Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics
Abstract
:1. Introduction
2. Polymer-Derived Ceramics Technology
2.1. Characteristics of Polymer-Derived Ceramics Technology
- i.
- The designability of the organic precursor structure can be used to tune the microstructure of ceramics; in other words, the structure, composition, and preparation process of the organic polymer precursor are adjusted to control the phase composition and structure of the final ceramic product [21].
- ii.
- The polymer precursors have good moldability and can be used to achieve the preparation of ceramics with complex shapes, including one-dimensional ceramic fibers [6], two-dimensional coatings [22], as well as three-dimensional micro-electro-mechanical systems (MEMS) [23] and ceramic composites [24]. The preparation of fibers takes advantage of the fusible nature of precursors [6,25]; the synthesis of the coating exploits the fluidity of the precursor to achieve a two-dimensional uniform structure on the surface of the material [26,27,28]. Polymer-derived ceramics technology can be applied in semiconductor preparation techniques, such as lithography, and in the synthesis of ceramic micro–nano devices through the design of the functional groups of the polymer, which provides a good processing route for the manufacture of MEMS [23]. The solubility of the precursor can also be used to impregnate the fiber precast [29,30,31]; after impregnation, the polymer is crosslinked, cured, and pyrolyzed at high-temperature into the ceramic matrix to fill voids in the precast (this preparation process is called PIP); after repeating the PIP process, a dense fiber-reinforced ceramic matrix composite is obtained.
- iii.
- The process temperature is relatively low. Traditional non-oxide ceramics, such as SiC and Si3N4, require a high sintering temperature, usually above 1600 °C, while PDCs can be sintered at temperatures as low as 900 °C [32].
- iv.
- Sintering aids are not needed. Due to the slow atomic diffusion caused by the properties of covalent bonds, sintering additives are often required in the preparation of non-oxide ceramics [33]. These additives form a liquid phase at high temperatures and accelerate the diffusion of atoms, thereby promoting the sintering of non-oxide ceramics [34,35]. However, the sintering additive residues at grain boundaries will impair the oxidation resistance [36,37] and the high-temperature mechanical properties of non-oxide ceramics (such as the high-temperature creep resistance) [38]. In contrast, PDCs technology can achieve the sintering of ceramic materials without sintering additives [39,40], and the resulting materials exhibit good resistance to high-temperature oxidation [41], as well as high-temperature creep properties [42].
- v.
- Excellent high-temperature performance. Since no sintering additives are required in the preparation of polymer-derived ceramics, a high-purity matrix is obtained after sintering; thus, the prepared material has good high-temperature properties, such as creep [42,43,44], oxidation [41,45], and corrosion [46,47] resistances.
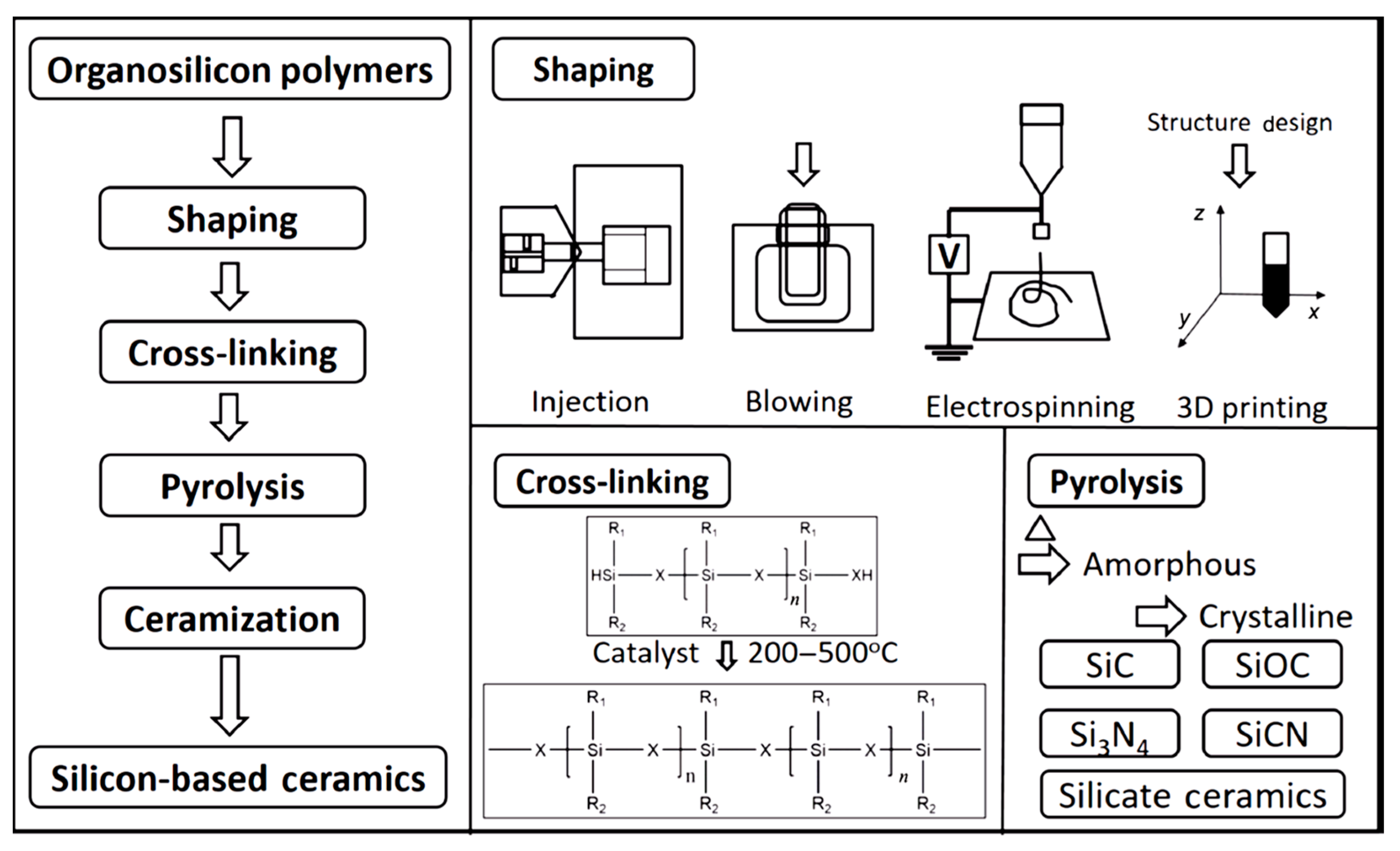
2.2. Procedure of Polymer-Derived Ceramics Technology
- i.
