Theoretical Examination of the Radiation Protecting Properties of CaTiO3 Material Sintered at Different Temperatures
Abstract
:1. Introduction
2. Experiment
2.1. Chemicals and Samples Fabrication
2.2. Characterization
2.3. Monte Carlo Simulation
3. Results and Discussion
3.1. Phase Identification
3.2. FTIR Study
3.3. Radiation Shielding Study
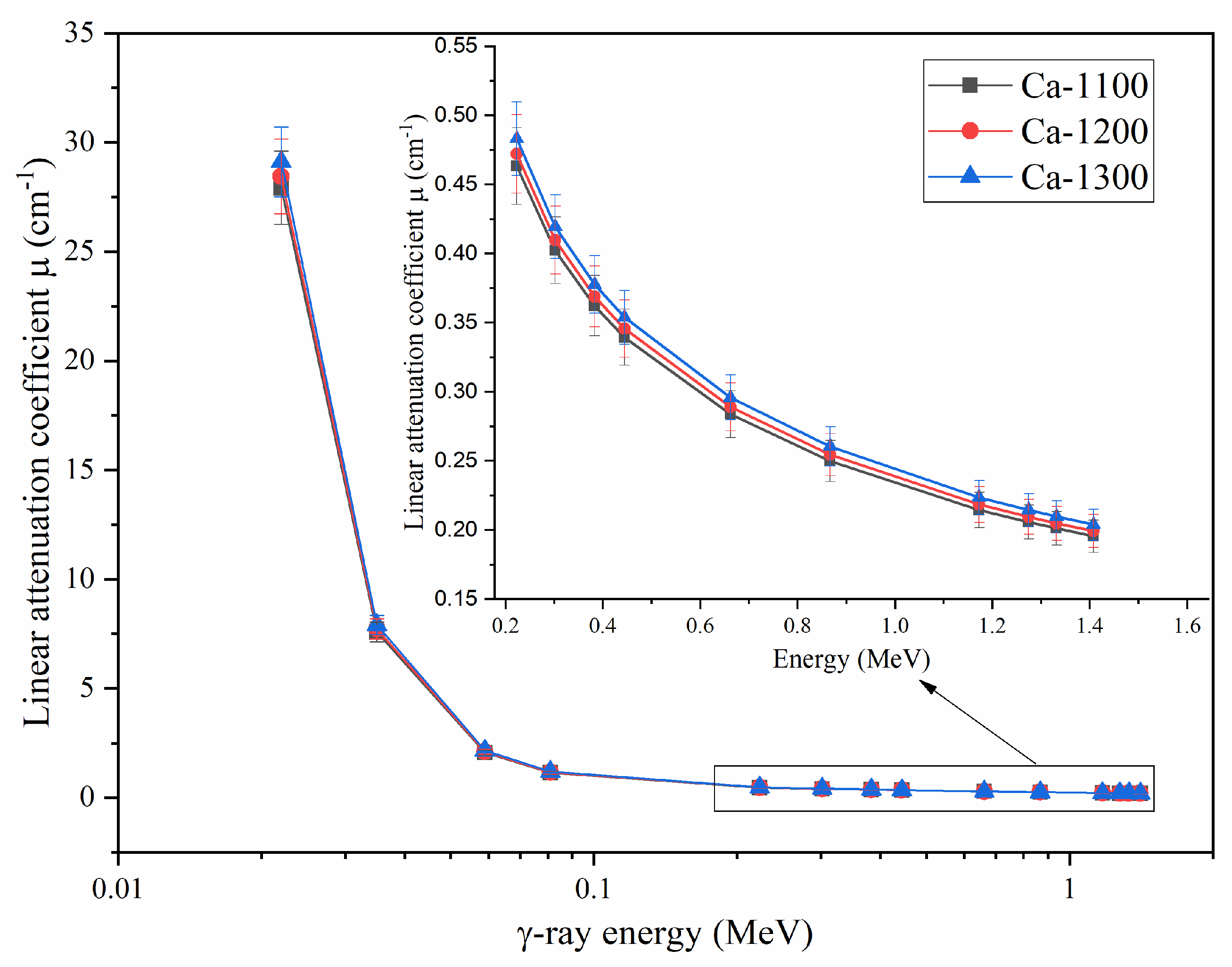
