Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Results and Discussions
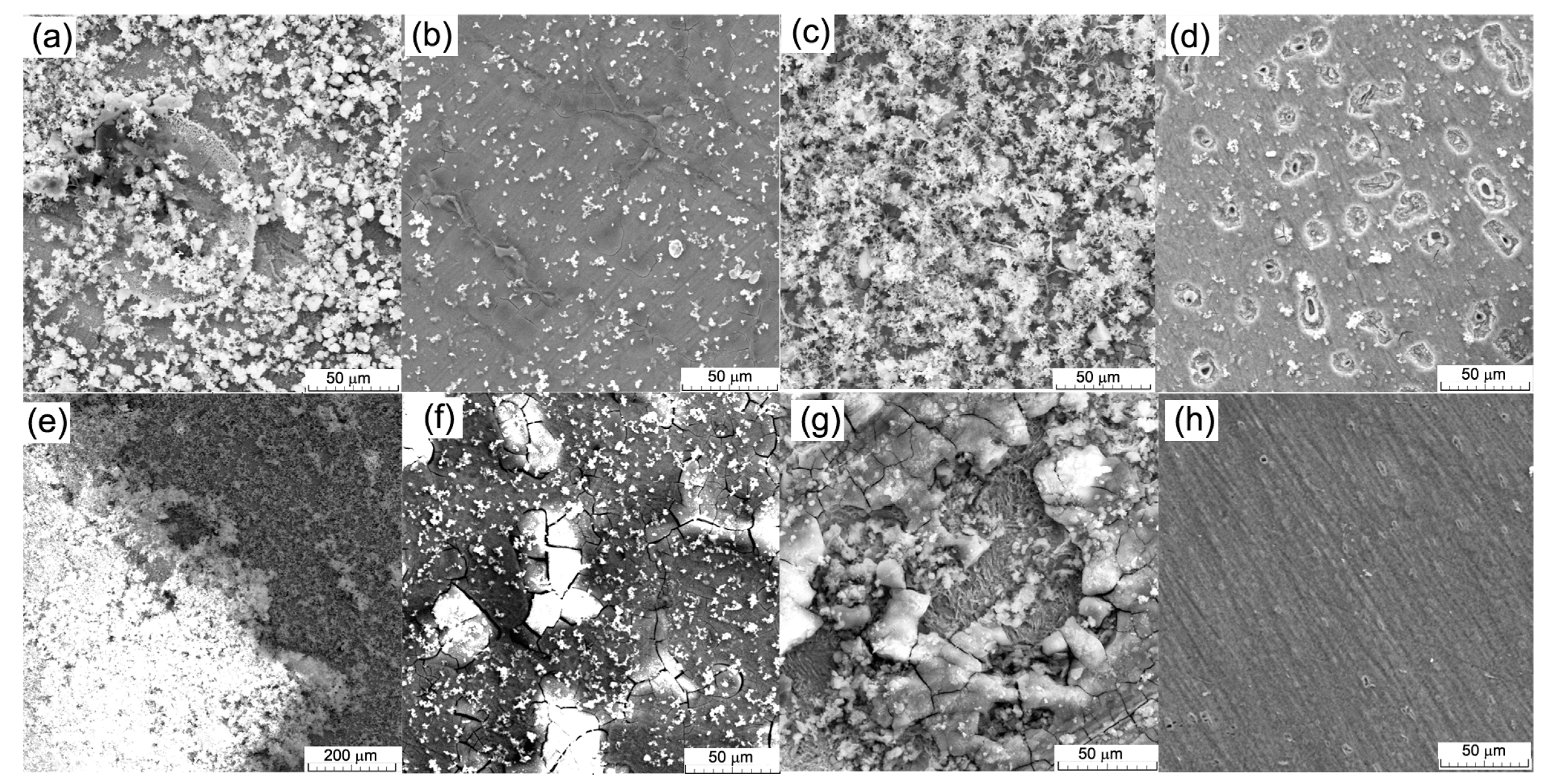
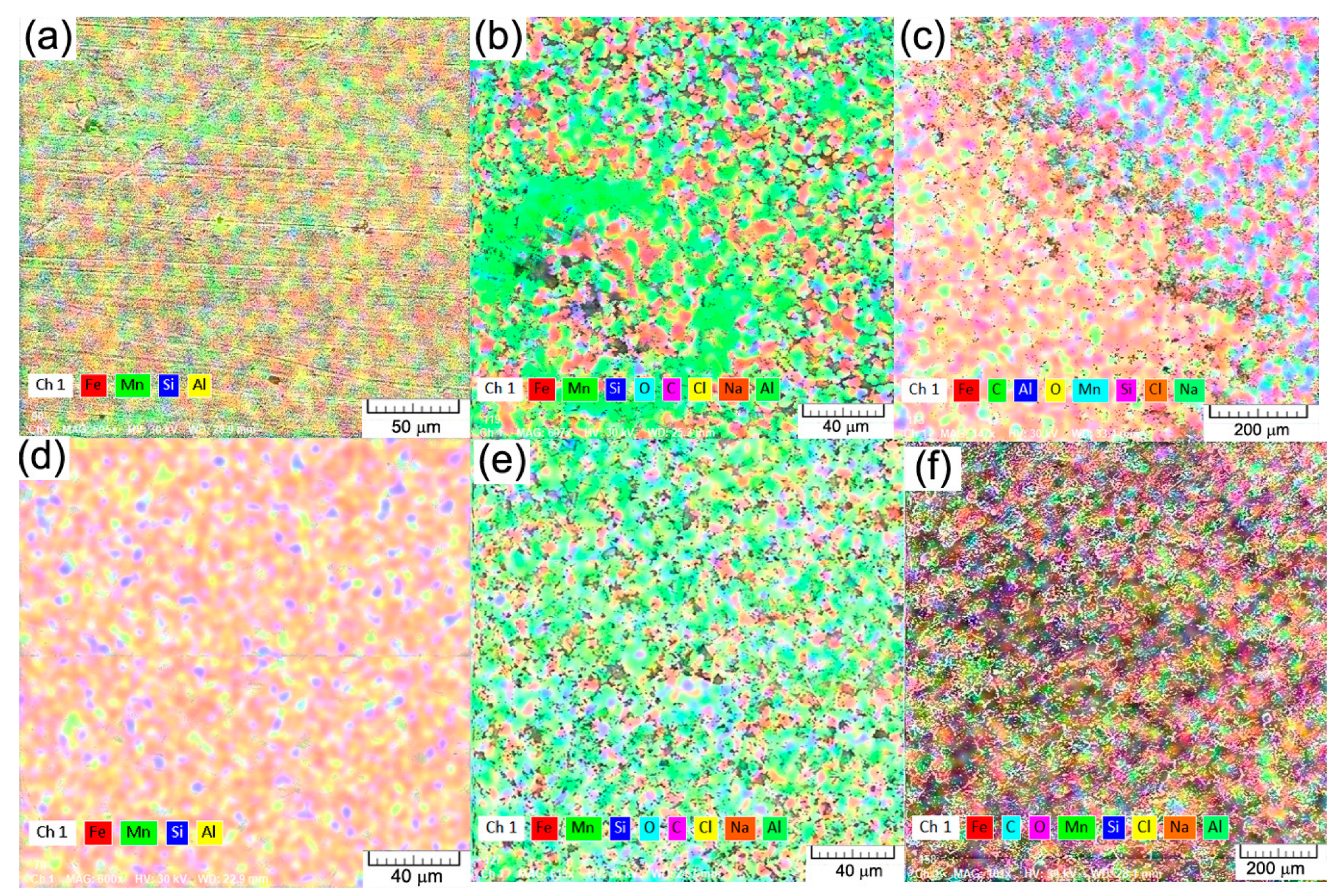
3.1. Microstructural and Chemical Analysis of FeMnSiAl Alloy
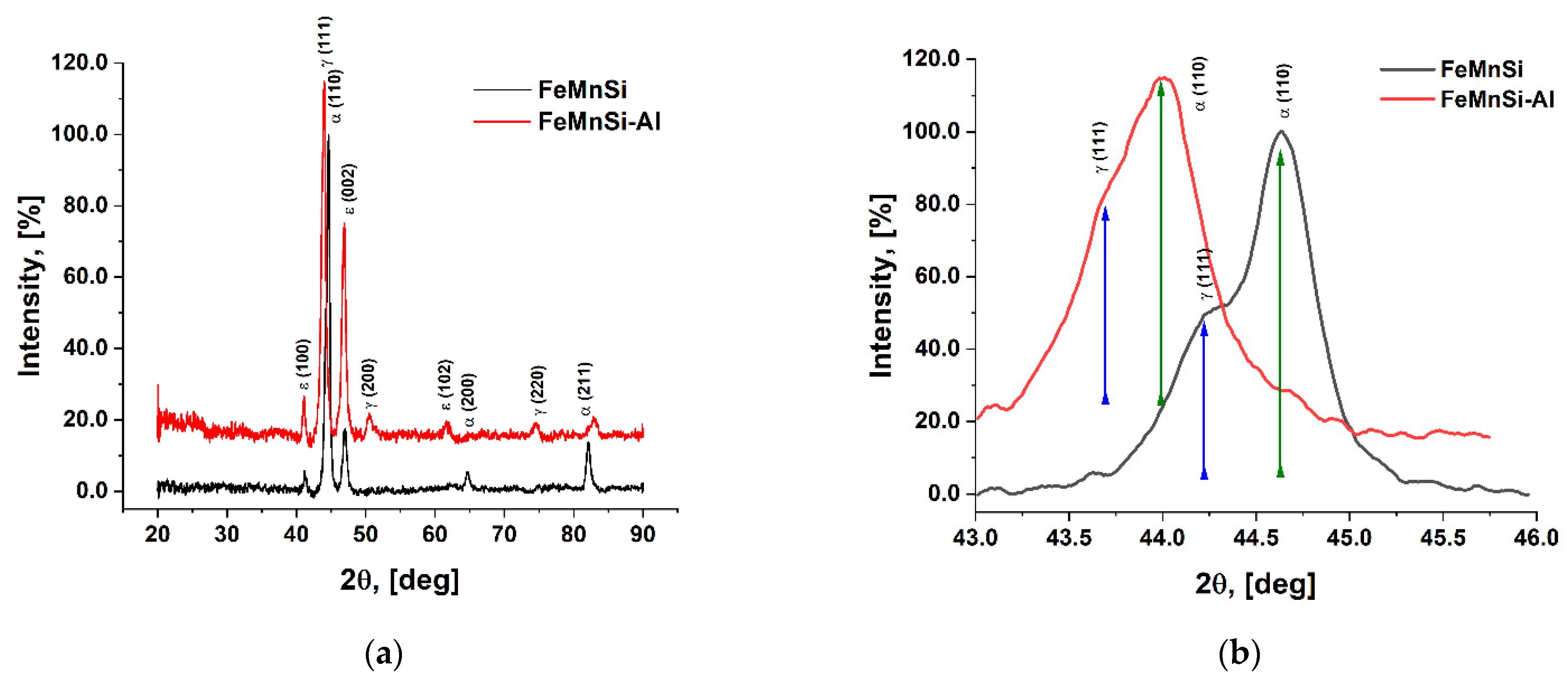
3.2. Structural Analysis by XRD
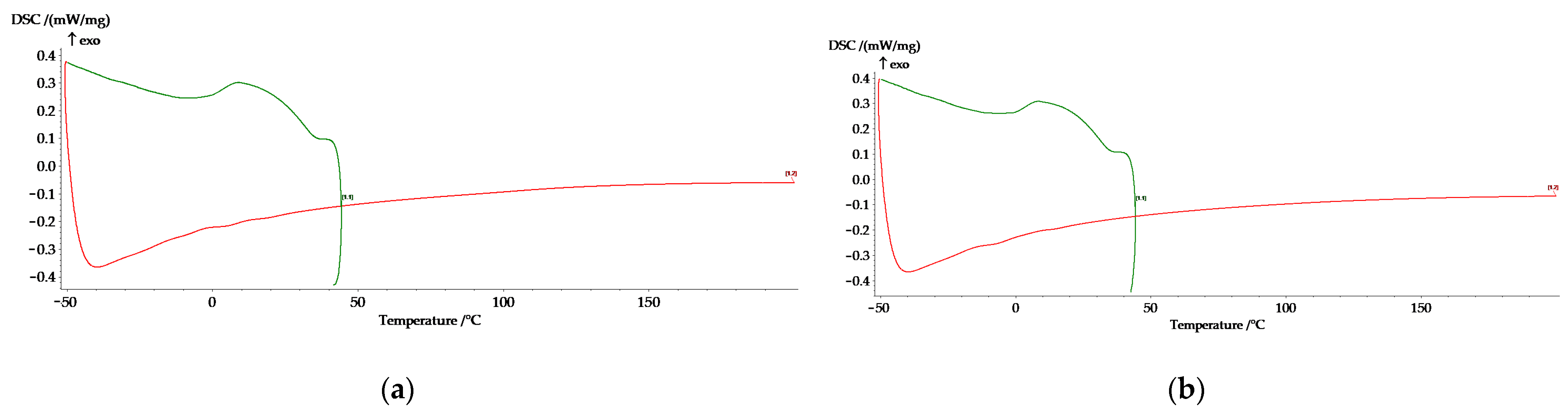
3.3. DSC Analysis for the FeMnSiAl Alloy
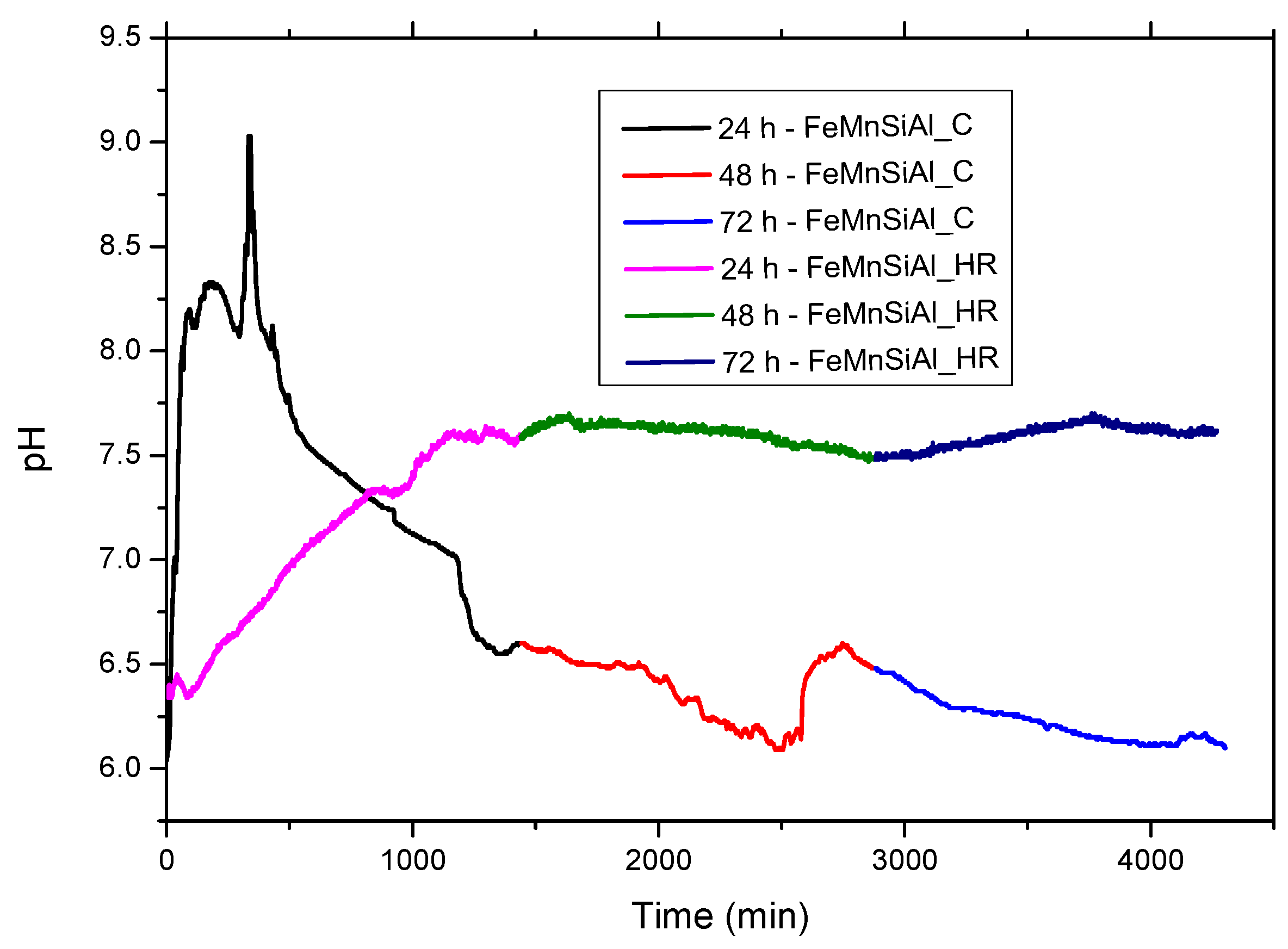
3.4. The pH Variation of the Electrolyte Solution and the Immersion Experiment
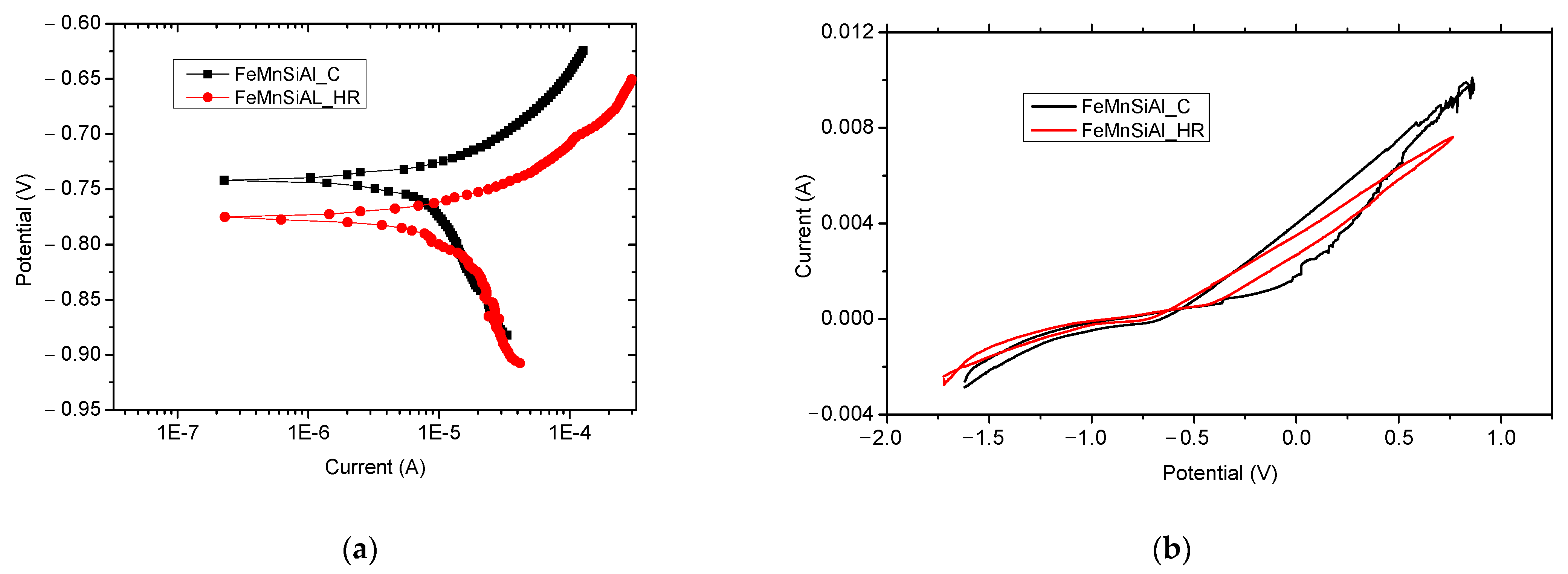
3.5. Electro-Chemical and EIS Analysis
4. Conclusions
- a homogeneous alloy was obtained through arc-melting and induction furnace re-melting;
- a solid-state transformation was recorded using DSC at cooling with the Ms temperature near the human body temperature, encouraging the potential of this material for medical applications;
- different corrosion stages of the Ringer solution were characterized through a mass loss analysis (the CR was determined) and chemical composition determinations (the presence of oxides from day one, the carbon growth based on the surface compounds with high stability, the appearance of chloride and sodium-based compounds with low stability on the surface, a combination of pitting with general corrosion was observed on the surface);
- along with the immersion period, the CR (electro-corrosion in case of galvanic couple) varies considering the complex layer properties formed on top of the surface;
- further investigation on the SME of this smart material with corrosion evolution must be fulfilled to implement it in medical applications; however, currently the results are promising in this direction. More investigation on cytotoxicity of the alloy must be performed and the effect of the aluminum alloying percentage on the possibility of influencing the appearance of different diseases, such as Alzheimer, can be taken in consideration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rapetto, C.; Leoncini, M. Magmaris: A new generation metallic sirolimus-eluting fully bioresorbable scaffold: Present status and future perspectives. J. Thorac. Dis. 2017, 9, S903. [Google Scholar] [CrossRef] [Green Version]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthcare Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [Green Version]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brugmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal-results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar]