- Synthesis: various small organic molecules are used as raw materials to obtain precursors with specific molecular weights by organic synthesis methods [20]. The precursor can be varied by selecting suitable small molecules and optimizing the synthesis process. Ceramics with different microstructures can be obtained by using different precursors, as well as different curing and cracking systems [45,46,47,48,49].
- ii.
- Shaping: polymers can be shaped directly with a variety of methods, such as injection molding, blow molding, extrusion molding, coating, electrospinning, 3D printing, etc., which further enable one-step molding of polymer-derived ceramics [50].
- iii.
- Crosslinking/curing: the main purpose of crosslinking is to make the polymer backbone connected [20]. Crosslinking methods include light and thermal curing processes. Thermal-curing crosslinking generally relies on curing agents to polymerize polymer precursors into a mesh structure at a certain temperature, forming a non-molten polymer [51]. In light-curing crosslinking, a polymer is doped with a curing agent and polymerized under illumination at a specific wavelength to obtain a non-molten polymer [52].
- iv.
- Pyrolysis/caramelization: these processes complete the transformation of the material from organic to inorganic, inducing qualitative changes in its internal structure and properties [20]. During the process, the organic groups of the precursor gradually vanish, and the polymer transforms into amorphous ceramics, with a typical pyrolyzing temperature of 900–1000 °C [50]. The phase composition, structure, and properties of amorphous ceramics obtained by pyrolysis are strongly dependent on the caramelization process.
- v.
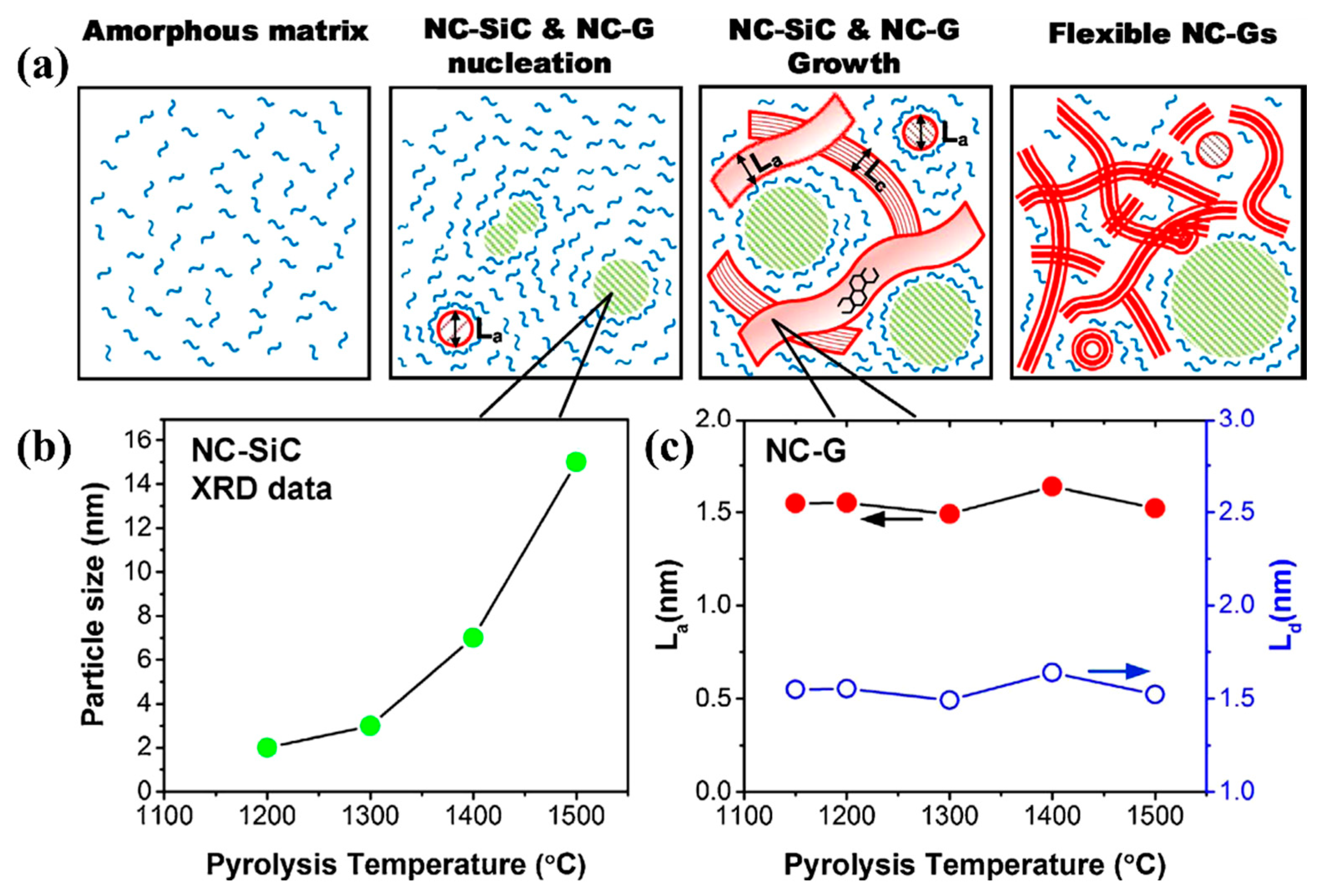
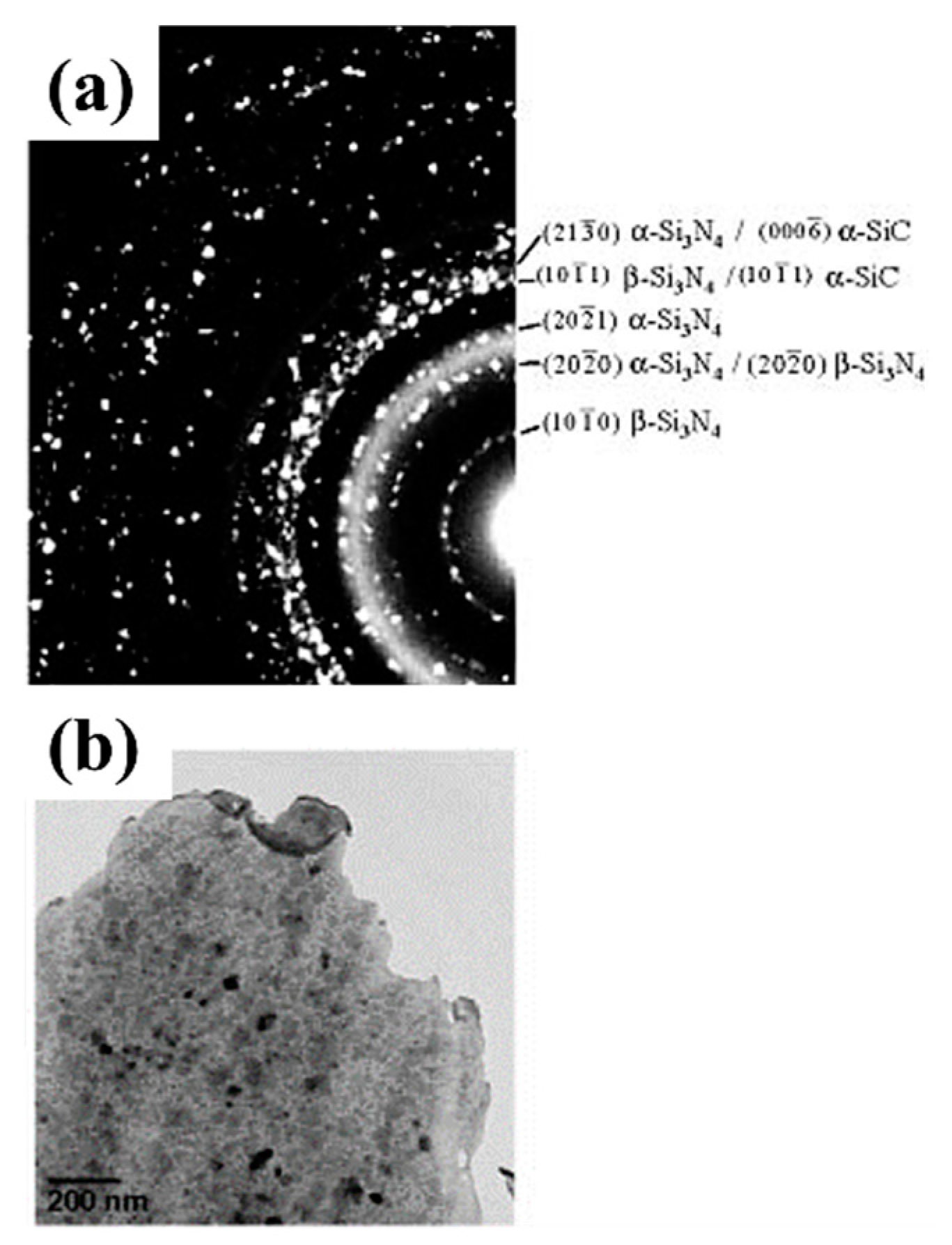
- Crystallization: typically, the polymer transforms into amorphous ceramics at a temperature between 900 and 1000 °C [38]. As the heat treatment temperature increases, the amorphous phase is gradually crystallized in the temperature range of 1200–1800 °C, and the crystalline ceramic material is finally obtained [20,38]. Several structural transformations are triggered by the amorphous → crystalline transition [20,29]: the amorphous disordered structure is rearranged with the relevant chemical bonds broken, and the structure gradually turns into crystalline as the temperature is increased; then, the rupture of chemical bonds and the atomic rearrangement cause the separation of the ceramic and carbon phases to form a multiphase ceramic system, which, in turn, promotes nucleation; the formed crystal nuclei gradually grow with increasing temperature and time. Take the C-enriched SiC produced by PDCs technology as an example; the amorphous → crystalline transition can be schematically drawn in Figure 2 [53]. Meanwhile, the amorphous → crystalline transition is usually accompanied by a decomposition reaction, along with the formation of a small amount of gaseous products.
3. Structure and Properties of Polymer-Derived Ceramics
3.1. High-Temperature Stability
3.2. Semiconducting and Electrical Properties
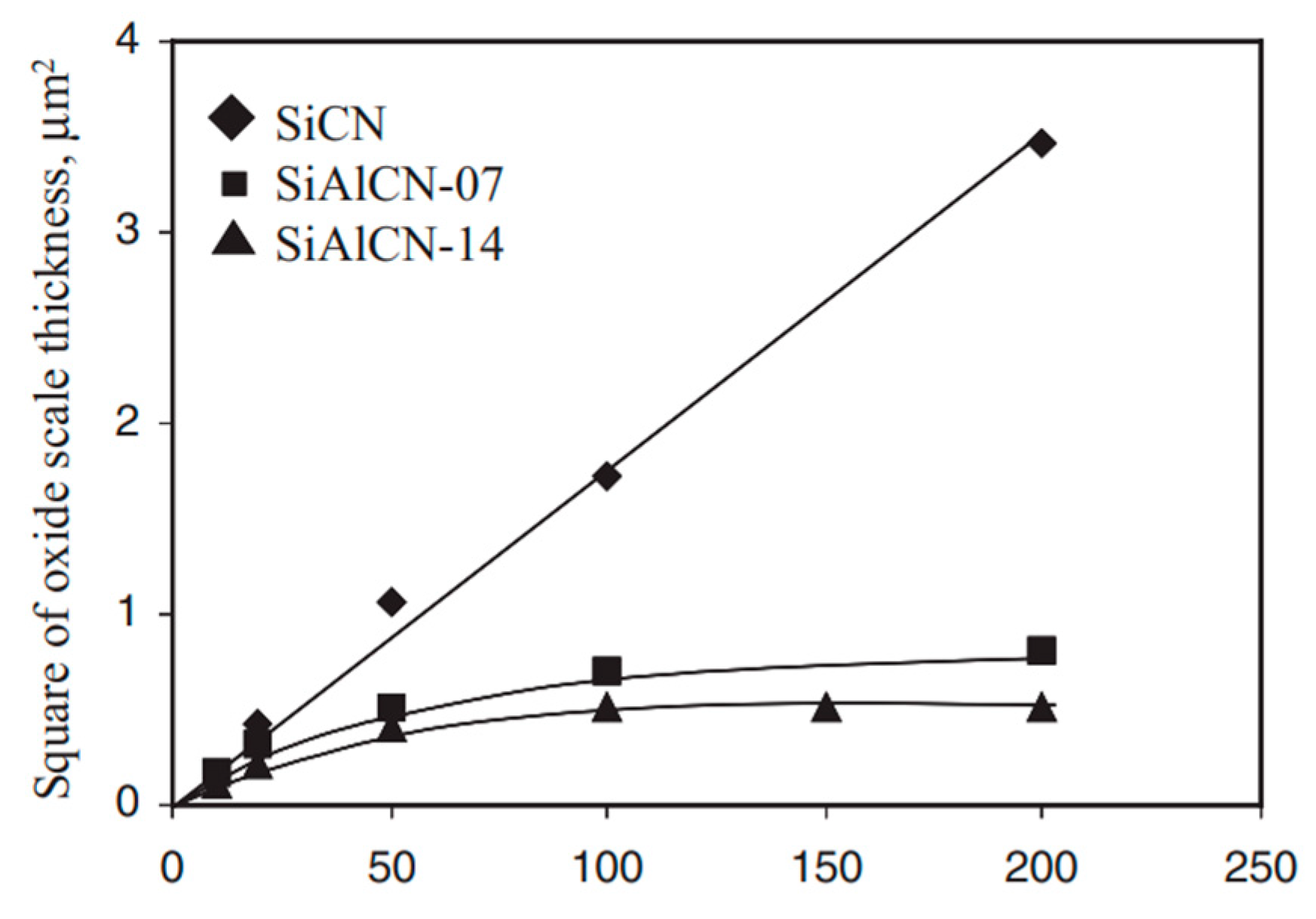
3.3. Oxidation and Corrosion Resistance
3.4. Light Transmission and Luminescence
3.5. Mechanical Properties
- i.
- Density and modulus: as the pyrolyzing temperature increases, the Si–H and C–H bonds in the system are broken, more Si–C network connections are formed by eliminating the hydrogen content in the system, and the density and elastic modulus increase accordingly [87].
- ii.
