Deposit Formation in a Coal-Fired Rotary Kiln for Fluxed Iron Ore Pellet Production: Effect of MgO Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
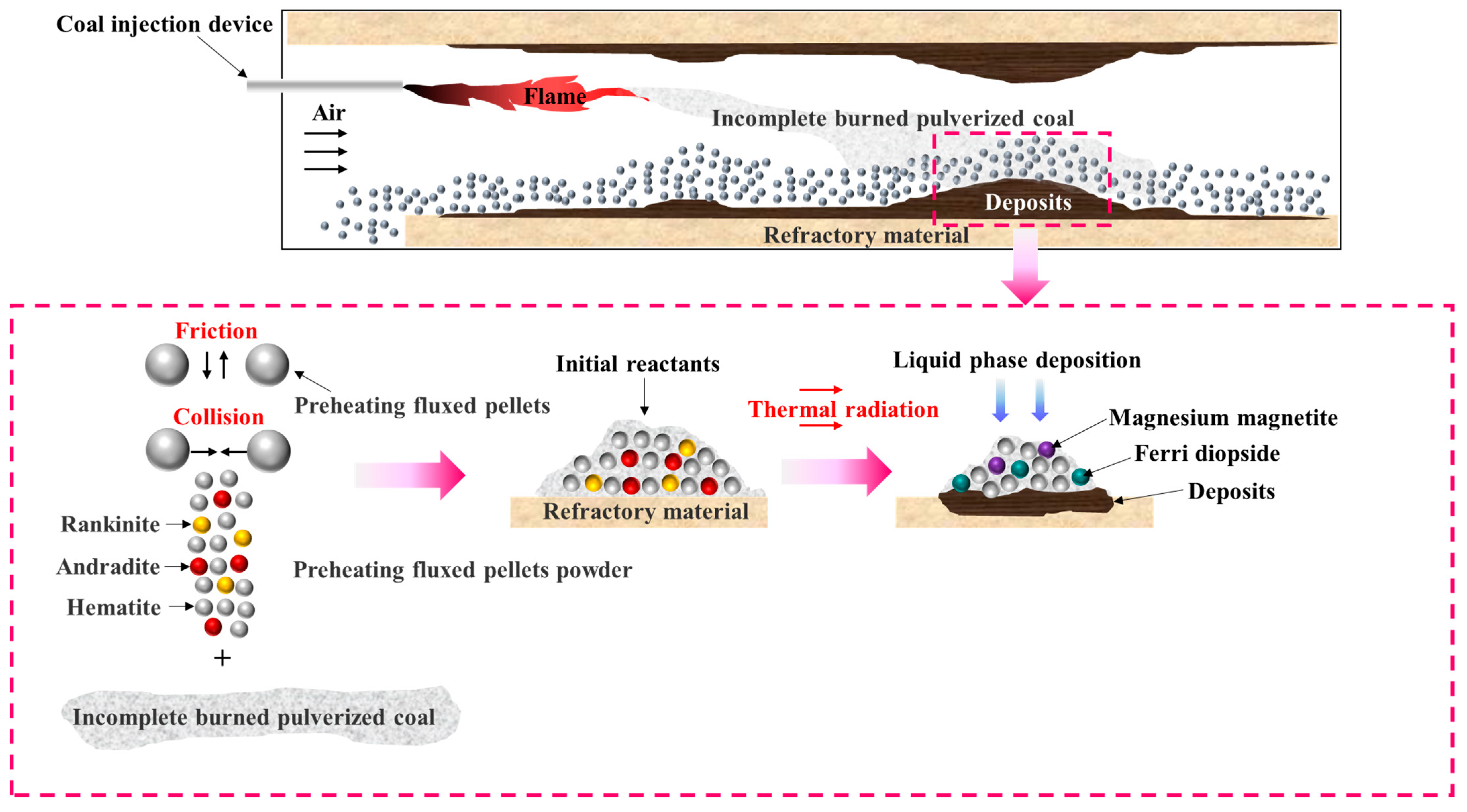
2.2.1. Preparation of the Initial Reactants for Deposit Formation
- (1)
- Burn-off rate of pulverized coal, which was calculated from Equation (1):
- (2)
- SCA percentage of pulverized coal input, which was worked out by Equation (2):
- (3)
- RVC percentage of pulverized coal input, which was calculated by Equation (3):
2.2.2. Preparation of Deposit Simulants
2.2.3. Evaluation Method for Softening-Melting Performance
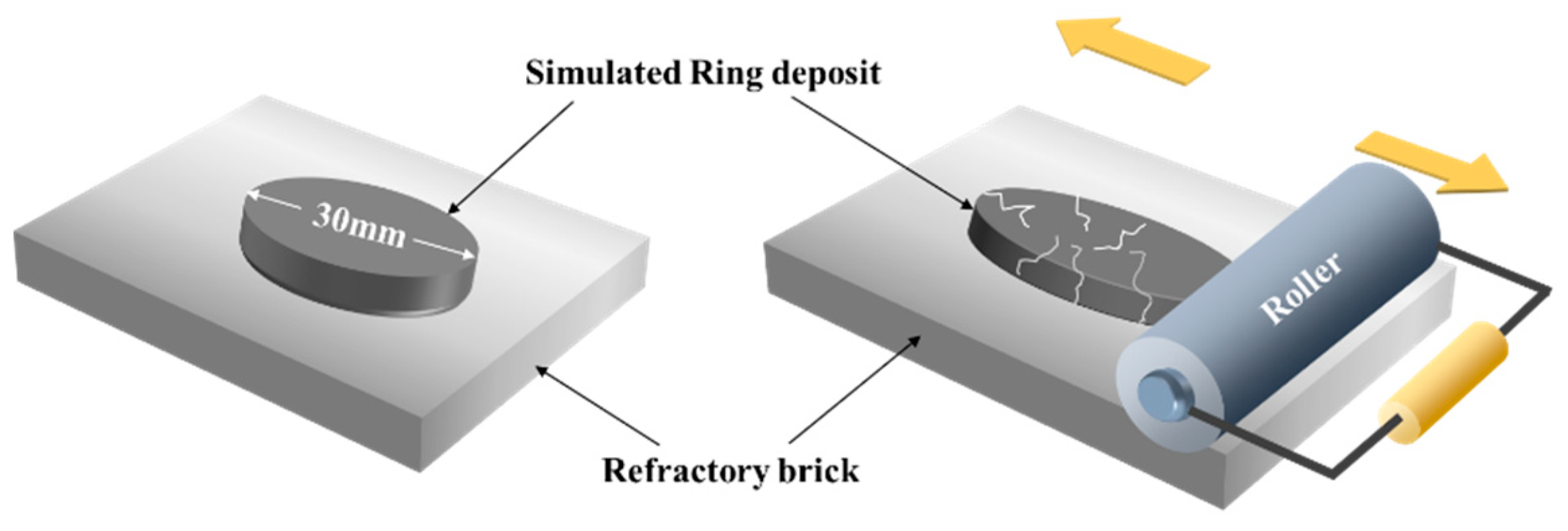
2.2.4. Evaluation Method for Adhesivity to Refractory Brick
2.3. Analysis and Characterization
3. Results
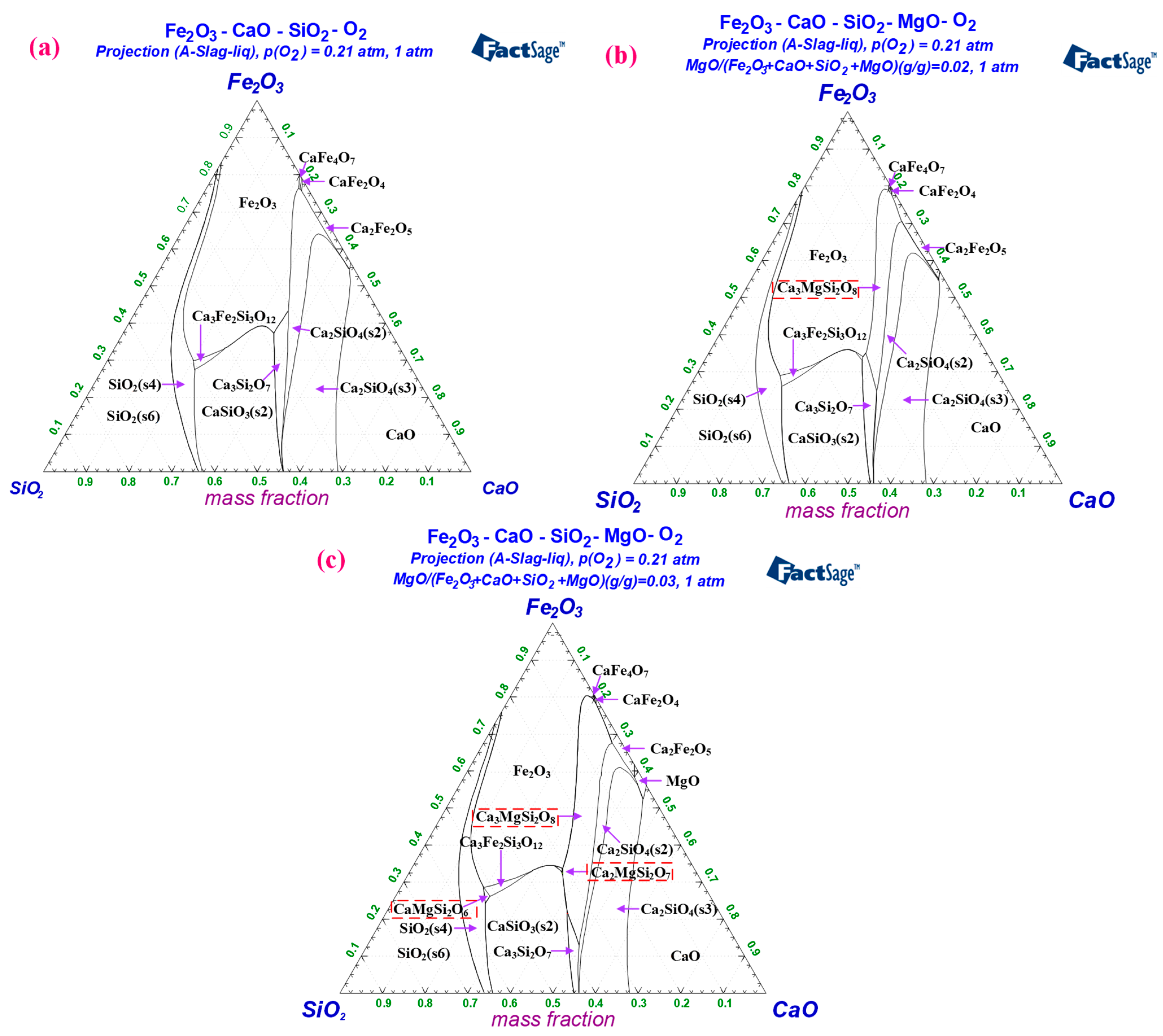
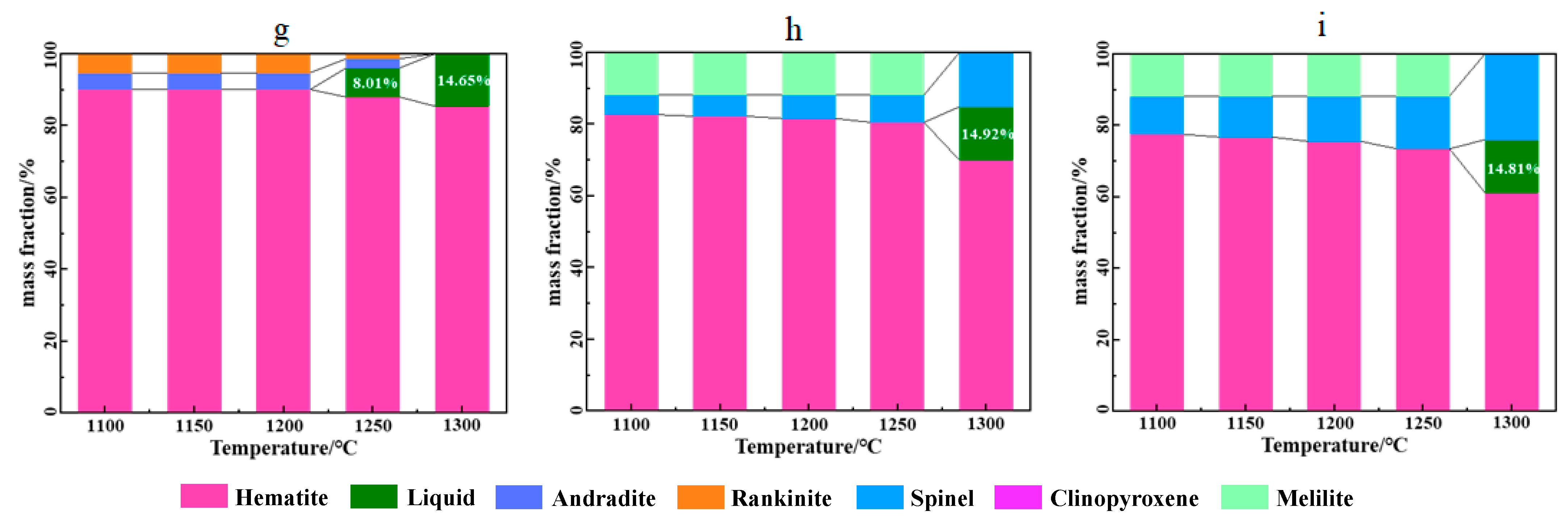
3.1. Phase Diagram Calculation of Preheating Fluxed Pellet Powder
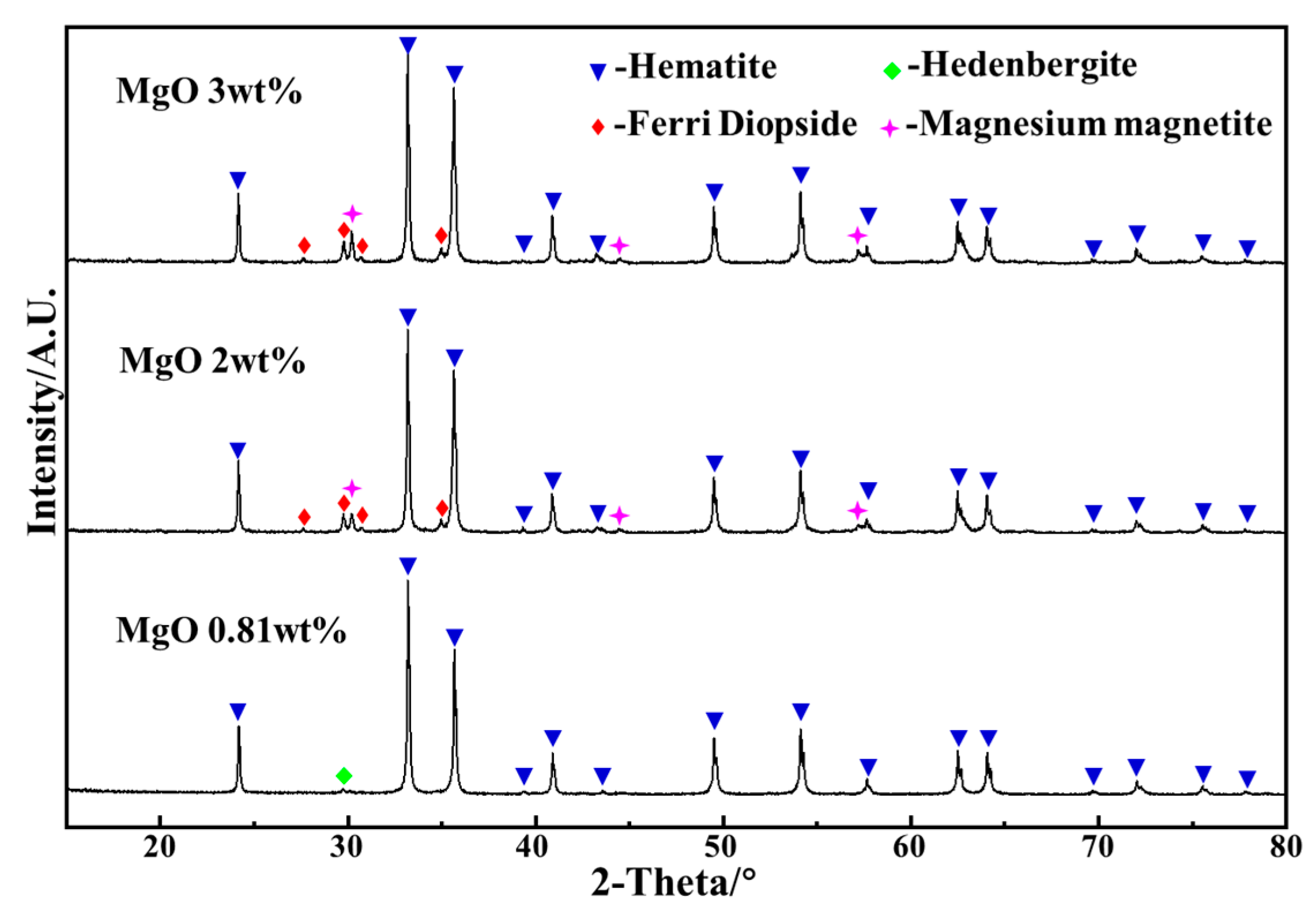
3.2. XRD Analysis of Deposit Simulants
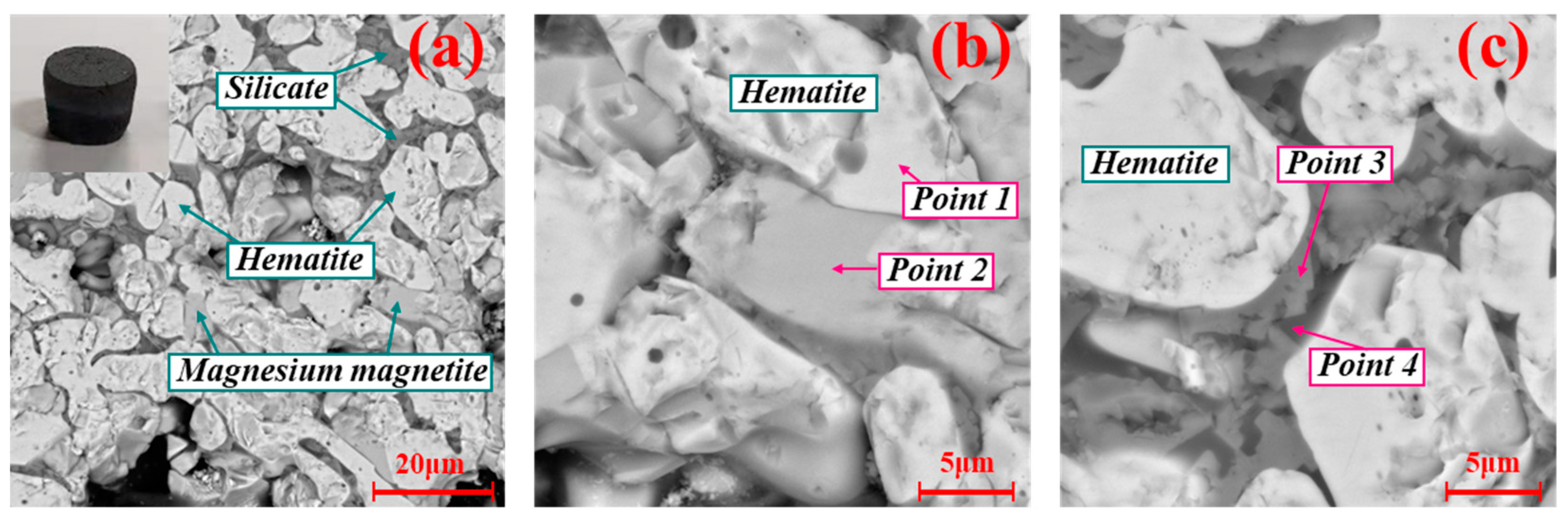
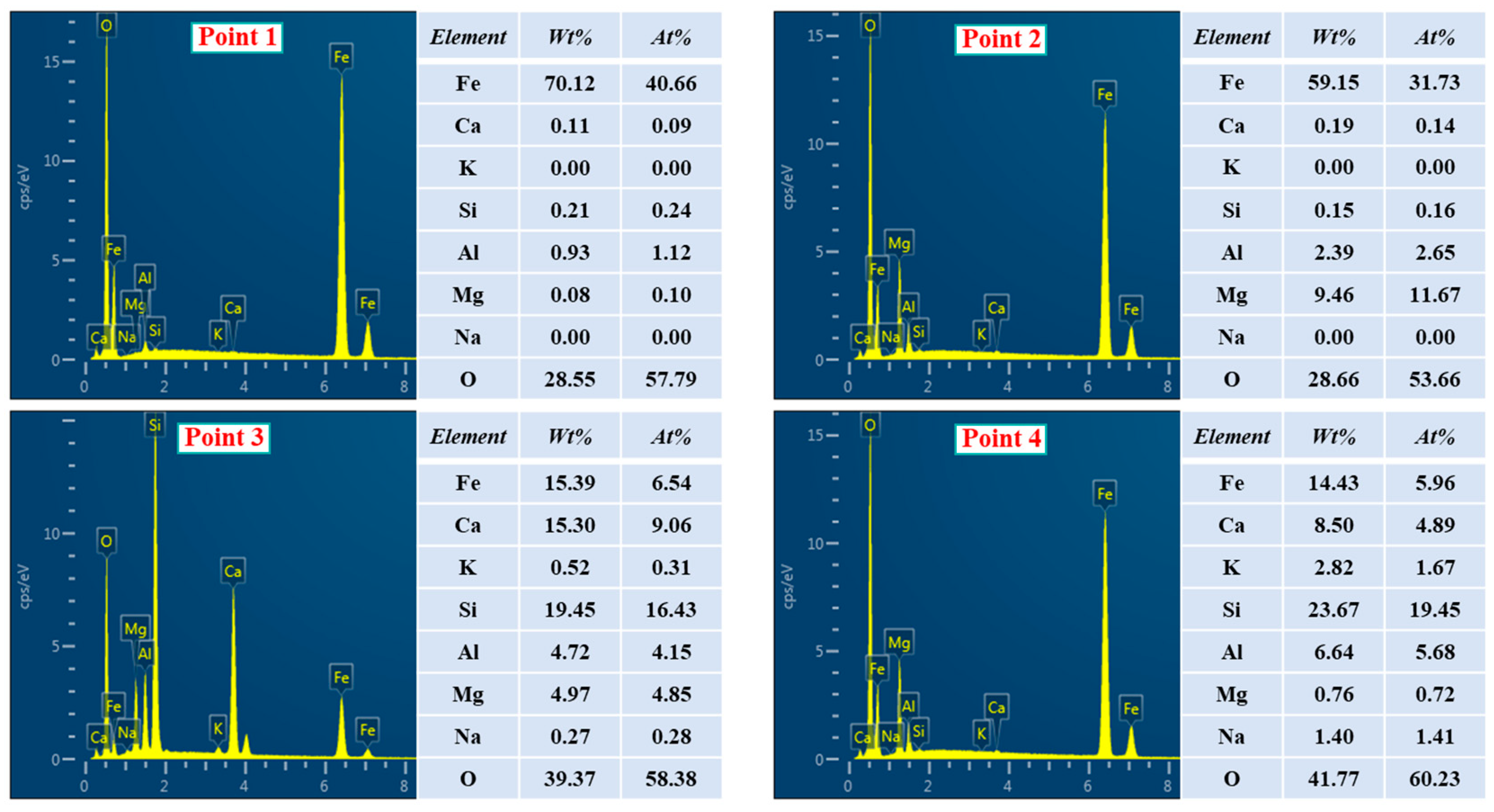
3.3. SEM-EDS Analysis of Deposit Simulants
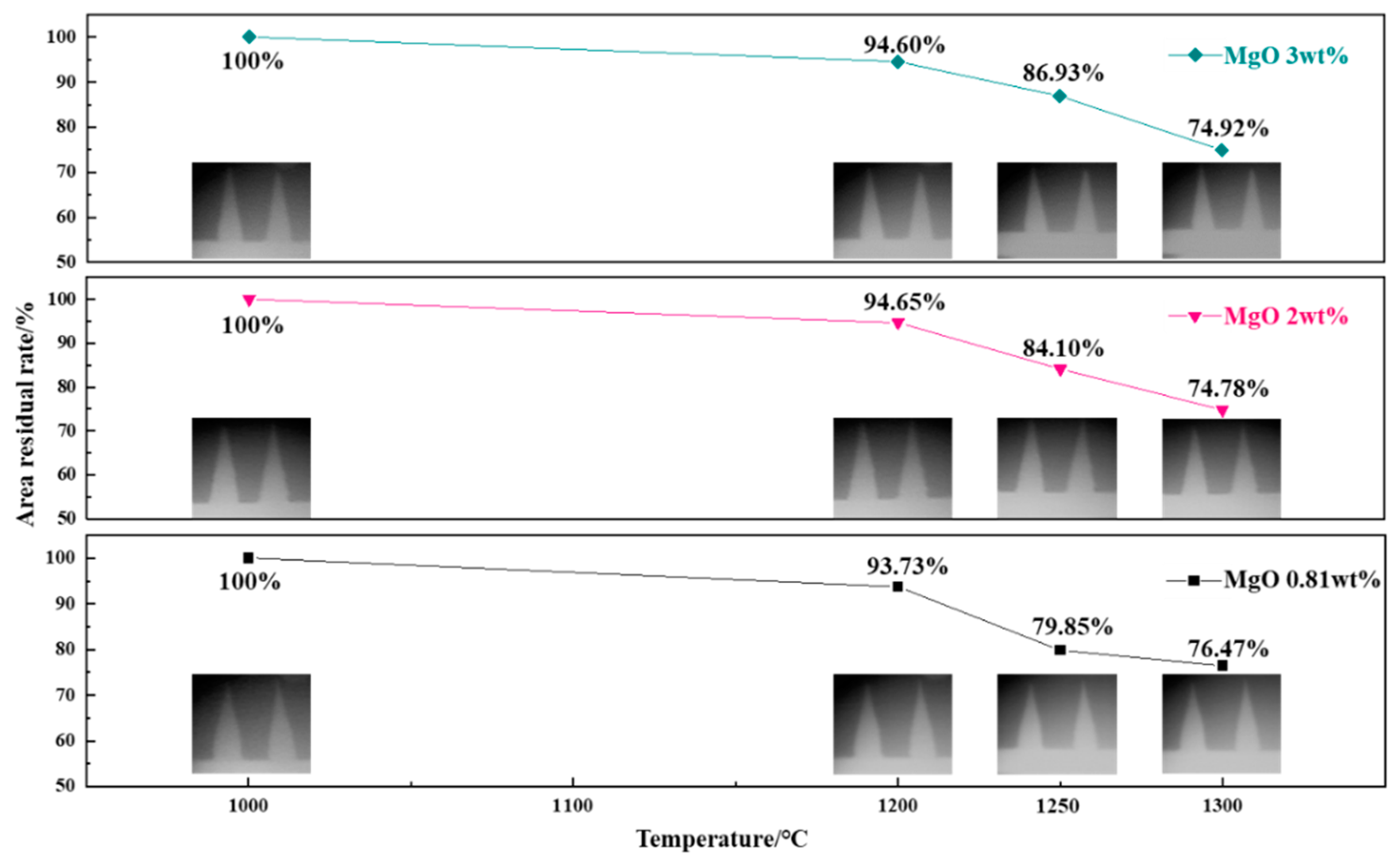
3.4. Effect of MgO on Softening-Melting Performance of Deposits
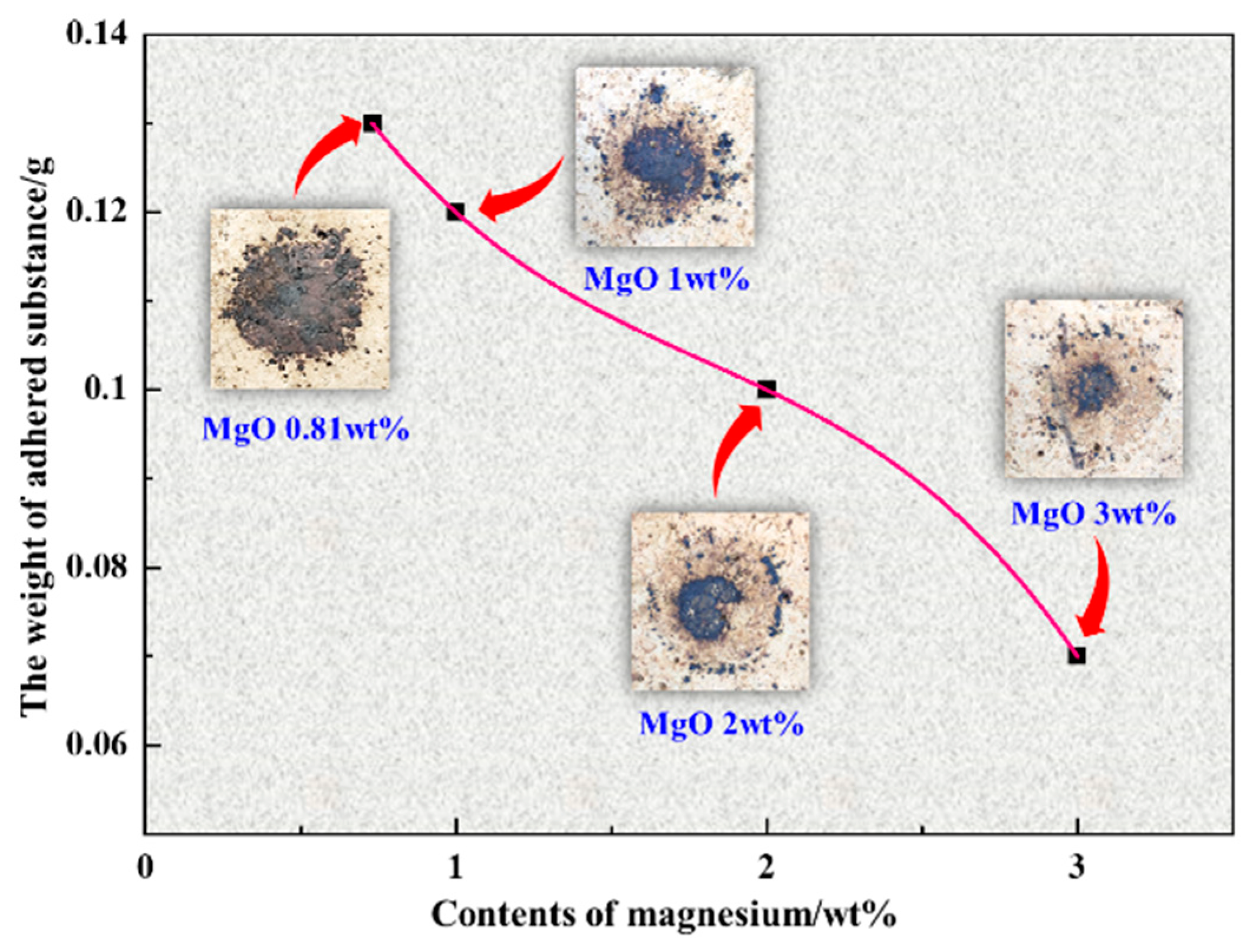
3.5. Effect of MgO on Adhesivity of Deposits to Refractory Brick
4. Discussion
5. Conclusions
- (1)
- Based on the calculation results of Factsage8.0 software, fluxed pellet powder with 0.8–1.2 binary basicity roasted at 1200–1250 °C would produce 2.30–8.01 wt% liquid phase, which led to the formation of initial deposits. As MgO content increased to 2–3 wt%, the formation of these liquid phases in fluxed pellet powder could be inhibited at 1200–1250 °C.
- (2)
