduce toxicity, and release in specific targeting sites, such as the brain, lung, liver, kidney, or colon [
4]. For pulmonary administration, the nebulized nanosuspension had a significantly higher inhalable fraction and also showed stronger mucus adhesion. Ocularly administered nanodropable dosage forms also have high drug loads and long-lasting drug effects [
35]. In conclusion, the application and combination of various routes of drug administration will provide the basis for nanocrystals to play an increasingly important role in disease treatment.
5. Conclusions and Perspective
In this work, we reviewed the preparation method for drug nanocrystals, containing top-down (e.g., WBM, HPH), bottom-up (e.g., LAS, SCF), and combinative (e.g., Nanoedge, SmartCrystal) technology. After that, characterization methods and application scope of nanocrystals including DLS, SEM, XPRD, etc. were discussed. Next, evaluation paths and examples on stability, cytotoxicity, and in vitro/in vivo dissolution rate were introduced, which can reflect the practical performance of drug nanocrystals. Lastly, the applicability of drug nanocrystals was broadly demonstrated by various routes (e.g., oral, parenteral).
Although great progress has been made in nanocrystal technology, there are still many difficulties to be faced. For drug nanocrystal preparation technology, it is necessary to continuously improve the productive efficiency and stability of nanocrystals. For this purpose, the continuous strengthening of basic research on nanocrystal preparation, such as the optimization of nanomedicine formulations and process parameters is required. Furthermore, on industrial production of nanocrystals, the transferability from laboratory scale to industrial scale should be seriously considered.
What follows is the characterization and evaluation of nanocrystal products including the properties of the nanoparticles themselves and in vivo/in vitro behaviors. Until now, the release of nanocrystals is not yet fully understood. More detailed preclinical in vivo experiments are needed to explain the mechanism of transportation and absorption of nanocrystals in vivo, both for efficacy and safety. Additionally, more robust and in-depth in vitro/in vivo correlation evaluations are also required.
In addition, for specific applications area of nanocrystals, the high drug-carrying capacity of nanocrystals makes them a hot spot for the study of targeted insoluble drug formulations. However, further research is still needed on targeted drug nanocrystal delivery formulations, rates, and mechanisms.
Overall, nanocrystal technology has the potential for industrial applications and the ability to take on insolubility problems. This makes it a prospering technology for optimizing the bioavailability of insoluble drugs. Therefore, continuous efforts are needed to introduce drug nanocrystals into the pharmaceutical market.
Author Contributions
Investigation, Q.R., M.W. and J.O.; writing—original draft preparation, Q.R.; writing—review, D.H. and Z.G.; writing—modifications, Q.R., M.W. and W.K.; supervision, J.O. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Shandong Provincial Key R&D Program (Major Key Technology Project) 2021CXGC01051 and Academic and technical leader training program for major disciplines in Jiangxi Province (20212BCJ23001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was financially supported by Shandong Provincial Key R&D Program (Major Key Technology Project) 2021CXGC01051 and Academic and technical leader training program for major disciplines in Jiangxi Province (20212BCJ23001). The financial support of Haihe Laboratory of Sustainable Chemical Transformations and the Institute of Shaoxing, Tianjin University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Chogale, M.M.; Ghodake, V.N.; Patravale, V.B. Performance parameters and characterizations of nanocrystals: A brief review. Pharmaceutics 2016, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Nair, A.; Kuentz, M.; Dressman, J.; Saal, C. Calculation of drug-polymer mixing enthalpy as a new screening method of precipitation inhibitors for supersaturating pharmaceutical formulations. Eur. J. Pharm. Sci. 2019, 132, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, I.S.; Hu, H.; Yin, L.; He, W. Drug nanocrystals: Fabrication methods and promising therapeutic applications. Int. J. Pharm. 2019, 562, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Dressman, J.B. The Developability Classification System: Application of Biopharmaceutics Concepts to Formulation Development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef]