- Hardness and fracture toughness: similar to the modulus of elasticity, the increase in the heat treatment temperature and the formation of more Si–C network links after dehydrogenation will increase the hardness. The fracture toughness exhibits a more complicated trend: many studies have shown that cracks in Si–C–N ceramics extend along regions of the material that have not yet been dehydrogenated. These regions exhibit lower strength compared to regions that have been dehydrogenated to form Si–C bonds [86]. As the temperature increases, the areas with lower strength gradually decrease, resulting in a tortuous crack propagation path at a certain scale, and the overall fracture toughness of the material increases.
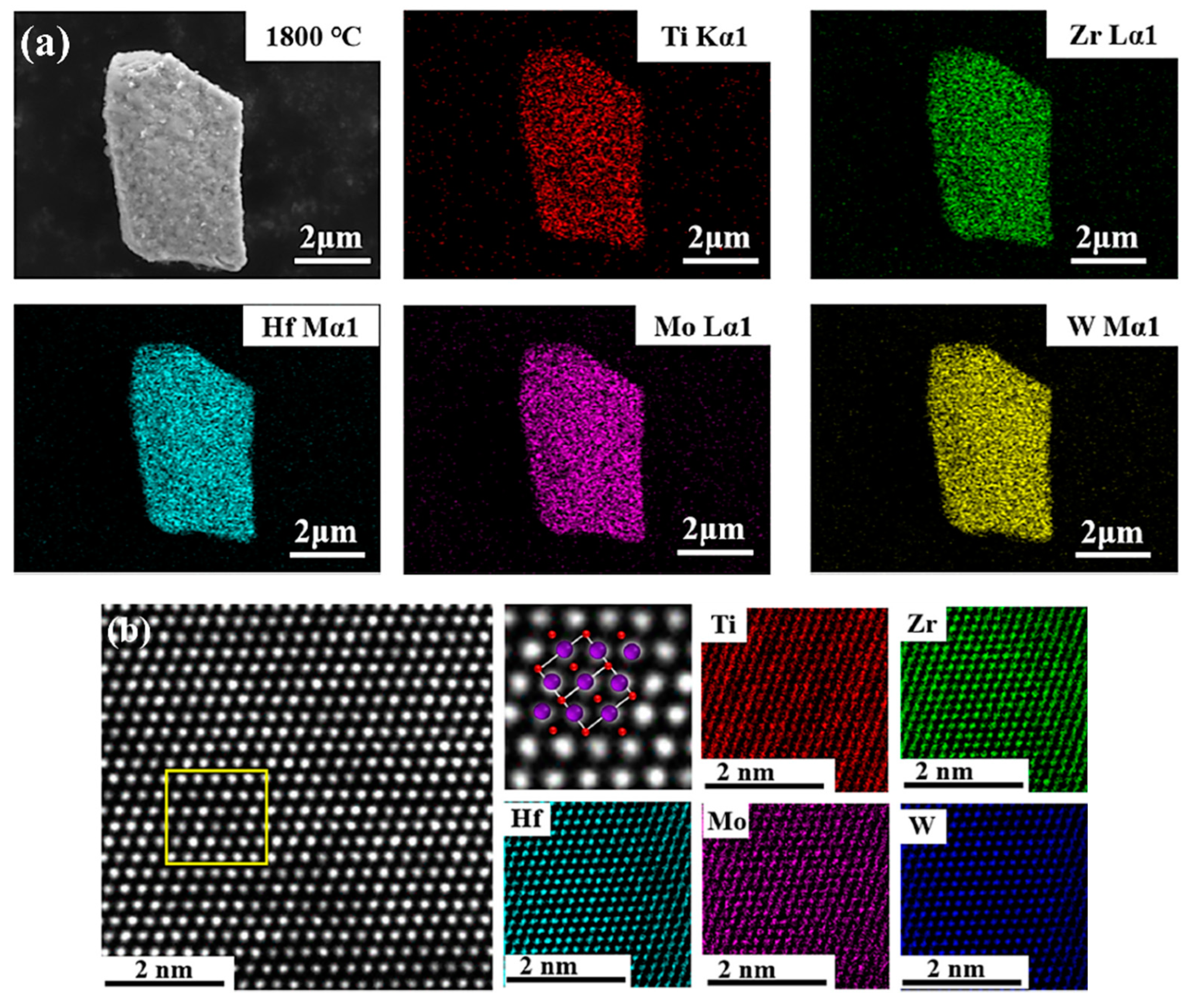
4. Development of PDCs Technology in the Field of High-Entropy Ceramics
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ainger, F.W.; Herbert, J.M. The Preparation of Phosphorus–Nitrogen Compounds as Non–Porous Solids; Academic Press: New York, NY, USA, 1960; pp. 168–182. [Google Scholar]
- Chantrell, P.G.; Popper, P. Inorganic Polymers and Ceramic; Academic Press: New York, NY, USA, 1960; pp. 87–103. [Google Scholar]
- Verbeek, W. Production of Shaped Articles of Homogeneous Mixtures of Silicon Carbide and Nitride. U.S. Patent 3853567, 8 November 1973. [Google Scholar]
- Verbeek, W.; Winter, G. Formkoerper aus siliciumcarbid und verfahren zu ihrer herstellung. Ger. Offen 1974, 7, 2236078. [Google Scholar]
- Fritz, G.; Raabe, B. Bildung siliciumorganischer verbindungen. v. die thermische zersetzung von Si(CH3)4 und Si(C2H5)4. Z. Anorg. Allg. Chem. 1956, 286, 149–167. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Imori, M. Continuous silicon carbide fiber of high tensile strength. Chem. Lett. 1975, 4, 931–934. [Google Scholar] [CrossRef]
- Xia, A.; Yin, J.; Chen, X.; Liu, X.; Huang, Z. Polymer-Derived Si-Based Ceramics: Recent Developments and Perspectives. Crystals 2020, 10, 824. [Google Scholar] [CrossRef]
- Liu, H.; Tian, C. Manufacturing Process of New Structural Ceramics-Polymer-Derived Method and its Application; China Machine Press: Beijing, China, 2010; pp. 11–12. [Google Scholar]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Comm. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scripta Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskites type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, J.; Zhang, X.; Yuan, M.; Zhao, Y.; Yan, J.; Hou, M.; Yan, J.; Kunz, M.; et al. Stability and Compressibility of cation-doped high-entropy oxide MgCoNiCuZnO5. J. Phys. Chem. C. 2019, 123, 17735–17744. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys—A new era of exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Eur. J. Control. 2006, 33, 633–648. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.K.; Shao, G.; Zhao, X.F.; Wang, Y.G. Multicomponent high-entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scripta Mater. 2020, 178, 382–386. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.K.; Cao, Y.J.; Shao, G.; Wang, Y.G. Multicomponent rare-earth cerate and zirconocerate ceramics for thermal barrier coating materials. J. Eur. Ceram. Soc. 2021, 41, 1720–1725. [Google Scholar] [CrossRef]
- Liu, J.; Ren, K.; Ma, C.Y.; Du, H.L.; Wang, Y.G. Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic. Ceram. Int. 2020, 46, 20576–20581. [Google Scholar] [CrossRef]
- Ma, B.S.; Zhu, Y.; Wang, K.W.; Sun, Z.Z.; Ren, K.; Wang, Y.G. Reactive flash sintering and electrical transport properties of high-entropy (MgCoNiCuZn)1−xLixO oxides. J. Am. Ceram. Soc. 2022, 105, 3765–3773. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer–derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Greil, P. Acive-filler-controlled pyrolysis of preceramic polymers. J. Am. Ceram. Soc. 1995, 78, 835–848. [Google Scholar] [CrossRef]