- The deposit simulants prepared by roasting the mixtures containing preheated pellet powder and incomplete burnt pulverized coal were mainly composed of hematite, magnesium magnetite, and ferri-diopside. The SEM-EDS analysis showed that the hematite and magnesium magnetite were bonded together by ferri-diopside to form the microstructure of deposit simulants. The Fe2+ formed by the combustion of residual carbon could be absorbed by magnesium oxide, which decreased the formation of low-melting-point hedenbergite, thereby inhibiting the decline in liquid phase formation temperature and slowing down the deposit formation.
- (3)
- With MgO added, the area residual rate of temperature cones prepared by preheated pellet powder and incomplete burnt pulverized coal at 1200–1250 °C was relatively higher than cones without extra MgO addition, which confirmed that MgO had a certain inhibitory effect on the formation of liquid phase. The results of the adhesivity experiment showed that the addition of MgO could reduce the adhesion weight of deposit simulants on the refractory bricks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, J.; Arunkumar, C.; Rajshekhar, Y.; Das, G.; Goswami, M.C.; Venugopalan, T. Development on Iron Ore Pelletization Using Calcined Lime and MgO Combined Flux Replacing Limestone and Bentonite. ISIJ Int. 2014, 54, 2169–2178. [Google Scholar] [CrossRef]
- Guo, H.; Shen, F.M.; Jiang, X.; Gao, Q.J.; Ding, G.G. Effects of MgO additive on metallurgical properties of fluxed-pellet. J. Cent. South Univ. 2019, 26, 3238–3251. [Google Scholar] [CrossRef]
- Fan, X.H.; Li, J.; Chen, X.L.; Wang, Y.; Gan, M. Temperature Field Simulation Model for Rotary Kiln of Iron Ore Oxidized Pellet. J. Iron Steel Res. Int. 2013, 20, 16–19. [Google Scholar] [CrossRef]