- He, J.; He, F.L.; Li, D.; Liu, Y.L.; Liu, Y.Y.; Ye, Y.J.; Yin, D.C. Advances in Fe-based bio-degradable metallic materials. RSC Adv. 2016, 6, 112819–112838. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; Schnakenburg, C.V. Long term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C 2019, 99, 838–852. [Google Scholar] [CrossRef]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron-manganese: New class of de-gradable metallic biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M. Biodegradable Fe-based alloys for use in osteo-synthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Loffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC(Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Gebert, A.; Kochta, F.; Voß, A.; Oswald, S.; Fernandez-Barcia, M.; Kühn, U.; Hufenbach, J. Corrosion studies on Fe-30Mn-1C alloy in chloride-containing solutions with view to biomedical application. Mater. Corros. 2018, 69, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Harjanto, S.; Pratesa, Y.; Suharno, B.; Syarif, J. Corrosion Behavior of Fe-Mn-C Alloy as Degradable Materials Candidate Fabricated via Powder Metallurgy Process. Adv. Mater. Res. 2012, 576, 386–389. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Tan, L.; Yang, K. Study on properties of a novel biodegradable Fe-30Mn-1C alloy. Acta Metall. Sinica 2011, 47, 1342–1347. [Google Scholar]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Hermawan, H. Biodegradable Metals—From Concept to Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sotoudeh Bagha, P.; Sheibani, S.; Khakbiz, M.; Ebrahimi-Barough, S.; Hermawan, H. Novel antibacterial biodegradable Fe-Mn-Ag alloys produced by mechanical alloying. Mater. Sci. Eng. C 2018, 88, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Niendorf, T.; Brenne, F.; Hoyer, P.; Schwarze, D.; Schaper, M.; Grothe, R.; Wiesener, M.; Grundmeier, G.; Maier, H.J. Processing of New Materials by Additive Manufacturing: Iron-Based Alloys Containing Silver for Biomedical Applications Metall. Mater. Trans. A 2015, 46, 2829–2833. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F.; Ruan, L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Malikova, P.; Dubinsky, S.; Korotitskiy, A.; Pustov, Y.; Prokoshkin, S. Martensitic Transformations and Mechanical and Corrosion Properties of Fe-Mn-Si Alloys for Biodegradable Medical Implants. Metall. Mater. Trans. A 2018, 49, 1006–1013. [Google Scholar] [CrossRef]
- Rahman, R.A.; Juhre, D.; Halle, T.; Mehmood, S.; Asghar, W. Types, DSC Thermal Characterization of Fe-Mn-Si based Shape Memory, Smart Materials and their Feasibility for Human Body in Terms of Austenitic Start Temperatures. J. Eng. Technol. 2019, 8, 185–206. [Google Scholar]
- Rahman, R.A.; Juhre, D.; Halle, T. Review of Types, Properties, and Importance of Ferrous Based Shape Memory Alloys. Korean J. Mater. Res. 2018, 28, 381–390. [Google Scholar] [CrossRef]
- Koyama, M.; Sawaguchi, T.; Ogawa, K.; Kikuchi, T.; Murakami, M. The effects of thermomechanical training treatment on the deformation characteristics of Fe–Mn–Si–Al alloys. Mater. Sci. Eng. A 2008, 497, 353–357. [Google Scholar] [CrossRef]
- Yang, H.K.; Tian, Y.Z.; Zhang, Z.J.; Zhang, Z.F. Different strain rate sensitivities between Fe–22Mn–0.6C and Fe– 30Mn–3Si–3Al twinning-induced plasticity steels. Mater. Sci. Eng. A 2016, 655, 251–255. [Google Scholar]
- Mahato, B.; Shee, S.K.; Sahu, T.; Ghosh Chowdhury, S.; Sahu, P.; Portere, D.A.; Karjalainene, L.P. An effective stacking fault energy viewpoint on the formation of extended defects and their contribution to strain hardening in a Fe–Mn–Si–Al twinning-induced plasticity steel. Acta Mater. 2015, 86, 69–79. [Google Scholar]
- Idrissi, H.; Renard, K.; Ryelandt, L.; Schryvers, D.; Jacques, P.J. On the mechanism of twin formation in Fe–Mn–C TWIP steels. Acta Mater. 2010, 58, 2464–2476. [Google Scholar] [CrossRef]
- Idrissi, H.; Renard, K.; Schryvers, D.; Jacques, P.J. TEM investigation of the formation mechanism of deformation twins in Fe–Mn–Si–Al TWIP steels. Philos. Mag. 2013, 93, 4378–4391. [Google Scholar] [CrossRef]