- Bill, J.; Heimann, D. Polymer-derived ceramic coatings on C/C-SiC composites. J. Eur. Ceram. Soc. 1996, 16, 1115–1120. [Google Scholar] [CrossRef]
- Schulz, M. Polymer derived ceramics in MEMS/NEMS—A review on production processes and application. Adv. Appl. Ceram. 2009, 108, 454–460. [Google Scholar] [CrossRef]
- Jones, R.; Szweda, R.; Petrak, D. Polymer derived ceramic matrix composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 569–575. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, Q.; Cheng, L.; Wang, Y.G. Polymer–derived SiOC–barium–strontium aluminosilicate coatings as an environmental barrier for C/SiC Composites. J. Am. Ceram. Soc. 2010, 93, 4148–4152. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, J. Corrosion of barium aluminosilicates by water–vapor: An investigation from first principles. Corros. Sci. 2009, 51, 2126–2129. [Google Scholar] [CrossRef]
- More, K.L.; Tortorelli, P.F. Evaluating the Stability of BSAS–Based EBCs in High Water–Vapor Pressure Environments; Power for Land, Sea, & Air: Vienna, Austria, 2004; pp. 1–8. [Google Scholar]
- Haug, T.; Knale, H.; Ehrmann, U. Processing, properties and structure development of polymer–derived fiber–reinforced SiC. J. Am. Ceram. Soc. 1989, 72, 103–110. [Google Scholar]
- Nakano, K.; Kamiya, A.; Nishino, Y.; Imura, T.; Chou, T.W. Fabrication and characterization of three–dimensional carbon fiber reinforced silicon carbide and silicon nitride composites. J. Am. Ceram. Soc. 1995, 78, 2811–2814. [Google Scholar] [CrossRef]
- Naslain, R. Design, preparation and properties of non–oxide CMCs for application in engines and nuclear reactors: An overview. Compos. Sci. Technol. 2004, 64, 155–170. [Google Scholar] [CrossRef]
- Riedel, R.; Seher, M.; Mayer, J.; Szabó, D.V. Polymer–derived Si–based bulk ceramics, Part I: Preparation, processing and properties. J. Am. Ceram. Soc. 1995, 15, 703–715. [Google Scholar] [CrossRef]
- Raju, K.; Yoon, D.H. Sintering additives for SiC based on the reactivity: A review. Ceram. Int. 2016, 42, 17947–17962. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, C.H. Toughening behavior of silicon carbide with additions of yttria and alumina. J. Am. Ceram. Soc. 1990, 73, 1431–1434. [Google Scholar] [CrossRef]
- Jang, C.W.; Kim, J.; Kang, S.L. Effect of sintering atmosphere on grain shape and grain growth in liquid-phase-sintered silicon carbide. J. Am. Ceram. Soc. 2002, 85, 1281–1284. [Google Scholar] [CrossRef]
- Costello, J.A.; Tressler, R.E. Oxidation of silicon carbide crystals and ceramics: In dry oxygen. J. Am. Ceram. Soc. 1986, 69, 674–681. [Google Scholar] [CrossRef]
- Singhal, C.; Lange, F.F. Effect of alumina content on the oxidation of hot–pressed silicon carbide. J. Am. Ceram. Soc. 1975, 58, 133–135. [Google Scholar] [CrossRef]
- Wang, Y.G. Polymer–Derived Si-Al-C-N Ceramics: Oxidation, Hot–Corrosion and Structural Evolution. Ph.D Thesis, Dissertation of the University of Central Florida, Orlando, FL, USA, 2006. [Google Scholar]
- He, J.; Gao, Y.; Wang, Y.G.; Fang, J.; An, L. Synthesis of ZrB2-SiC nanocomposite powder via polymeric precursor route. Ceram. Int. 2017, 43, 1602–1607. [Google Scholar] [CrossRef]
- He, J.; Cao, Y.; Zhang, Y.; Wang, Y.G. Mechanical properties of ZrB2–SiC ceramics prepared by polymeric precursor route. Ceram. Int. 2018, 44, 6520–6526. [Google Scholar] [CrossRef]
- Raj, R.; An, L.; Shah, S.; Riedel, R.; Fasel, C.; Kleebe, H.J. Oxidation kinetics of an amorphous silicon carbonitride ceramic. J. Am. Ceram. Soc. 2001, 84, 1803–1810. [Google Scholar] [CrossRef]
