Exploration of Optical Properties of Novel Pyrene Derivatives Modified by Click Functionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Method
2.2. Experimental Method
2.2.1. Synthesis of Trimethyl(pyren-1-ylethynyl) Silane (1)
2.2.2. Synthesis of 1-Ethynylpyrene (2)
2.2.3. Synthesis of N,N-Dihexadecyl-4-(pyren-1-ylethynyl)aniline (PY-C16)
2.2.4. Synthesis of 2-(4-(Dihexadecylamino)phenyl)-3-(pyren-1-yl)buta-1,3-diene-1,1,4,4-tetracarbo Nitrile (PY-C16-TCNE)
2.2.5. Synthesis of 2-(4-(3,3-Dicyano-1-(4-(dihexadecylamino)phenyl)-2-(pyren-1-yl)allylidene)cyclohexa-2,5-dien-1-ylidene)malononitrile (PY-C16-TCNQ)
3. Results and Discussion
3.1. UV-Vis and PL Spectroscopy
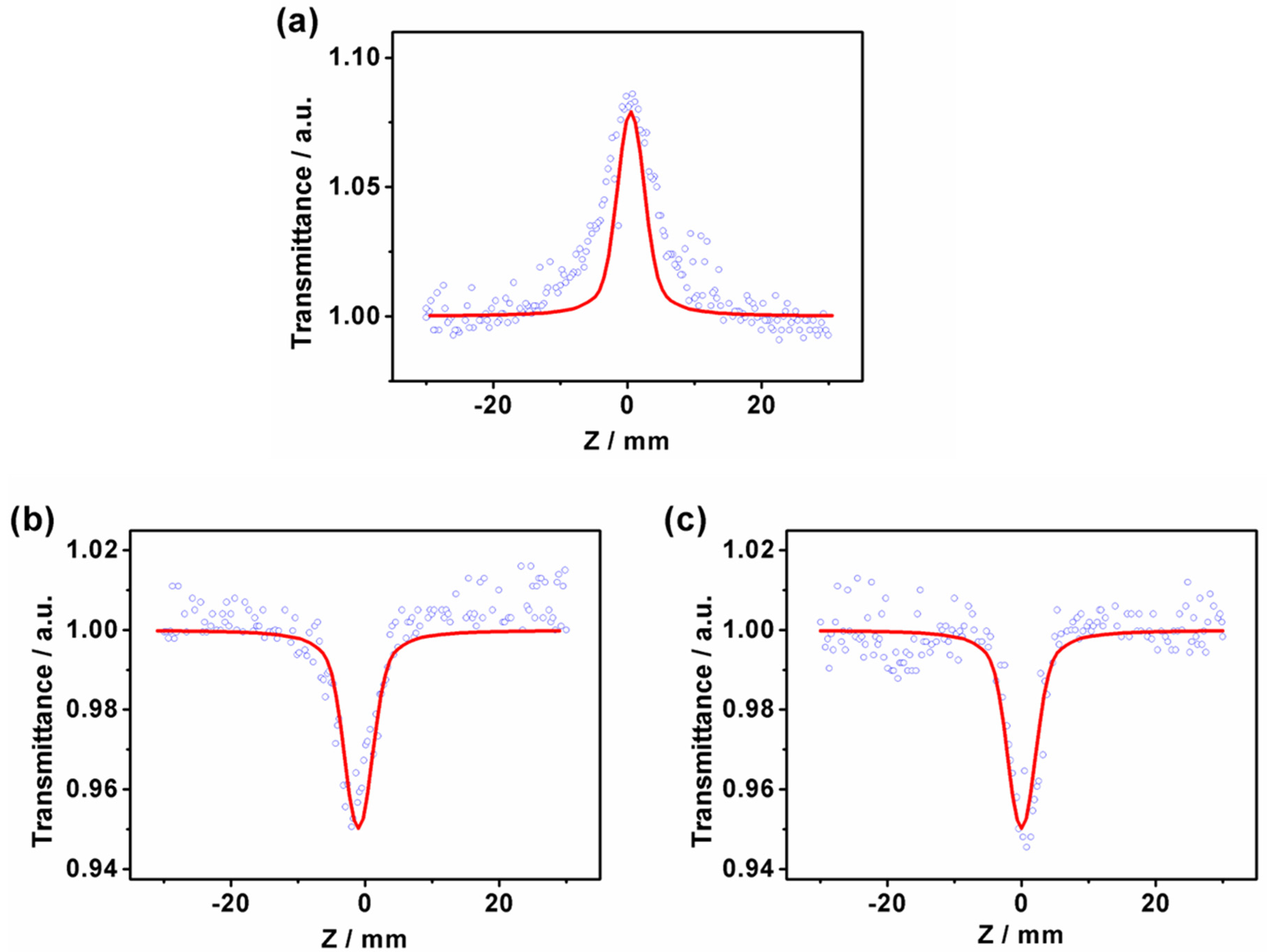
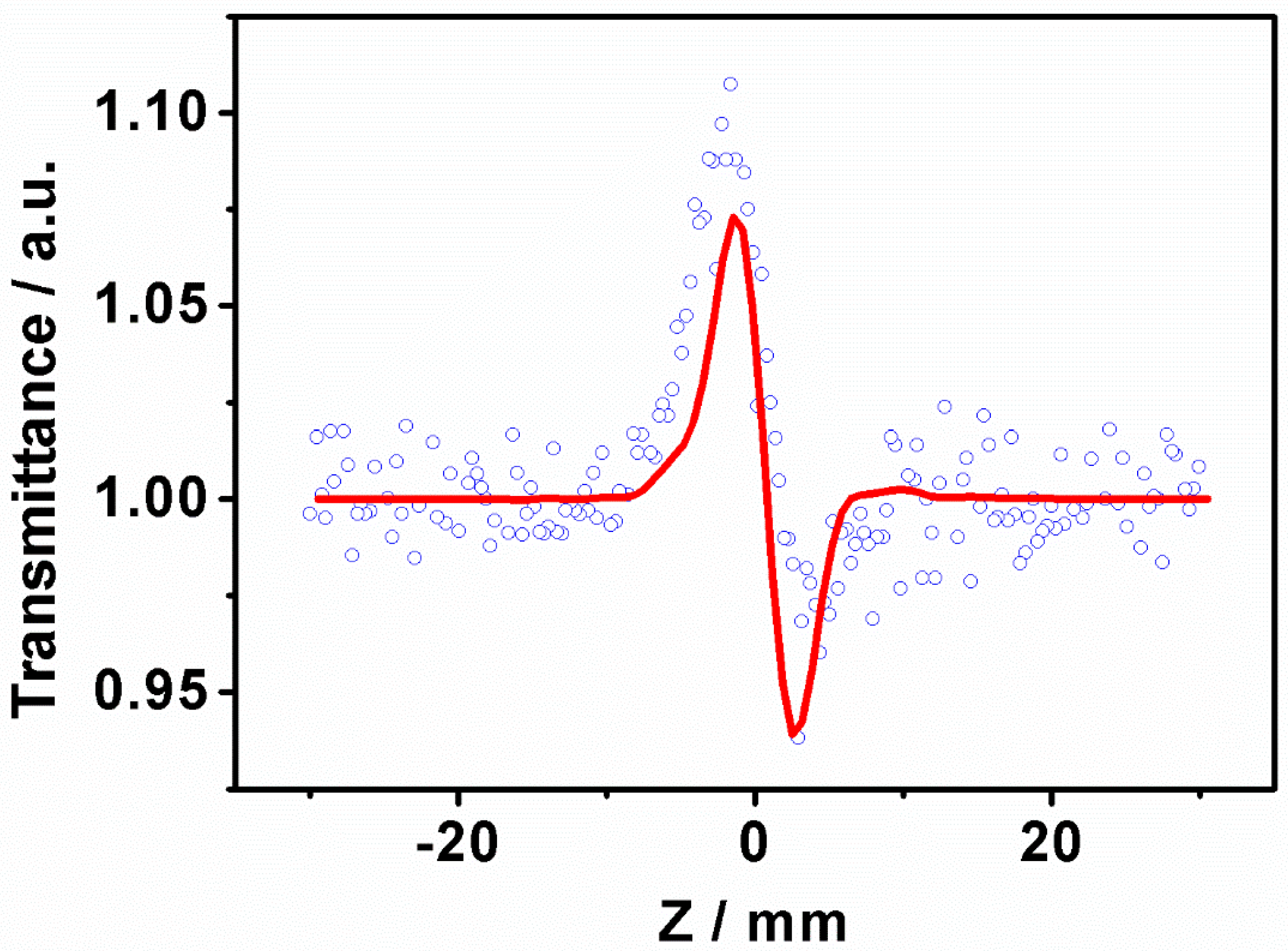
3.2. Z-Scan Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byer, R. Nonlinear optics and solid-state lasers: 2000. IEEE J. Sel. Top. Quantum Electron. 2000, 6, 911–930. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Luo, J.; Jen, A.K.-Y. High-performance organic second- and third-order nonlinear optical materials for ultrafast information processing. J. Mater. Chem. C 2020, 8, 15009–15026. [Google Scholar] [CrossRef]
- Sang, X.; Chu, P.L.; Yu, C. Applications of Nonlinear Effects in Highly Nonlinear Photonic Crystal Fiber to Optical Communications. Opt. Quantum Electron. 2005, 37, 965–994. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.F.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Chanclou, P.; Thual, M.; Lostec, J.; Gadonna, M. Double external cavity laser diode for DWDM applications. J. Opt. A Pure Appl. Opt. 1999, 2, L6–L8. [Google Scholar] [CrossRef]
- Deng, G.; Chun, X.; Li, Z.; Zhang, X.; Yang, Y.; Huo, F.; Zhang, G.; Fedorchuk, A.; Kityk, I. Synthesis and features of nonlinear optical switches based on dithienylethene unit. Tetrahedron Lett. 2018, 59, 3448–3452. [Google Scholar] [CrossRef]
- Tomiyasu, M.; Egami, C. Hollow tube fabrication in photoresist film using continuous wave laser. Opt. Rev. 2011, 18, 357–359. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Babu, M.; Kalluraya, B.; Chandrasekharan, K. Third-order nonlinear optical response of newly synthesized accepter/donor substituted propylidene aryloxy acet hydrazide. Optik 2012, 123, 21–25. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; He, J.; He, Z.; Wu, H. The important role of tetraphenylethene on designing bichromophores for organic nonlinear optical materials. Mater. Lett. 2021, 291, 129521. [Google Scholar] [CrossRef]