- Feng, Y.P.; Blanquer, A.; Fornell, J.; Zhang, H.; Solsona, P.; Baró, M.D.; Suriñach, S.; Ibáñez, E.; García-Lecina, E.; Wei, X.; et al. Novel Fe-Mn-Si-Pd alloys: Insights into mechanical, magnetic, corrosion resistance and biocompatibility performances. J. Mater. Chem. B 2016, 4, 6402–6412. [Google Scholar] [CrossRef] [Green Version]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Chen, G.; Peng, H.; Tang, S.; Zhou, Z.; Pei, F.; Shen, B. In vitro and 48 weeks in vivo performances of 3D printed porous Fe-30Mn biodegradable scaffolds. Acta Biomater. 2021, 121, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In vitro and in vivo corrosion properties of new iron–manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res. B 2015, 103, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Biswal, A.K.; Sahoo, S.K. Processing and characterization of Fe-35Mn biodegradable metallic materials. Mater. Today Proc. 2020, 33, 5284–5289. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Pasternak, K. Silicon in medicine and therapy. J. Elem. 2011, 16, 489–497. [Google Scholar] [CrossRef]
- Rahman, R.A.U.; Juhre, D.; Halle, T. Review of applications of ferrous based shape memory smart materials in engineering and in biomedical sciences. Pak. J. Eng. Appl. Sci. 2019, 24, 32–49. [Google Scholar]
- Bosiers, M.; Peeters, P.; D’Archambeau, O.; Hendriks, J.; Pilger, E.; Duber, C.; Zeller, T.; Gussmann, A.; Lohle, P.N.M.; Minar, E.; et al. AMS INSIGHT—Absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc. Intervent. Radiol. 2009, 32, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe–Mn alloys for stents. J. Biomed. Mater. Res. A 2010, 93, 1–11. [Google Scholar] [CrossRef]
- Kocijan, A.; Paulin, I.; Donik, C.; Hocevar, M.; Zelic, K.; Godec, M. Influence of different production processes on the biodegradability of an FeMn17 alloy. Mater. Tehnol. 2016, 50, 805–811. [Google Scholar]
- Kirindi, T.; Sari, U. The effects of austenite phase deformation on microstructure and magnetic properties in Fe-13.4%Mn-5.2%Mo alloy. J. Mater. Sci. 2011, 46, 6378–6383. [Google Scholar]
- Sari, U.; Kirindi, T.; Yueksel, M.; Agan, S. Influence of Mo and Co on the magnetic properties and martensitic transformation characteristics of a Fe-Mn alloy. J. Alloys Compd. 2009, 476, 160–163. [Google Scholar] [CrossRef]
- Guerrero, L.M.; La Roca, P.; Malamud, F.; Baruj, A.; Sade, M. Composition effects on the fcc-hcp martensitic transformation and on the magnetic ordering of the fcc structure in Fe-Mn-Cr alloys. Mater. Des. 2017, 116, 127–135. [Google Scholar] [CrossRef]