- Fan, X.H.; Yang, G.M.; Chen, X.L.; Gao, L.; Huang, X.X.; Li, X. Predictive models and operation guidance system for iron ore pellet induration in traveling grate-rotary kiln process. Comput. Chem. Eng. 2015, 79, 80–90. [Google Scholar] [CrossRef]
- Stjernberg, J.; Jonsson, C.Y.C.; Wiinikka, H.; Lindblom, B.; Bostrom, D.; Ohman, M. Deposit Formation in a Grate-Kiln Plant for Iron-Ore Pellet Production. Part 2: Characterization of Deposits. Energy Fuels 2013, 27, 6171–6184. [Google Scholar] [CrossRef]
- Eriksson, M.; Carlborg, M.; Brostrom, M. Characterization of Ring Deposits Inside a Quicklime Producing Long Rotary Kiln. Energy Fuels 2019, 33, 11731–11740. [Google Scholar] [CrossRef]
- Belgacem, S.; Galai, H.; Tiss, H. From a mineralogical analytical view to a mechanism evaluation of cement kiln rings. Eng. Fail. Anal. 2019, 95, 289–299. [Google Scholar] [CrossRef]
- Guo, Y.F.; Wang, S.; He, Y.; Jiang, T.; Chen, F.; Zheng, F.Q. Deposit formation mechanisms in a pulverized coal fired grate for hematite pellet production. Fuel Process. Technol. 2017, 161, 33–40. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Liu, K.; Yang, Z.; Liu, Y.J.; Jiang, Y.; Chen, F.; Zheng, F.Q.; Yang, L.Z. The Deposit Formation Mechanism in Coal-Fired Rotary Kiln for Iron Ore Pellet Production: A Review. Crystals 2021, 11, 974. [Google Scholar] [CrossRef]
- Zan, H.F.; Chen, X.P.; Zhong, W.Q.; Ma, J.L.; Liu, D.Y.; Lian, G.Q.; Geng, P.F.; Liang, C. Coal combustion emissions and ash formation characteristics during oxy-fuel combustion in a 100 kWth pressurized circulating fluidized bed. Fuel Process. Technol. 2022, 228, 107140. [Google Scholar] [CrossRef]