- Riedel, R.; Ruwisch, L.M.; An, L.; Raj, R. Amorphous silicoboron carbonitride ceramics with anomalously high resistance to creep. J. Am. Ceram. Soc. 1998, 81, 3341–3344. [Google Scholar] [CrossRef]
- An, L.; Riedel, R.; Konetschny, C.; Kleebe, H.J.; Raj, R. Newtonian viscosity of amorphous silicon carbonitride at high temperature. J. Am. Ceram. Soc. 1998, 81, 1349–1352. [Google Scholar] [CrossRef]
- Thum, G.; Canel, J.; Bill, J.; Aldinger, F. Compression creep behavior of precursor-derived Si–C–N ceramics. J. Eur. Ceram. Soc. 1999, 19, 2317–2323. [Google Scholar]
- Wang, Y.G.; Fan, Y.; Zhang, L.; Zhang, W.; An, L. Polymer–derived SiAlCN ceramics resist to oxidation at 1400 °C. Scr. Mater. 2006, 55, 295–297. [Google Scholar] [CrossRef]
- Wang, Y.G.; Fei, W.; Fan, Y.; Zhang, L.; Zhang, W.; An, L. A silicoaluminum carbonitride ceramic resist oxidation/corrosion in water vapor. J. Mater. Res. 2006, 21, 1625–1628. [Google Scholar] [CrossRef]
- An, L.; Wang, Y.G.; Bharadwaj, L.; Zhang, L.; Fan, Y.; Jiang, D.; Sohn, Y.; Desai, V.H.; Kapat, J.; Chow, L.C. Silicoaluminum carbonitride with anomalously high resistance to oxidation and hot corrosion. Adv. Eng. Mater. 2004, 6, 337–340. [Google Scholar] [CrossRef]
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon–containing polymer–derived ceramic nanocomposites (PDC–NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu, C. Fabrication and properties of ceramic 1D nanostructures from preceramic polymers: A review. Adv. Appl. Ceram. 2011, 110, 188–204. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Cheng, L.; Yu, Z.; Huang, M.; Tu, H.; Xia, H. Effect of curing and pyrolysis processing on the ceramic yield of a highly branched polycarbosilane. J. Mater. Sci. 2009, 44, 721–725. [Google Scholar] [CrossRef]
- Hauser, A.Q.; Honnef, K.; Hanemann, T. Crosslinking behavior of UV-cured polyorganosilazane as polymer-derived ceramic precursor in ambient and nitrogen atmosphere. Polymers 2021, 13, 2424. [Google Scholar] [CrossRef]
- Zhang, L.G.; Wang, Y.S.; Wei, Y.; Xu, W.; Fang, D.; Zhai, L.; Lin, K.C.; An, L. A silicon carbonitride ceramic with anomalously high piezoresistivity. J. Am. Ceram. Soc. 2008, 91, 1346–1349. [Google Scholar] [CrossRef]
- Liu, W.; Cao, Y.; Cheng, L.; Wang, Y. Design of polymer-derived SiC for nuclear applications from the perspective of heterogeneous interfaces. J. Eur. Ceram. Soc. 2018, 38, 469–478. [Google Scholar] [CrossRef]
- Packirisamy, S.; Sreejith, K.J.; Devapal, D.; Swaminathan, B. Handbook of Advanced Ceramics and Composites: Polymer-Derived Ceramics and Their Space Applications; Springer Nature: Cham, Switzerland, 2020; pp. 999–1000. [Google Scholar]
- Gunji, T.; Sopyan, I.; Abe, Y. Synthesis of polytitanosiloxanes and their transformation to SiO2–TiO2 ceramic fibers. J. Polym. Sci. A Polym. Chem. 1994, 32, 3133–3313. [Google Scholar] [CrossRef]
- Liu, C.; Pan, R.; Hong, C.; Zhang, X.; Han, W.; Han, J.; Du, S. Effects of Zr on the precursor architecture and high-temperature nanostructure evolution of SiOC polymer derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 395–402. [Google Scholar] [CrossRef]
- Hotza, D.; Nishihora, R.K.; Machado, R.A.F.; Geffroy, P.M.; Chartier, T.; Bernard, S. Tape casting of preceramic polymers towards advanced ceramics: A review. Int. J. Ceram. Eng. Sci. 2019, 1, 21–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Quaranta, A.; Sorarù, G.D. Synthesis and luminescent properties of novel Eu2+-doped silicon oxycarbide glasses. Opt. Mater. 2004, 24, 601–605. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Raj, R. Fundamental research in structural ceramics for service near 2000 °C. J. Am. Ceram. Soc. 1993, 76, 2147–2174. [Google Scholar] [CrossRef]