- Pan, M.M.; Zhang, X.M.; Zhou, D.; Misra, R.D.K.; Chen, P.; Su, X. Bin On the significance of C and Co on shape memory performance of Fe–Mn–Si–Cr–Ni shape memory alloy. Mater. Sci. Eng. A 2020, 786, 139412. [Google Scholar] [CrossRef]
- Cotes, S.; Fernández Guillermet, A.; Sade, M. Phase stability and fcc/hcp martensitic trans-formation in Fe-Mn-Si alloys Part I. Experimental study and systematics of the Ms and As temperatures. J. Alloys Compd. 1998, 278, 231–238. [Google Scholar] [CrossRef]
- Gurau, C.; Gurau, G.; Tolea, F.; Popescu, B.; Banu, M.; Bujoreanu, L.G. The Effect of the In-Situ Heat Treatment on the Martensitic Transformation and Specific Properties of the Fe-Mn-Si-Cr Shape Memory Alloys Processed by HSHPT Severe Plastic Deformation. Materials 2021, 14, 4621. [Google Scholar] [CrossRef]
- Collazo, A.; Figueroa, R.; Mariño-Martínez, C.; Pérez, C. Micro-structure and Thermomechanical Characterization of Fe-28Mn-6Si-5Cr Shape Memory Alloy. Metals 2021, 11, 649. [Google Scholar] [CrossRef]
- Feng, Y.P.; Gaztelumendi, N.; Fornell, J.; Zhang, H.Y.; Solsona, P.; Baro, M.D.; Surinach, S.; Ibanez, E.; Barrios, L.; Pellicer, E.; et al. Mechanical properties, corrosion performance and cell viability studies on newly developed porous Fe-Mn-Si-Pd alloys. J. Alloys Compd. 2017, 724, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Stanford, N.; Dunne, D.P. Effect of Si on the reversibility of stress-induced martensite in Fe-Mn-Si shape memory alloys. Acta Mater. 2010, 58, 6752–6762. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals, Mater. Sci. Eng. R. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Qi, Y.; Li, X.; He, Y.; Zhang, D.; Ding, J. Mechanism of acceleration of iron corrosion by a polylactide coating. ACS Appl. Mater. Interfaces 2019, 11, 202–218. [Google Scholar] [CrossRef]
- Revie, W.R.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John and Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Suárez Muñoz, M.; Melián Rodríguez, C.; Gelen Rudnikas, A.; Díaz Rizo, O.; Martínez-Santos, M.; Ruiz-Romera, E.; Fagundo Castillo, J.R.; Pérez-Gramatges, A.; Martínez-Villegas, N.V.; Blanco Padilla, D.; et al. Physico-chemical characterization, elemental speciation and hydrogeochemical modeling of river and peloid sediments used for therapeutic uses. Appl. Clay Sci. 2015, 104, 36–47. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Dehghan-manshadi, A.; Shahbazi, M.; Venezuela, J.; Tran, X.Q.; Song, J.; Liu, N.; Xu, C.; Ye, Q.; Wen, C. Exploring the Role of Manganese on the Microstructure, Mechanical Properties, Biodegradability, and Biocompatibility of Porous Iron-Based Scaffolds. ACS. Biomater. Sci. Eng. 2019, 5, 1686–1702. [Google Scholar] [CrossRef] [PubMed]
- Donik, C.; Kraner, J.; Kocijan, A.; Paulin, I.; Godec, M. Evolution of the ε and γ phases in biodegradable Fe–Mn alloys produced using laser powder-bed fusion. Sci. Rep. 2021, 11, 19506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.M.; Geanta, V.; Cimpoesu, R.; Munteanu, C.; Lohan, N.M.; Zegan, G.; Cernei, E.R.; Ionita, I.; Cimpoesu, N.; Ioanid, N. In-Vitro Analysis of FeMn-Si Smart Biodegradable Alloy. Materials 2022, 15, 568. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on bio-degradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Ravanbakhsh, S.; Paternoster, C.; Barucca, G.; Mengucci, P.; Gambaro, S.; Lescot, T.; Chevallier, P.; Fortin, M.A.; Mantovani, D. Improving the radiopacity of Fe-Mn biodegradable metals by magnetron-sputtered W-Fe-Mn-C coatings: Application for thinner stents. Bioact. Mater. 2021, 12, 64–70. [Google Scholar] [CrossRef]
- Kawashima, K.; Asami, K.; Hashimoto, K. Effect of manganese on the corrosion behaviour of chromium-bearing amorphous metal-metalloid alloys. Sci. Rep. Res. Inst. Tohoku Univ. Phys. Chem. Metall. 1981, 29, 276–283. [Google Scholar]
- Ijsseling, F.P. Application of electrochemical methods of corrosion rate determination to system involving corrosion product layers. Br. Corros. J. 1986, 21, 95–101. [Google Scholar] [CrossRef]
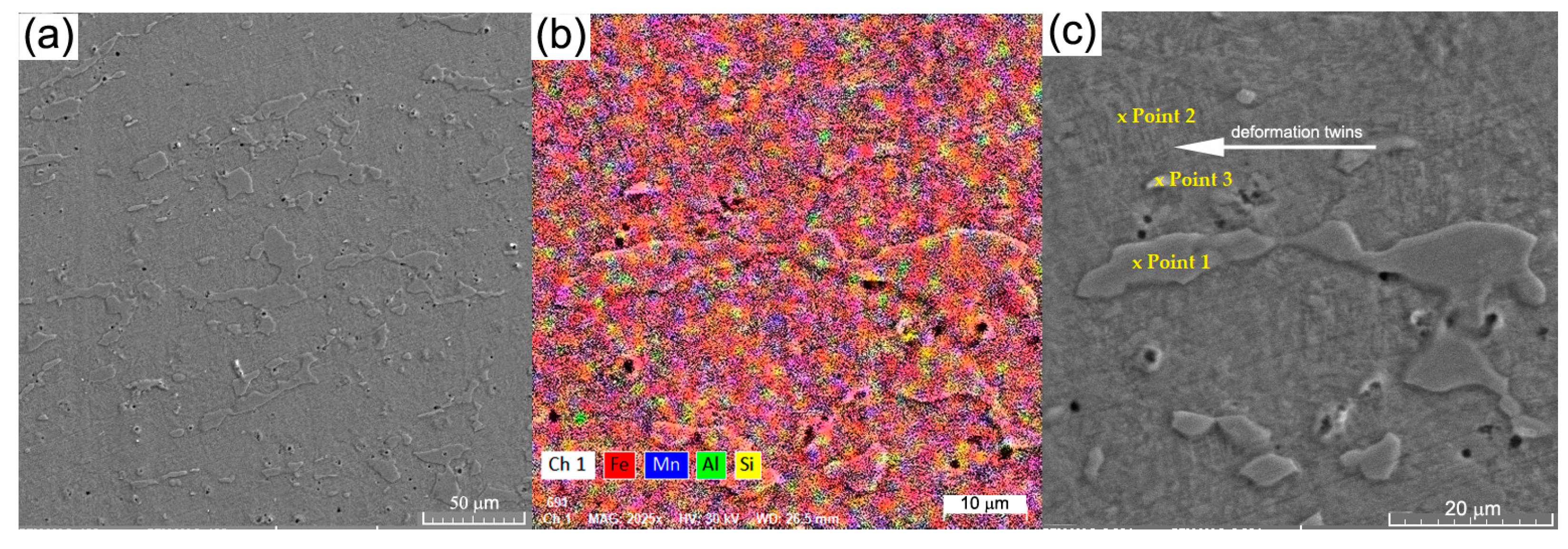

| Chem. Comp./Area | Fe | Mn | Si | Al | ||||
|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| General (1 mm2) | 80.25 | 75.79 | 14.25 | 13.66 | 3.05 | 5.72 | 2.47 | 4.82 |
| Point 1 | 81.23 | 76.43 | 12.87 | 12.31 | 3.00 | 5.86 | 2.9 | 5.64 |
| Point 2 | 81.01 | 76.47 | 13.40 | 12.85 | 3.12 | 5.86 | 2.46 | 4.81 |
| Point 3 | 78.80 | 74.94 | 16.42 | 15.87 | 2.91 | 5.5 | 1.88 | 3.69 |
| EDS error % | 1.9 | 0.47 | 0.2 | 0.19 | ||||