- Yi, L.Y.; Zhang, N.; Liang, Z.K.; Wang, L.; Xiao, H.R.; Huang, Z.C. Coal ash induced ring formation in a pilot scale rotary kiln for low-grade iron ore direct reduction process: Characterization and mechanism. Fuel 2022, 310, 122342. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Zhou, T.; Chen, Y.; Hou, N.; Piao, G.; Kobayashi, N.; Itaya, Y.; Mori, S. The effect of iron-bearing mineral melting behavior on ash deposition during coal combustion. Proc. Combust. Inst. 2011, 33, 2853–2861. [Google Scholar] [CrossRef]
- Van Dyk, J.C.; Benson, S.A.; Laumb, M.L.; Waanders, B. Coal and coal ash characteristics to understand mineral transformations and slag formation. Fuel 2009, 88, 1057–1063. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Gupta, S.; Lindblom, B.; Jonsson, S.; French, D.; Sahajwalla, V. Assessment of ash deposition tendency in a rotary kiln using Thermo-mechanical analysis and Experimental Combustion Furnace. Fuel 2014, 135, 301–307. [Google Scholar] [CrossRef]
- Sato, N.; Priyanto, D.E.; Ueno, S.; Matsuzawa, Y.; Ueki, Y.; Yoshiie, R.; Naruse, I. Growth and gravity shedding of ash deposition layer in pulverized coal combustors. Fuel Process. Technol. 2015, 134, 1–10. [Google Scholar] [CrossRef]
- Zhong, R.H.; Yi, L.Y.; Huang, Z.C.; Shen, X.H.; Jiang, T. Sticking mechanism of low grade iron ore-coal composite in rotary kiln reduction. Powder Technol. 2018, 339, 625–632. [Google Scholar] [CrossRef]
- Stjernberg, J.; Ion, J.C.; Antti, M.-L.; Nordin, L.-O.; Lindblom, B.; Oden, M. Extended studies of degradation mechanisms in the refractory lining of a rotary kiln for iron ore pellet production. J. Eur. Ceram. Soc. 2012, 32, 1519–1528. [Google Scholar] [CrossRef]