- Riedel, R.; Passing, G.; Schonfelder, H.; Brook, R.J. Synthesis of dense silicon–based ceramics at low temperature. Nature 1992, 355, 714–717. [Google Scholar] [CrossRef]
- Riedel, R.; Kienzle, A.; Dressler, W.; Ruwisch, L.; Bill, J.; Aldinger, F. A silicoboron carbonitride ceramic stable to 2000 °C. Nature 1996, 382, 796–798. [Google Scholar] [CrossRef]
- Meraa, G.; Riedel, R.; Poli, F.; Müller, K. Carbon-rich SiCN ceramics derived from phenyl–containing poly(silylcarbodiimides). J. Eur. Ceram. Soc. 2009, 29, 2873–2883. [Google Scholar] [CrossRef]
- Wang, Z.C.; Aldinger, F.; Riedel, R. Novel silicon–boron–carbon–nitrogen materials thermally stable up to 2200 °C. J. Am. Ceram. Soc. 2001, 84, 2179–2183. [Google Scholar] [CrossRef]
- Yajima, S. Special heat-resisting materials from organometallic polymers. Am. Ceram. Soc. Bull. 1983, 62, 893–915. [Google Scholar]
- Cordelair, J.; Greil, P. Electrical conductivity measurements as a microprobe for structure transitions in polysiloxane derived Si–O–C ceramics. J. Eur. Ceram. Soc. 2000, 20, 1947–1957. [Google Scholar] [CrossRef]
- Wang, K.; Ma, B.; Li, X.; Wang, Y.G.; An, L. Structural evolutions in polymer–derived carbon–rich amorphous silicon carbide. J. Phys. Chem. A 2015, 119, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, B.; Li, X.; Wang, Y.; An, L. Effect of pyrolysis temperature on the structure and conduction of polymer–derived SiC. J. Am. Ceram. Soc. 2014, 97, 2135–2138. [Google Scholar] [CrossRef]
- Raj, R.; Toma, L.; Janssen, E.; Nuffer, J.; Melz, T.; Hanselka, H. Piezoresistive effect in SiOC ceramics for integrated pressure sensors. J. Am. Ceram. Soc. 2010, 93, 920–924. [Google Scholar]
- Riedel, R.; Kleee, H.J.; Schbrifelder, H.; Aldinger, F. A covalent micro/ nano composite resistant to high temperature oxidation. Nature 1995, 374, 526–528. [Google Scholar] [CrossRef]
- Wang, Y.G.; An, L.; Fan, Y.; Zhang, L.; Burton, S.; Gao, Z. Oxidation of polymer–derived SiAlCN ceramics. J. Am. Ceram. Soc. 2005, 88, 3075–3080. [Google Scholar] [CrossRef]
- Wang, Y.G.; Fei, W.; An, L. Oxidation/corrosion of polymer–derived SiAlCN ceramics in water vapor. J. Am. Ceram. Soc. 2006, 89, 1079–1082. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.G.; Luo, L.; An, L. Oxidation behaviour of ZrB2–SiC (AlY) ceramics at 1700 °C. J. Eur. Ceram. Soc. 2016, 36, 3769–3774. [Google Scholar] [CrossRef]
- Zhang, H.; Pantano, C.G. Synthesis and characterization of silicon oxycarbide glasses. J. Am. Ceram. Soc. 1990, 73, 958–963. [Google Scholar] [CrossRef]
- Sorarù, G.D. Silicon oxycarbide glasses from gels. J. Sol–Gel Sci. Technol. 1994, 2, 843–848. [Google Scholar] [CrossRef]
- Sorarù, G.D.; Andrea, G.D.; Campostrini, R.; Babonneau, F. Si–O–C glasses from gels. In Sol–Gel Science and Technology; Pope, E.J.A., Sakka, S., Klein, L., Eds.; American Ceramic Society: Westerville, OH, USA, 1994; pp. 135–146. [Google Scholar]
- Sorarù, G.D.; Andrea, G.D.; Campostrini, R.; Babonneau, F.; Mariotto, G. Structural characterization and high temperature behaviour of silicon oxycarbide glasses prepared from Sol–Gel precursors containing Si–H bonds. J. Am. Ceram. Soc. 1995, 78, 379–387. [Google Scholar] [CrossRef]