- Al-Kassas, R.; Bansal, M.; Shaw, J. Nanosizing techniques for improving bioavailability of drugs. J. Control. Release 2017, 260, 202–212. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Kumar, S.; Bhargava, D.; Thakkar, A.; Arora, S. Drug carrier systems for solubility enhancement of BCS class II drugs: A critical review. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 217–256. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals—Versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef]
- Zheng, A.; Shi, J. Research progress in nanocrystal drugs. J. Int. Pharm. Res. 2012, 39, 177–183. [Google Scholar]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing--oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, X.; Zhu, Q.; Wu, W.; Chen, Z.; Li, Y.; Lu, Y. Enhanced transdermal delivery of meloxicam by nanocrystals: Preparation, in vitro and in vivo evaluation. Asian J. Pharm. 2018, 13, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Liu, Y.; Xie, J.; Chen, Y.; Yang, M. Review and prospect on preparation technology of drug nanocrystals in the past thirty years. Acta Pharm. Sin. 2018, 53, 529–537. [Google Scholar]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of Drug Nanocrystal Technologies on Oral Drug Delivery of Poorly Soluble Drugs. Pharm. Res. 2013, 30, 307–324. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.; Dong, X.; Zhao, W.; Wu, W. The in vivo fate of nanocrystals. Drug Discov. Today 2017, 22, 744–750. [Google Scholar] [CrossRef]
- Mou, D.; Liao, Y.; Zhou, X.; Wan, J.; Yang, X. Advances in Nanocrystal Medicine. Her. Med. 2020, 39, 1257–1261. [Google Scholar]
- Zhou, Y.; Du, J.; Wang, L.; Wang, Y. Nanocrystals Technology for Improving Bioavailability of Poorly Soluble Drugs: A Mini-Review. J. Nanosci. Nanotechnol. 2017, 17, 18–28. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Wu, W. Injected nanocrystals for targeted drug delivery. Acta Pharm. Sin. B 2016, 6, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Bitterlich, A.; Laabs, C.; Krautstrunk, I.; Dengler, M.; Juhnke, M.; Grandeury, A.; Bunjes, H.; Kwade, A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur. J. Pharm. Biopharm. 2015, 92, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, W.; Lu, Y. What is the future for nanocrystal-based drug-delivery systems? Ther. Deliv. 2020, 11, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Patel, M.M. Preparation and Characterization of Nanoparticles for Solubility and Dissolution Rate Enhancement of Meloxicam. Int. Res. J. Pharm. 2011, 1, 42–49. [Google Scholar]
- Liu, T.; Müller, R.H.; Möschwitzer, J.P. Effect of drug physico-chemical properties on the efficiency of top-down process and characterization of nanosuspension. Expert Opin. Drug Deliv. 2015, 12, 1741–1754. [Google Scholar] [CrossRef]
- Jarvis, M.; Krishnan, V.; Mitragotri, S. Nanocrystals: A perspective on translational research and clinical studies. Bioeng. Transl. Med. 2019, 4, 5–16. [Google Scholar] [CrossRef]
- Funahashi, I.; Kondo, K.; Ito, Y.; Yamada, M.; Niwa, T. Novel contamination-free wet milling technique using ice beads for poorly water-soluble compounds. Int. J. Pharm. 2019, 563, 413–425. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Li, M.; Qian, H. Research progress of nanomedicine. Pharm. Clin. Res. 2020, 28, 51–55. [Google Scholar]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Müller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef]
- Raghava Srivalli, K.M.; Mishra, B. Drug nanocrystals: A way toward scale-up. Saudi Pharm. J. 2016, 24, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Cheng, M.; Liu, Q.; Liu, W.; Gao, C.; Feng, J.; Jin, Y.; Tu, L. Improving Oral Bioavailability of Luteolin Nanocrystals by Surface Modification of Sodium Dodecyl Sulfate. AAPS PharmSciTech 2021, 22, 133. [Google Scholar] [CrossRef]
- Joshi, K.; Chandra, A.; Jain, K.; Talegaonkar, S. Nanocrystalization: An Emerging Technology to Enhance the Bioavailability of Poorly Soluble Drugs. Pharm. Nanotechnol. 2019, 7, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Asahi, T.; Masuhara, H. Formation of 10 nm-sized Oxo(phtalocyaninato)vanadium(IV) Particles by Femtosecond Laser Ablation in Water. Chem. Lett. 2004, 33, 724–725. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, Y.; Zhang, Z.; Zhang, H.; Gao, X. Research progress on preparation technology of nanocrystal drugs. Acta Pharm. Sin. 2021, 56, 1902–1910. [Google Scholar]
- Guo, Z.; Zhang, M.; Li, H.; Wang, J.; Kougoulos, E. Effect of ultrasound on anti-solvent crystallization process. J. Cryst. Growth 2005, 273, 555–563. [Google Scholar] [CrossRef]
- Chen, J. The Pharmacokinetics Evaluation and Preparation of Hydrochlorothiazide and Valsartan Nanocrystals. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2016. [Google Scholar]
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181–182, 1–34. [Google Scholar] [CrossRef]
- Technical Guidelines for Nanomedicine Quality Control Research (for Trial Implementation). Available online: https://www.nmpa.gov.cn/ (accessed on 18 March 2021).