- Tsuji, H.; Tachino, N. Ring Formation in the Smelting of Saprolite Ni-ore in a Rotary Kiln for Production of Ferro-nickel Alloy: Examination of the Mechanism. ISIJ Int. 2012, 52, 1951–1957. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, J.L.; Liu, Z.J. Rings growth behavior within a pre-reduction rotary kiln: The layered structure and formation mechanism. Powder Technol. 2019, 356, 73–82. [Google Scholar] [CrossRef]
- Tao, J.; Guo-qiang, H.; Min, G.; Guang-hui, L.; Xiao-hui, F.; Li-shun, Y. Forming Mechanism of Rings in Rotary-Kiln for Oxidized Pellet. J. Iron Steel Res. Int. 2009, 16, 292–297. [Google Scholar]
- Wang, S.; Guo, Y.F.; Fan, J.J.; Jiang, T.; Chen, F.; Zheng, F.Q.; Yang, L.Z. Deposits in a coal fired grate-kiln plant for hematite pellet production: Characterization and primary formation mechanisms. Powder Technol. 2018, 333, 122–137. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, Y.; Jiang, T.; Li, Q.; Xu, B. Effect of coal ash on ring behavior of iron-ore pellet powder in kiln. Powder Technol. 2018, 323, 195–202. [Google Scholar] [CrossRef]
- Fan, X.-h.; Wang, Y.; Chen, X.-l. Mathematical models and expert system for grate-kiln process of iron ore oxide pellet production. Part II: Rotary kiln process control. J. Cent. South Univ. 2012, 19, 1724–1727. [Google Scholar] [CrossRef]
- Larsson, I.A.S.; Marjavaara, B.D.; Lundstrom, T.S. Simulation of the flow field in an iron ore pelletizing kiln. Miner. Metall. Process. 2016, 33, 144–148. [Google Scholar] [CrossRef]
- Zhang, X.G.; Lei, Y.Y.; Chen, H.; Zhang, L.; Zhou, Y.C. Multivariate Time-Series Modeling for Forecasting Sintering Temperature in Rotary Kilns Using DCGNet. IEEE Trans. Ind. Inform. 2021, 17, 4635–4645. [Google Scholar] [CrossRef]