- Karakuscu, A.; Guider, R.; Paves, L.; Sorarù, G.D. White kuminescence from sol–gel-derived SiOC thin films. J. Am. Ceram. Soc. 2009, 92, 2969–2974. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Omori, M.; Okamura, K. Development of a silicon carbide fiber with high tensile strength. Nature 1976, 261, 683–685. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Iimura, M.; Yajima, S. Synthesis of continuous silicon carbide fibre. Part. 2 conversion of polycarbosilane fibre into silicon carbide fibres. J. Mater. Sci. 1980, 15, 720–728. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Okamura, K. Synthesis of continuous silicon carbide fibre. J. Mater. Sci. 1983, 18, 3633–3648. [Google Scholar] [CrossRef]
- Yajima, S.; Iwai, T.; Yamamura, T.; Okamura, K.; Hasegawa, Y. Synthesis of a polytitanocarbosilane and its conversion into inorganic compounds. J. Mater. Sci. 1981, 16, 1349–1355. [Google Scholar] [CrossRef]
- Yamamura, T.; Ishikawa, T.; Shibuya, M.; Hisayuki, T.; Okamura, K. Development of a new continuous Si–Ti–C–O fibre using an organometallic polymer precursor. J. Mater. Sci. 1988, 23, 2589–2594. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kohtoku, Y.; Kumagawa, K.; Yamamura, T.; Nasagawa, T. High–strenght, alkali–resistant sintered SiC fibre stable up to 2200 °C. Nature 1998, 391, 773–775. [Google Scholar] [CrossRef]
- Moraes, K.V.; Interrante, L.V. Processing, fracture toughness, and vickers hardness of allylhydridopolycarbosilane–derived silicon carbide. J. Am. Ceram. Soc. 2003, 86, 342–346. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Ferber, D.L.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Raj, R.; et al. Polymer derived Si–B–C–N ceramics: 30 years of research. Adv. Eng. Mater. 2018, 20, 1800360. [Google Scholar] [CrossRef]
- Kodama, H.; Miyoshi, T. Study of fracture behavior of very fine–grained silicon carbide ceramics. J. Am. Ceram. Soc. 1990, 73, 3081–3086. [Google Scholar] [CrossRef]
- Niihara, K. New design concept of structural ceramics: Ceramic nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Kumar, R.; Cai, Y.; Gerstel, P.; Rixecker, G.; Aldinger, F. Processing, crystallization and characterization of polymer derived nano–crystalline Si–B–C–N ceramics. J. Mater. Sci. 2006, 41, 7088–7095. [Google Scholar] [CrossRef]
- Bill, J.; Kamphowe, T.W.; Müller, A.; Wichmann, T.; Zern, A.; Jalowieki, A.; Mayer, J.; Weinmann, M.; Schuhmacher, J.; Müller, K.; et al. Precursor–derived Si–(B–)C–N ceramics: Thermolysis, amorphous state and crystallization. Appl. Organomet. Chem. 2001, 15, 777. [Google Scholar] [CrossRef]
- Ye, B.L.; Wen, T.Q.; Nguyen, M.; Hao, L.; Wang, C.; Chu, Y.H. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high-entropy ceramics. Acta Mater. 2019, 170, 15–23. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Sang, X.; Unocic, R.R.; Kinch, R.T.; Liu, X.; Hu, J.; Liu, H.; Dai, S. Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy. Adv. Mater. 2018, 30, 1707512. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Rojas, J.; Ahlborg, N.; Lim, K.; Toney, M.F.; Jin, H.; Chueh, W.C.; Majumdar, A. The use of poly-cation oxides to lower the temperature of two-step thermochemical water splitting. Energy Environ. Sci. 2018, 11, 2172–2178. [Google Scholar] [CrossRef]
- Braun, J.L.; Rost, C.M.; Lim, M.; Giri, A.; Olson, D.H.; Kotsonis, G.N.; Stan, G.; Brenner, D.W.; Maria, J.P.; Hopkins, P.E. Charge-induced disorder controls the thermal conductivity of entropy-stabilized oxides. Adv. Mater. 2018, 30, 1805004. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Hirai, T.; Yagi, K.; Nakai, K.; Okamoto, K.; Murai, D.; Okamoto, H. High-entropy polymer blends utilizing in situ exchange reaction. Polymer 2022, 240, 124483. [Google Scholar] [CrossRef]