- Jagadesan, A.; Sivakumar, N.; Arjunan, S.; Parthipan, G. Growth, structural, optical, thermal and dielectric behaviour of a novel organic nonlinear optical (NLO) material: Benzimidazolium trichloroacetate monohydrate. Opt. Mater. 2020, 109, 110285. [Google Scholar] [CrossRef]
- Rosenne, S.; Grinvald, E.; Shirman, E.; Neeman, L.; Dutta, S.; Bar-Elli, O.; Ben-Zvi, R.; Oksenberg, E.; Milko, P.; Kalchenko, V.; et al. Self-Assembled Organic Nanocrystals with Strong Nonlinear Optical Response. Nano Lett. 2015, 15, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, Z.; Sun, H. Design of zinc porphyrin-perylene diimide donor-bridge-acceptor chromophores for large second-order nonlinear optical response: A theoretical exploration. Int. J. Quantum Chem. 2017, 118, e25536. [Google Scholar] [CrossRef]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2020, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Lechner, V.M.; Nappi, M.; Deneny, P.J.; Folliet, S.; Chu, J.C.K.; Gaunt, M.J. Visible-Light-Mediated Modification and Manipulation of Biomacromolecules. Chem. Rev. 2021, 122, 1752–1829. [Google Scholar] [CrossRef]
- Shamsiya, A.; Bahulayan, D. D–A systems based on oxazolone–coumarin triazoles as solid-state emitters and inhibitors of human cervical cancer cells (HeLa). New J. Chem. 2021, 46, 480–489. [Google Scholar] [CrossRef]
- Tang, W.; Becker, M.L. “Click” reactions: A versatile toolbox for the synthesis of peptide-conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039. [Google Scholar] [CrossRef]
- He, W.; Washino, Y.; Murata, K.; Nozaki, N.; Matsumoto, H.; Michinobu, T. [2+2] Cycloaddition-retroelectrocyclization reactivity and thin film transistor performances of carbazole-based platinum polyyne polymers. Mater. Chem. Phys. 2022, 281, 125861. [Google Scholar] [CrossRef]
- Michinobu, T. Click Functionalization of Aromatic Polymers for Organic Electronic Device Applications. Macromol. Chem. Phys. 2015, 216, 1387–1395. [Google Scholar] [CrossRef]
- Michinobu, T.; Diederich, F. The [2+2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem. Int. Ed. 2018, 57, 3552–3577. [Google Scholar] [CrossRef]