- Sefidari, H.; Lindblom, B.; Wiinikka, H.; Nordin, L.-O.; Mouzon, J.; Bhuiyan, I.U.; Ohman, M. The effect of disintegrated iron-ore pellet dust on deposit formation in a pilot-scale pulverized coal combustion furnace. Part I: Characterization of process gas particles and deposits. Fuel Process. Technol. 2018, 177, 283–298. [Google Scholar] [CrossRef]
- Guo, Y.F.; Liu, K.; Chen, F.; Wang, S.; Zheng, F.Q.; Yang, L.Z.; Liu, Y.J. Effect of basicity on the reduction swelling behavior and mechanism of limestone fluxed iron ore pellets. Powder Technol. 2021, 393, 291–300. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhang, J.L.; Liu, Z.J.; Li, Y.; Xu, C.Y. Effect of CaO and MgO additives on the compressive strength of pellets: Exploration on the decisive stage during induration. Powder Technol. 2021, 390, 496–503. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Z.; Fan, J.; Wang, S.; Chen, F.; Yang, L.; Liu, Y.; Liu, K. Effects of Pellet Basicity on the Simulated Deposit Formation in Coal-Fired Rotary Kilns for Iron Ore Pellet Production. ACS Omega 2022, 7, 4640–4647. [Google Scholar] [CrossRef]
- Deraz, N.M.; Abd-Elkader, O.H. Investigation of Magnesium Ferrite Spinel Solid Solution with Iron-Rich Composition. Int. J. Electrochem. Sci. 2013, 8, 9071–9081. [Google Scholar]
- Yang, Z.C.; Liu, Z.G.; Chu, M.S.; Gao, L.H.; Feng, C.; Tang, J. Effect of Basicity on Metallurgical Properties of Fluxed Pellets with High MgO Content. ISIJ Int. 2021, 61, 1431–1438. [Google Scholar] [CrossRef]
- Shen, F.M.; Gao, Q.J.; Jiang, X.; Wei, G.; Zheng, H.Y. Effect of magnesia on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 431–437. [Google Scholar] [CrossRef]