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Ding, Y.; Kang, B.; Wang, J. Preparation and in vitro evaluation of budesonide inhalation nanosuspension. Chin. J. Pharm. 2017, 48, 1131–1137. [Google Scholar]
- Han, X.; Wang, M.; Ma, Z.; Xue, P.; Wang, Y. A new approach to produce drug nanosuspensions CO2-assisted effervescence to produce drug nanosuspensions. Colloids Surf. B. Biointerfaces 2016, 143, 107–110. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Wang, J.; Wang, Y. Preparation, characterization and in vivo evaluation of amorphous tacrolimus nanosuspensions produced using CO2-assisted in situ nanoamorphization method. Int. J. Pharm. 2016, 505, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Wang, Y.H.; Guo, F.; Wang, X.M.; Zheng, C. Synthesis of Nanoparticles with Novel Technology: High-Gravity Reactive Precipitation. Ind. Eng. Chem. Res. 2000, 39, 948–954. [Google Scholar] [CrossRef]
- Chiou, H.; Li, L.; Hu, T.; Chan, H.-K.; Chen, J.-F.; Yun, J. Production of salbutamol sulfate for inhalation by high-gravity controlled antisolvent precipitation. Int. J. Pharm. 2007, 331, 93–98. [Google Scholar] [CrossRef]
- Yin, Y.; Deng, H.; Wu, K.; He, B.; Dai, W.; Zhang, H.; Le, Y.; Wang, X.; Zhang, Q. A multiaspect study on transcytosis mechanism of sorafenib nanogranules engineered by high-gravity antisolvent precipitation. J. Control. Release 2020, 323, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Development and Bioavailability Evaluation in Hens of Florfenicol Nanocrystals. Master’s Thesis, Northwest A&F University, Xianyang, China, 2021. [Google Scholar]
- Kipp, J.E.; Wong, J.C.T.; Doty, M.J.; Rebbeck, C.L. Microprecipitation Method for Preparing Submicron Suspensions. 2001. Available online: https://patents.google.com/patent/US7037528B2/en (accessed on 18 March 2021).
- Pardhi, V.P.; Verma, T.; Flora, S.J.S.; Chandasana, H.; Shukla, R. Nanocrystals: An Overview of Fabrication, Characterization and Therapeutic Applications in Drug Delivery. Curr. Pharm. Des. 2018, 24, 5129–5146. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Romero, G.B.; Chen, R.; Keck, C.M.; Müller, R.H. Industrial concentrates of dermal hesperidin smartCrystals®—Production, characterization & long-term stability. Int. J. Pharm. 2015, 482, 54–60. [Google Scholar]
- Gholap, A.; Borude, S.; Mahajan, A.; Amol, M.; Gholap, D. Smart Crystals Technology: A Review. Pharmacologyonline 2011, 3, 238–243. [Google Scholar]
- Li, Y.; Wang, Y.; Yue, P.F.; Hu, P.Y.; Wu, Z.F.; Yang, M.; Yuan, H.L. A novel high-pressure precipitation tandem homogenization technology for drug nanocrystals production—A case study with ursodeoxycholic acid. Pharm. Dev. Technol. 2014, 19, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J.; Müller, R.H. New method for the effective production of ultrafine drug nanocrystals. J. Nanosci. Nanotechnol. 2006, 6, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. Preparation and Formulation of Novel Drug Nanoparticles Based on High Efficient Solubilization. Master’s Thesis, Qingdao University of Science & Technology, Qingdao, China, 2021. [Google Scholar]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Wadhawan, J.; Parmar, P.K.; Bansal, A.K. Nanocrystals for improved topical delivery of medium soluble drug: A case study of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 65, 102662. [Google Scholar] [CrossRef]
- Martena, V.; Shegokar, R.; Di Martino, P.; Müller, R.H. Effect of four different size reduction methods on the particle size, solubility enhancement and physical stability of nicergoline nanocrystals. Drug Dev. Ind. Pharm. 2014, 40, 1199–1205. [Google Scholar] [CrossRef]
- Morakul, B.; Suksiriworapong, J.; Chomnawang, M.T.; Langguth, P.; Junyaprasert, V.B. Dissolution enhancement and in vitro performance of clarithromycin nanocrystals produced by precipitation–lyophilization–homogenization method. Eur. J. Pharm. Biopharm. 2014, 88, 886–896. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Zhang, H.; Shen, Z.; Yun, J.; Chen, J. Facile Preparation of Danazol Nanoparticles by High-Gravity Anti-solvent Precipitation (HGAP) Method. Chin. J. Chem. Eng. 2009, 17, 318–323. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Tian, C.; Ran, X.; Zhu, L.; Chen, B.; Zhao, J. Preparation and characterization of albendazole nanocrystals. Chin. J. Hosp. Pharm. 2020, 40, 260–264. [Google Scholar]