- Michinobu, T. Click-Type Reaction of Aromatic Polyamines for Improvement of Thermal and Optoelectronic Properties. J. Am. Chem. Soc. 2008, 130, 14074–14075. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Click chemistry functionalization improving the wideband optical-limiting performance of fullerene derivatives. Phys. Chem. Chem. Phys. 2016, 18, 7341–7348. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Nonlinear optical properties of symmetrical and asymmetrical porphyrin derivatives with click chemistry modification. Dye. Pigment. 2016, 134, 155–163. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Q.; Deng, H.; Zhao, W.; Chen, P.; Tang, R.; Li, Q.; Li, Z. Janus NLO dendrimers with different peripheral functional groups: Convenient synthesis and enhanced NLO performance with the aid of the Ar–ArF self-assembly. J. Mater. Chem. C 2019, 7, 7344–7351. [Google Scholar] [CrossRef]
- Liu, J.; Huo, F.; He, W. Construction of a simple crosslinking system and its influence on the poling efficiency and oriental stability of organic electro-optic materials. RSC Adv. 2020, 10, 6482–6490. [Google Scholar] [CrossRef]
- Mi, Y.; Liang, P.; Jin, Z.; Wang, D.; Yang, Z. Synthesis and Third-Order Nonlinear Optical Properties of Triphenylene Derivatives Modified by Click Chemistry. ChemPhysChem 2013, 14, 4102–4108. [Google Scholar] [CrossRef]
- Miao, Z.; Chu, Y.; Wang, L.; Zhu, W.; Wang, D. Nonlinear Optical and Ion Sensor Properties of Novel Molecules Conjugated by Click Chemistry. Polymers 2022, 14, 1516. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Long, G.; Li, Y.; Ganguly, R.; Zhang, M.; Aratani, N.; Yamada, H.; Liu, M.; Zhang, Q. Pyrene-Containing Twistarene: Twelve Benzene Rings Fused in a Row. Angew. Chem. Int. Ed. 2018, 57, 13555–13559. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Han, Y.; Song, T.; Zhao, X.; Xiao, J. Synthesis, physical properties and electroluminescence of functionalized pyrene derivative. Dye. Pigment. 2019, 167, 22–28. [Google Scholar] [CrossRef]
- Xu, Z.; Singh, N.J.; Lim, J.; Pan, J.; Na Kim, H.; Park, S.; Kim, K.S.; Yoon, J. Unique Sandwich Stacking of Pyrene-Adenine-Pyrene for Selective and Ratiometric Fluorescent Sensing of ATP at Physiological pH. J. Am. Chem. Soc. 2009, 131, 15528–15533. [Google Scholar] [CrossRef]