- Qian, X.; Han, D.; Zheng, L.; Chen, J.; Tyagi, M.; Li, Q.; Du, F.; Zheng, S.; Huang, X.; Zhang, S.; et al. High-entropy polymer produces a giant electrocaloric effect at low fields. Nature 2021, 600, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.P.; Yang, Q.; McCormack, S.J.; Kriven, W.M. High-entropy, phase-constrained, lanthanide sesquioxide. J. Am. Ceram. Soc. 2020, 103, 569–576. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, F.; Qiu, W.; Ye, L.; Han, W.; Zhao, W.; Zhou, H.; Zhao, T. Synthesis of rare earth containing single-phase multicomponent metal carbides via liquid polymer precursor route. J. Am. Ceram. Soc. 2020, 103, 6081–6087. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zhang, Y.; Chen, F.; Han, W.; Qiu, W.; Zhao, T. Synthesis of high entropy carbide ceramics via polymer precursor route. Ceram. Int. 2022, 48, 15939–15945. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Cestari, A. Sol-gel methods for synthesis of aluminosilicates for dental applications. J. Dent. 2016, 55, 105–113. [Google Scholar] [CrossRef]
- Shankhwar, N.; Srinivasan, A. Evaluation of sol–gel based magnetic 45S5 bioglass and bioglass–ceramics containing iron oxide. Mater. Sci. Eng. C 2016, 62, 190–196. [Google Scholar] [CrossRef]
- Li, F.; Lu, Y.; Wang, X.G.; Bao, W.; Liu, J.X.; Xu, F.; Zhang, G.J. Liquid precursor-derived high-entropy carbide nanopowders. Ceram. Int. 2019, 45, 22437–22441. [Google Scholar] [CrossRef]
- Du, B.; Liu, H.; Chu, Y. Fabrication and characterization of polymer-derived high-entropy carbide ceramic powders. J. Am. Ceram. Soc. 2020, 103, 4063–4068. [Google Scholar] [CrossRef]

| Processing Parameters | Conventional Route | PDC Route |
|---|---|---|
| Ceramic Raw Material | Ceramic powders, such as alumina, zirconia, silicon carbide, or aluminum nitride | Precursor polymers, such as polysiloxanes or polysilazanes, with passive or active fillers |
| Mixing/Milling | Powders are mixed, generally in a ball mill, to liquid + dispersant for breaking up agglomerates; binders and plasticizers are added homogenized | Synthesis: solid or liquid are dissolved, with the aid of different equipment, in a solvent; fillers, crosslinkers, and others are added and homogenized |
| Shaping | Cutting or press into desired shapes | |
| Thermal Treatments | Debinding at middle temperatures and sintering at high temperatures are needed | Crosslinking at low temperatures (as low as room temperature) and pyrolysis at high temperatures are needed; eventually, crystallization at higher temperatures is accomplished; composite materials may be produced with partial pyrolysis of precursors |
| Ceramic Products | Dense parts with a residual porosity and controlled shrinkage, or, less often, macroporous parts; all kinds of oxide and non-oxide ceramics may be fabricated | Near net shape parts with the use of active/passive fillers, or controlled porosity with the aid of pore formers; mostly silicon-based ceramics are fabricated (SiC, SiOC, SiOCN…) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Song, M.; Chen, K.; Kan, D.; Zhu, M. Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics. Crystals 2022, 12, 1292. https://doi.org/10.3390/cryst12091292
He J, Song M, Chen K, Kan D, Zhu M. Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics. Crystals. 2022; 12(9):1292. https://doi.org/10.3390/cryst12091292
Chicago/Turabian StyleHe, Jiabei, Mengshan Song, Kaiyun Chen, Dongxiao Kan, and Miaomiao Zhu. 2022. "Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics" Crystals 12, no. 9: 1292. https://doi.org/10.3390/cryst12091292
APA StyleHe, J., Song, M., Chen, K., Kan, D., & Zhu, M. (2022). Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics. Crystals, 12(9), 1292. https://doi.org/10.3390/cryst12091292