- Chen, X.; Young, T.J.; Sarkari, M.; Williams, R.O.; Johnston, K.P. Preparation of cyclosporine A nanoparticles by evaporative precipitation into aqueous solution. Int. J. Pharm. 2002, 242, 3–14. [Google Scholar] [CrossRef]
- Huang, T.; Lin, Q.; Qian, Y.; Xu, X.; Zhou, J. Preparation and Characteristics of Celecoxib Nanocrystals. Chin. J. Pharm. 2015, 46, 358–363. [Google Scholar]
- Jin, S.; Yuan, H.; Jin, S.; Lv, Q.; Bai, J.; Han, J. Preparationof baicalin nanocrystal pellets and preliminary study on its pharmacokinetics. China J. Chin. Mater. Med. 2013, 38, 1156–1159. [Google Scholar]
- Zuo, X. Preparation of New Nnanopharmaceutical Combination Method and Its Preparation. Master’s Thesis, Qingdao University of Science & Technology, Qingdao, China, 2019. [Google Scholar]
- Zhang, H.; Meng, Y.; Wang, X.; Dai, W.; Wang, X.; Zhang, Q. Pharmaceutical and pharmacokinetic characteristics of different types of fenofibrate nanocrystals prepared by different bottom-up approaches. Drug Deliv. 2014, 21, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, H.; Tian, B.; Yuan, K.; Pan, H.; Ma, S.; Yang, X.; Pan, W. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation-ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech 2012, 13, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.-h.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef]
- Latif, R.; Makar, R.R.; Hosni, E.A.; El Gazayerly, O.N. The potential of intranasal delivery of nanocrystals in powder form on the improvement of zaleplon performance: In-vitro, in-vivo assessment. Drug Dev. Ind. Pharm. 2021, 47, 268–279. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Y.; Zhang, A.; Wang, X.; Zhao, Z.; Zhang, Y.; Yin, T.; Gou, J.; Wang, Y.; He, H.; et al. Rod-shaped nintedanib nanocrystals improved oral bioavailability through multiple intestinal absorption pathways. Eur. J. Pharm. Sci. 2022, 168, 106047. [Google Scholar] [CrossRef]
- Shah, S.M.H.; Ullah, F.; Khan, S.; Shah, S.M.M.; Isreb, M. Fabrication and Evaluation of Smart Nanocrystals of Artemisinin for Antimalarial and Antibacterial Efficacy. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 251–262. [Google Scholar] [CrossRef]
- Pu, X.; Sun, J.; Li, M.; He, Z. Formulation of Nanosuspensions as a New Approach for the Delivery of Poorly Soluble Drugs. Curr. Nanosci. 2009, 5, 417–427. [Google Scholar] [CrossRef]
- Soisuwan, S.; Teeranachaideekul, V.; Wongrakpanich, A.; Langguth, P.; Junyaprasert, V.B. In vitro performances and cellular uptake of clarithromycin nanocrystals produced by media milling technique. Powder Technol. 2018, 338, 471–480. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, Y.; Luo, Y.; Liu, Y.; Tu, L.; Ma, Y.; Yue, P.; Yang, M. Design and evaluation of inhalable nanocrystals embedded microparticles with enhanced redispersibility and bioavailability for breviscapine. Powder Technol. 2021, 377, 128–138. [Google Scholar] [CrossRef]
- Wang, Y.; Xuan, J.; Zhao, G.; Wang, D.; Ying, N.; Zhuang, J. Improving stability and oral bioavailability of hydroxycamptothecin via nanocrystals in microparticles (NCs/MPs) technology. Int. J. Pharm. 2021, 604, 120729. [Google Scholar] [CrossRef] [PubMed]
- Ančić, D.; Oršolić, N.; Odeh, D.; Tomašević, M.; Pepić, I.; Ramić, S. Resveratrol and its nanocrystals: A promising approach for cancer therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.T.; Thomas, B.R.; Vekilov, P.G. Molecular mechanisms of crystallization and defect formation. Phys. Rev. Lett. 2000, 85, 353–356. [Google Scholar] [CrossRef]
- Du, J.; Li, X.; Zhao, H.; Zhou, Y.; Wang, L.; Tian, S.; Wang, Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int. J. Pharm. 2015, 495, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Parhi, B.; Bharatiya, D.; Swain, S.K. Application of quercetin flavonoid based hybrid nanocomposites: A review. Saudi Pharm. J. 2020, 28, 1719–1732. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Tear, S.P.; Lewis, J.; David, H.; Hassellöv, M. Detection and characterization of engineered nanoparticles in food and the environment. Food Addit. Contam. Part A 2008, 25, 795–821. [Google Scholar] [CrossRef]
- Doyle, W.M. Principles and Applications of Fourier Transform Infra-Red (FTIR) Process Analysis. 2017. Available online: https://www.semanticscholar.org/paper/Principles-and-Applications-of-Fourier-Transform-(-Doyle/8b9108726fe76043badeecd1c75ed6e72352b8a1 (accessed on 18 March 2021).