- Sefidari, H.; Wiinikka, H.; Lindhlom, B.; Nordin, L.O.; Wu, G.; Yazhenskikh, E.; Muller, M.; Ma, C.; Ohman, M. Comparison of high-rank coals with respect to slagging/deposition tendency at the transfer-chute of iron-ore pelletizing grate-kiln plants: A pilot-scale experimental study accompanied by thermochemical equilibrium modeling and viscosity estimations. Fuel Process. Technol. 2019, 193, 244–262. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Fan, J.J.; He, Y.; Jiang, T.; Chen, F.; Zheng, F.Q.; Yang, L.Z. Initial stage of deposit formation process in a coal fired grate-rotary kiln for iron ore pellet production. Fuel Process. Technol. 2018, 175, 54–63. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Chen, F.; He, Y.; Jiang, T.; Zheng, F.Q. Combustion Reaction of Pulverized Coal on the Deposit Formation in the Kiln for Iron Ore Pellet Production. Energy Fuels 2016, 30, 6123–6131. [Google Scholar] [CrossRef]
- Fan, X.H. Principle and Technology of Iron Ore Matching for Sintering; Metallurgical Industry Press: Beijing, China, 2013. [Google Scholar]
- Sefidari, H.; Lindblom, B.; Wiinikka, H.; Nordin, L.O.; Lennartsson, A.; Mouzo, J.; Bhuiyan, I.U.; Ohman, M. The effect of disintegrated iron-ore pellet dust on deposit formation in a pilot-scale pulverized coal combustion furnace. Part II: Thermochemical equilibrium calculations and viscosity estimations. Fuel Process. Technol. 2018, 180, 189–206. [Google Scholar] [CrossRef]


| Raw Material | TFe | FeO | SiO2 | CaO | Al2O3 | MgO | TiO2 | K2O | Na2O | S |
|---|---|---|---|---|---|---|---|---|---|---|
| iron concentrate | 64.83 | 9.82 | 3.55 | 0.50 | 1.07 | 0.77 | - | 0.06 | 0.06 | 0.04 |
| Preheated pellet | 62.10 | - | 4.35 | 4.20 | 1.32 | 0.81 | - | 0.09 | 0.08 | 0.04 |