| FeMnSi Alloy | FeMnSi-Al Alloy | Phase | Miller Indices | |||
|---|---|---|---|---|---|---|
| Peak | Angle (2θ) | Angle (2θ) | h | k | l | |
| 1 | 41.2377 | 41.0715 | ε-Fe(ss) | 1 | 0 | 0 |
| 2 | 44.2085 | 43.5743 | γ-Fe(ss) | 1 | 1 | 1 |
| 3 | 44.6560 | 43.9838 | α-Fe(ss) | 1 | 1 | 0 |
| 4 | 46.9847 | 46.8430 | ε-Fe(ss) | 0 | 0 | 2 |
| 5 | - | 50.4888 | γ-Fe(ss) | 2 | 0 | 0 |
| 6 | - | 61.6867 | ε-Fe(ss) | 1 | 0 | 2 |
| 7 | 64.6965 | - | α-Fe(ss) | 2 | 0 | 0 |
| 8 | - | 74.5462 | γ-Fe(ss) | 2 | 2 | 0 |
| 9 | 82.1256 | 82.8594 | α-Fe(ss) | 2 | 1 | 1 |
| Sample | Ms [°C] | M50 [°C] | Mf [°C] | DH [KJ/kg] |
|---|---|---|---|---|
| C sample | 35.7 | 9.2 | 0.1 | 11.87 |
| HR sample | 35.1 | 8.1 | 0.3 | 9.55 |
| Immersion Time/State of the Samples | 1 Day | 3 Days | 7 Days | |||
|---|---|---|---|---|---|---|
| Cast Sample (C) | Hot-Rolled Sample (HR) | Cast Sample (C) | Hot-Rolled Sample (HR) | Cast Sample (C) | Hot-Rolled Sample (HR) | |
| Initial mass (mg) | 5637.6 | 597.0 | 5681.5 | 553.9 | 5660.0 | 547.9 |
| Mass after immersion (mg) | 5638.4 (+0.8) | 597.2 (+0.2) | 5676.6 (−4.9) | 553.5 (−0.4) | 5654.5 (−5.5) | 545.9 (−2.0) |
| Mass after ultrasonic cleaning (mg) | 5637.0 (−0.6) | 596.5 (−0.5) | 5675.0 (−6.5) | 551.9 (−2.0) | 5652.3 (−7.7) | 544.5 (−3.4) |
| CR (mm/year) (samples areas C = 4.2 cm2 and L = 2.7 cm2) | 0.070 | 0.091 | 0.253 | 0.121 | 0.128 | 0.088 |
| Chemical Elements/State of the Samples | Fe | Mn | Si | Al | O | C | Cl | Na | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | |||
| 1 day (I) | C | I | 43.29 | 19.25 | 8.05 | 3.64 | 1.38 | 1.22 | 2.21 | 2.03 | 32.59 | 50.57 | 10.40 | 21.50 | 1.18 | 0.82 | 0.90 | 0.98 |
| I+ UC | 47.74 | 23.07 | 10.84 | 5.32 | 1.63 | 1.56 | 2.29 | 2.29 | 25.27 | 42.61 | 10.52 | 23.64 | 1.22 | 0.93 | 0.49 | 0.57 | ||
| HR | I | 46.92 | 21.49 | 7.52 | 3.50 | 1.09 | 0.99 | 1.30 | 1.23 | 33.01 | 52.77 | 8.93 | 19.02 | 0.94 | 0.67 | 0.30 | 0.33 | |
| I+ UC | 49.62 | 24.05 | 12.04 | 5.93 | 1.28 | 1.24 | 1.61 | 1.62 | 21.92 | 37.10 | 13.24 | 29.85 | 0.28 | 0.22 | - | - | ||
| 7 days (I) | C | I | 44.20 | 17.70 | 0.31 | 0.13 | 0.24 | 0.19 | 0.10 | 0.08 | 41.82 | 58.47 | 12.04 | 22.42 | 0.73 | 0.46 | 0.56 | 0.54 |
| I+ UC | 42.32 | 18.96 | 7.82 | 3.56 | 1.98 | 1.77 | 4.19 | 3.88 | 33.08 | 51.73 | 8.87 | 18.48 | 0.69 | 0.49 | 1.05 | 1.14 | ||