- Gao, Q. Preparation and Characterization of ITZ Capsules Based on Nanocrystal Technology. Master’s Thesis, Hebei University of Science and Technology, Shijiazhuang, China, 2015. [Google Scholar]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Agarwal, V.; Kaushik, N.; Sharma, P.K. Nanocrystal Approaches for Poorly Soluble Drugs and their Role in Development of Marketed Formulation. Drug Deliv. Lett. 2021, 11, 275–294. [Google Scholar] [CrossRef]
- Blom, K.; Senkowski, W.; Jarvius, M.; Berglund, M.; Rubin, J.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknäs, M.; et al. The anticancer effect of mebendazole may be due to M1 monocyte/macrophage activation via ERK1/2 and TLR8-dependent inflammasome activation. Immunopharmacol. Immunotoxicol. 2017, 39, 199–210. [Google Scholar] [CrossRef]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical nanocrystals: A promising approach for improved topical drug delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-c.; Dong, L.; Jia, A.; Chang, X.-m.; Xue, H. Preparation of solid lipid nanoparticles loaded with traditional Chinese medicine by high-pressure homogenization. J. South. Med. Univ. 2006, 26, 541–544. [Google Scholar]
- Guo, M.; Dong, Y.; Wang, Y.; Ma, M.; He, Z.; Fu, Q. Fabrication, characterization, stability and in vitro evaluation of nitrendipine nanocrystals by media milling. Powder Technol. 2019, 358, 20–28. [Google Scholar] [CrossRef]
- Al Shaal, L.; Mueller, R.H.; Shegokar, R. smartCrystal combination technology—Scale up from lab to pilot scale and long term stability. Pharmazie 2010, 65, 877–884. [Google Scholar]
- Luo, K.; Li, X.; Luo, J.; Yang, L.; Lin, H.; Mou, Q. Characterization of Silymarin Nanocrystal Pellets and Investigation of Its Stability. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 7–11. [Google Scholar]
- Assad, M.; Jackson, N. Biocompatibility Evaluation of Orthopedic Biomaterials and Medical Devices: A Review of Safety and Efficacy Models. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 281–309. [Google Scholar]
- Sheng, H.; Zhang, Y.; Nai, J.; Wang, S.; Dai, M.; Lin, G.; Zhu, L.; Zhang, Q. Preparation of oridonin nanocrystals and study of their endocytosis and transcytosis behaviours on MDCK polarized epithelial cells. Pharm. Biol. 2020, 58, 518–527. [Google Scholar] [CrossRef]
- Choi, J.-S. Design of Cilostazol Nanocrystals for Improved Solubility. J. Pharm. Innov. 2020, 15, 416–423. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Xiong, S.; Luo, J.; Li, Y.; Zhao, Y.; Wang, Q.; Zhang, Z.; Chen, X.; Chen, T. Highly stabilized nanocrystals delivering Ginkgolide B in protecting against the Parkinson’s disease. Int. J. Pharm. 2020, 577, 119053. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yue, P.; Dan, J.; Xu, J.; Zheng, Q.; Yang, M. Research Progress of in Vitro Release Evaluation Methods for Nano Preparation. Chin. Pharm. J. 2016, 51, 861–866. [Google Scholar]
- Soroushnia, A.; Ganji, F.; Vasheghani-Farahani, E.; Mobedi, H. Preparation, optimization, and evaluation of midazolam nanosuspension: Enhanced bioavailability for buccal administration. Prog. Biomater. 2021, 10, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.A.; Imam, S.S.; Muheem, A.; Chettupalli, A.; Al-Abbasi, F.A.; Nadeem, M.S.; Kazmi, I.; Afzal, M.; Alshehri, S. Nanocrystals: Characterization Overview, Applications in Drug Delivery, and Their Toxicity Concerns. J. Pharm. Innov. 2022, 17, 237–248. [Google Scholar] [CrossRef]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).