| Limestone | 0.52 | 0.14 | 2.32 | 51.82 | 0.46 | 2.63 | - | 0.17 | 0.03 | 0.03 |
| Magnesium oxide | 0.01 | - | 0.07 | 0.01 | - | 99.60 | - | - | - | 0.19 |
| Bentonite | 3.09 | 0.20 | 60.01 | 3.73 | 12.52 | 3.00 | - | 1.40 | 1.80 | 0.02 |
| Deposit sample | 59.74 | 0.76 | 6.67 | 2.98 | 2.93 | 0.69 | - | 0.24 | 0.17 | 0.02 |
| Refractory bricks | 1.23 | - | 16.98 | 1.75 | 71.26 | 0.24 | 1.89 | - | - | - |
| Coal ash | 4.04 | 0.10 | 51.85 | 8.57 | 26.09 | 1.12 | - | 1.22 | 0.75 | 0.58 |
| Raw Material | Mad, % | Aad, % | Vad, % | FCad, % |
|---|---|---|---|---|
| Pulverized coal | 1.81 | 12.89 | 24.34 | 60.96 |
| Removed volatile pulverized coal (RVC) | 0.70 | 16.52 | 1.94 | 80.84 |
| Self-prepared coal ash (SCA) | 0.14 | 99.44 | 0.41 | 0.01 |
| Burn-Off Rate/% | SCA/wt% | RVC/wt% |
|---|---|---|
| 90 | 60.61 | 39.39 |
| Preheated Pellet Powder/g | SCA/g | RVC/g |
|---|---|---|
| 9.434 | 0.334 | 0.514 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, K.; Wang, S.; Chen, F.; Yang, Z.; Yang, L.; Li, D. Deposit Formation in a Coal-Fired Rotary Kiln for Fluxed Iron Ore Pellet Production: Effect of MgO Content. Crystals 2022, 12, 1214. https://doi.org/10.3390/cryst12091214
Guo Y, Liu K, Wang S, Chen F, Yang Z, Yang L, Li D. Deposit Formation in a Coal-Fired Rotary Kiln for Fluxed Iron Ore Pellet Production: Effect of MgO Content. Crystals. 2022; 12(9):1214. https://doi.org/10.3390/cryst12091214
Chicago/Turabian StyleGuo, Yufeng, Kuo Liu, Shuai Wang, Feng Chen, Zhuang Yang, Lingzhi Yang, and Dongyue Li. 2022. "Deposit Formation in a Coal-Fired Rotary Kiln for Fluxed Iron Ore Pellet Production: Effect of MgO Content" Crystals 12, no. 9: 1214. https://doi.org/10.3390/cryst12091214