- Iwasaki, T.; Murakami, S.; Takeda, Y.; Fukuhara, G.; Tohnai, N.; Yakiyama, Y.; Sakurai, H.; Kambe, N. Molecular Packing and Solid-State Photophysical Properties of 1,3,6,8-Tetraalkylpyrenes. Chem.–A Eur. J. 2019, 25, 14817–14825. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jia, J.; Sun, J.; Yin, A.; Zhao, M.; Song, Y. Study of ultrafast nonlinear optical response and transient dynamics of pyrene derivatives with intramolecular charge transfer characteristics. Opt. Mater. 2022, 128, 112378. [Google Scholar] [CrossRef]
- Khan, M.U.; Khalid, M.; Khera, R.A.; Akhtar, M.N.; Abbas, A.; ur Rehman, M.F.; Lu, C. Influence of acceptor tethering on the performance of nonlinear optical properties for pyrene-based materials with A-π-D-π-D architecture. Arabian J. Chem. 2022, 15, 103673. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, S.-W. A pyrene-functionalized polytyrosine exhibiting aggregation-induced emission and capable of dispersing carbon nanotubes and hydrogen bonding with P4VP. Polymer 2018, 156, 10–21. [Google Scholar] [CrossRef]
- Locritani, M.; Yu, Y.; Bergamini, G.; Baroncini, M.; Molloy, J.K.; Korgel, B.A.; Ceroni, P. Silicon Nanocrystals Functionalized with Pyrene Units: Efficient Light-Harvesting Antennae with Bright Near-Infrared Emission. J. Phys. Chem. Lett. 2014, 5, 3325–3329. [Google Scholar] [CrossRef]
- Peng, M.; Wang, Y.; Zhang, X. Pyrene-containing dyes: Reversible click/declick reaction, optical and aggregation behaviors. Dye. Pigment. 2020, 179, 108375. [Google Scholar] [CrossRef]
- Koenig, J.D.B.; Farahat, M.E.; Welch, G.C. Development of Tetrameric N-Annulated Perylene Diimides Using “Click” Chemistry. ChemSusChem 2022, 15, e202200492. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–organic frameworks catalyzed C–C and C–heteroatom coupling reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef]
- Crouch, R.D. Selective deprotection of Silyl ethers crossmark. Tetrahedron 2013, 69, 2383–2417. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Chae, S.; Shim, J.Y.; Lee, D.Y.; Kim, I.; Kim, H.J.; Park, S.H.; Suh, H. Conjugated polymers containing pyrimidine with electron withdrawing substituents for organic photovoltaics with high open-circuit voltage. Polymer 2015, 83, 50–58. [Google Scholar] [CrossRef]