| HR | I | 39.29 | 16.78 | 7.05 | 3.06 | 1.64 | 1.39 | 2.60 | 2.29 | 35.04 | 52.23 | 10.61 | 21.07 | 2.04 | 1.37 | 1.74 | 1.81 | |
| I+ UC | 47.39 | 21.44 | 10.82 | 4.98 | 1.54 | 1.38 | 1.88 | 1.76 | 19.57 | 30.91 | 18.79 | 39.53 | - | - | - | - | ||
| EDS error% | 1.38 | 0.35 | 0.12 | 0.21 | 4.91 | 4.48 | 0.09 | 0.20 | ||||||||||
| Alloy | E(I = 0) (mV) | icorr (µA) | vcorr mpy | βc (mV/dec) | βa (mV/dec) |
|---|---|---|---|---|---|
| FeMnSiAl (C) 0 days | 741 | 10.11 | 54.168 | 322 | 61 |
| 3 days | 671 | 69.43 | 371.9 | 1664 | 260 |
| 9 days | 590 | 23.51 | 125 | 516 | 293 |
| FeMnSiAl (HR) 0 days | −775 | 14.76 | 79.094 | 327 | 70 |
| 3 days | 775 | 16.22 | 86.86 | 374 | 77 |
| FeMnSiAl (C) | FeMnSiAl (HR) | ||||
|---|---|---|---|---|---|
| R(QR) |  | ||||
| 0 Days | 3 Days | 9 Days | 0 Days | 3 Days | |
| Rs (ohm.cm2) | 104.5 | 73.07 | 48.99 | 170.3 | 167.2 |
| Q 103 (S.sn/cm2) | 0.5745 | 0.3483 | 0.453 | 0.5394 | 0.5566 |
| n | 0.6914 | 0.5475 | 0.6468 | 0.6585 | 0.6476 |
| Rct (ohm.cm2) | 1275 | 4108 | 3937 | 706 | 819.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, A.-M.; Voiculescu, I.; Cimpoeșu, R.; Istrate, B.; Chelariu, R.; Cimpoeșu, N.; Zegan, G.; Panaghie, C.; Lohan, N.M.; Axinte, M.; et al. Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy. Crystals 2023, 13, 109. https://doi.org/10.3390/cryst13010109
Roman A-M, Voiculescu I, Cimpoeșu R, Istrate B, Chelariu R, Cimpoeșu N, Zegan G, Panaghie C, Lohan NM, Axinte M, et al. Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy. Crystals. 2023; 13(1):109. https://doi.org/10.3390/cryst13010109
Chicago/Turabian StyleRoman, Ana-Maria, Ionelia Voiculescu, Ramona Cimpoeșu, Bogdan Istrate, Romeu Chelariu, Nicanor Cimpoeșu, Georgeta Zegan, Cătălin Panaghie, Nicoleta Monica Lohan, Mihai Axinte, and et al. 2023. "Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy" Crystals 13, no. 1: 109. https://doi.org/10.3390/cryst13010109
APA StyleRoman, A.-M., Voiculescu, I., Cimpoeșu, R., Istrate, B., Chelariu, R., Cimpoeșu, N., Zegan, G., Panaghie, C., Lohan, N. M., Axinte, M., & Murariu, A. M. (2023). Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy. Crystals, 13(1), 109. https://doi.org/10.3390/cryst13010109