- Sun, Z.; Zeng, Z.; Wu, J. Zethrenes, Extended p-Quinodimethanes, and Periacenes with a Singlet Biradical Ground State. Accounts Chem. Res. 2014, 47, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- An, B.-K.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2011, 45, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Cornil, J.; Beljonne, D.; Calbert, J.P.; Brédas, J.L. Interchain interactions in organic π-conjugated materials: Impact on electronic structure, optical response, and charge transport. Adv. Mater. 2001, 13, 1053–1067. [Google Scholar] [CrossRef]
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Self-Assembly of Intramolecular Charge-Transfer Compounds into Functional Molecular Systems. Acc. Chem. Res. 2014, 47, 1186–1198. [Google Scholar] [CrossRef]




| Solvent | λabs,1 (max/nm) | λabs,2 (max/nm) | λem (max/nm) | Stokes Shift (cm−1) |
|---|---|---|---|---|
| petroleum ether(PE) | 414 | 388 | 430 | 898 |
| hexane | 414 | 386 | 430 | 898 |
| toluene | 416 | 392 | 470 | 2761 |
| dichloromethane (DCM) | 414 | 392 | 515 | 4737 |
| EtOAc | 408 | 388 | 500 | 4509 |
| CHCl3 | 412 | 392 | 490 | 3863 |
| N,N-Dimethylformamide (DMF) | 414 | 388 | 555 | 6136 |
| Samples | Β × 10−12 (m/W) | n2 × 10−19 (m2/W) | χ(3) × 10−13 (esu) |
|---|---|---|---|
| PY-C16 | −2.1 | −1.7 | 4.8 |
| PY-C16-TCNE | 0.8 | − | 1.8 |
| PY-C16-TCNQ | 1.4 | − | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhao, Y.; Mi, Y.; Zhao, Y.; Guo, Z.; Zhang, H.; Wang, D.; Miao, Z. Exploration of Optical Properties of Novel Pyrene Derivatives Modified by Click Functionalization. Crystals 2022, 12, 1295. https://doi.org/10.3390/cryst12091295
Yu Y, Zhao Y, Mi Y, Zhao Y, Guo Z, Zhang H, Wang D, Miao Z. Exploration of Optical Properties of Novel Pyrene Derivatives Modified by Click Functionalization. Crystals. 2022; 12(9):1295. https://doi.org/10.3390/cryst12091295
Chicago/Turabian StyleYu, Yang, Yuzhen Zhao, Yongsheng Mi, Yang Zhao, Zhun Guo, Huimin Zhang, Dong Wang, and Zongcheng Miao. 2022. "Exploration of Optical Properties of Novel Pyrene Derivatives Modified by Click Functionalization" Crystals 12, no. 9: 1295. https://doi.org/10.3390/cryst12091295
APA StyleYu, Y., Zhao, Y., Mi, Y., Zhao, Y., Guo, Z., Zhang, H., Wang, D., & Miao, Z. (2022). Exploration of Optical Properties of Novel Pyrene Derivatives Modified by Click Functionalization. Crystals, 12(9), 1295. https://doi.org/10.3390/cryst12091295