3.3.1. Linear Attenuation Coefficient (µ)
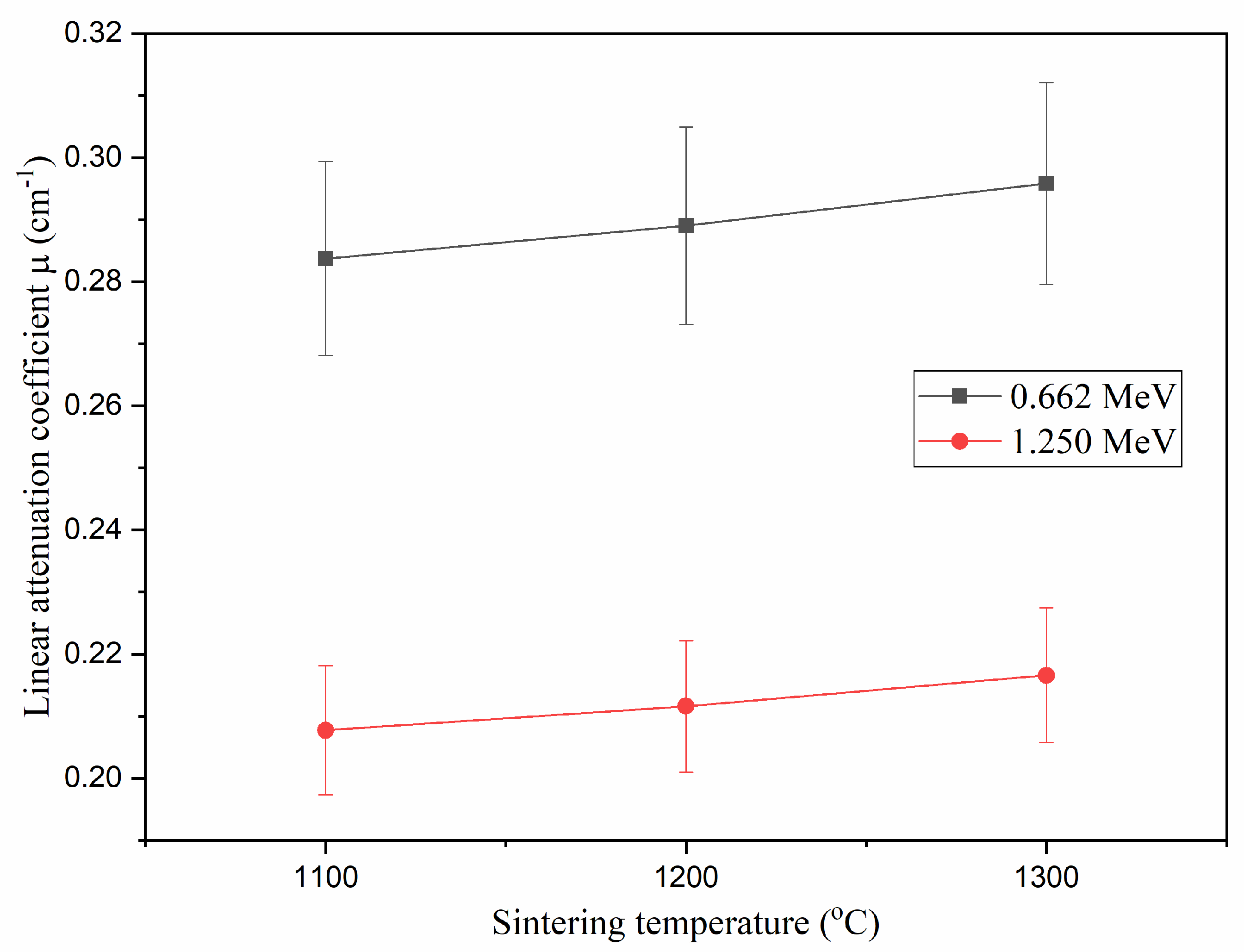
3.3.2. Half-Value Thickness (Δ0.5)
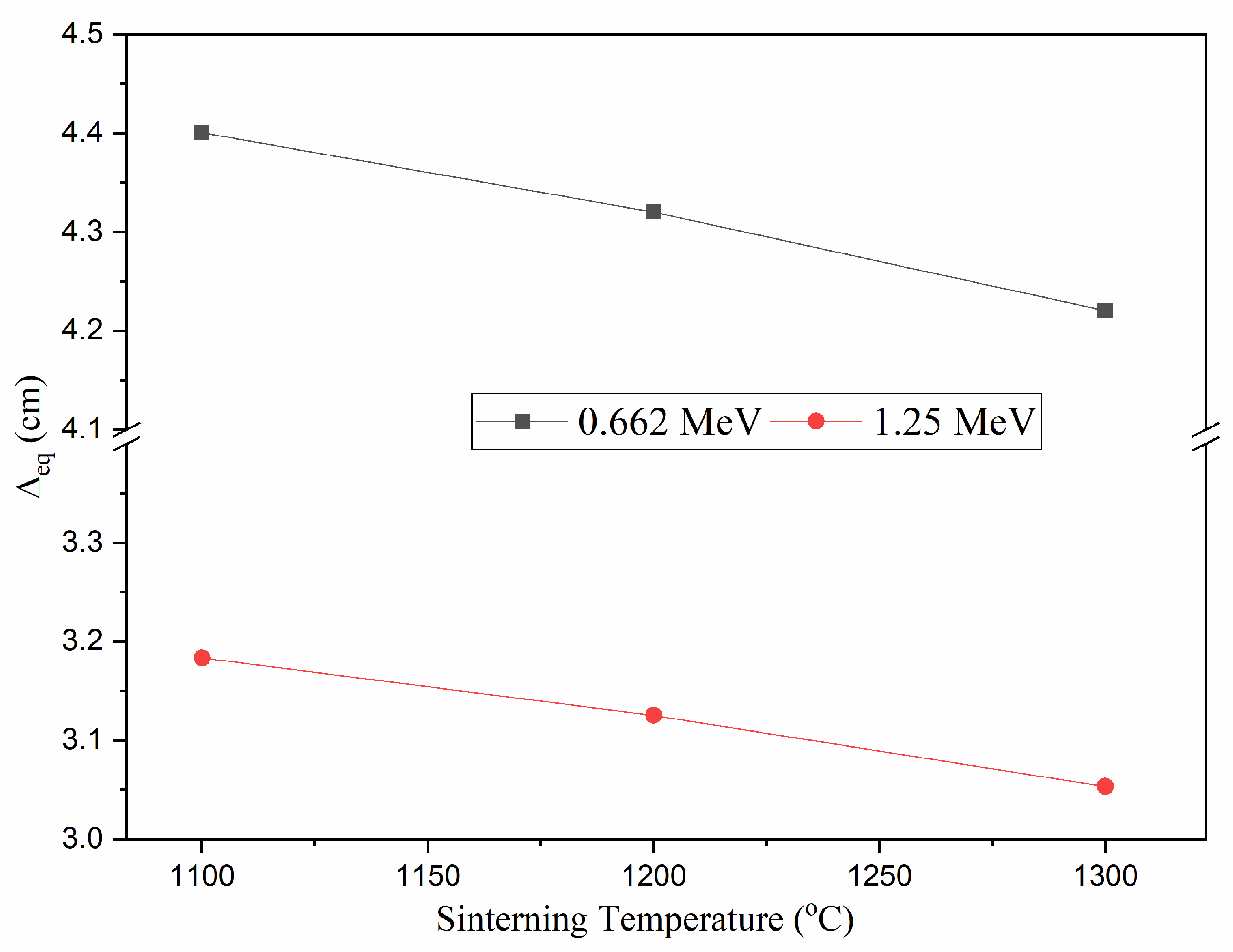
3.3.3. Equivalent Thickness (Δeq)
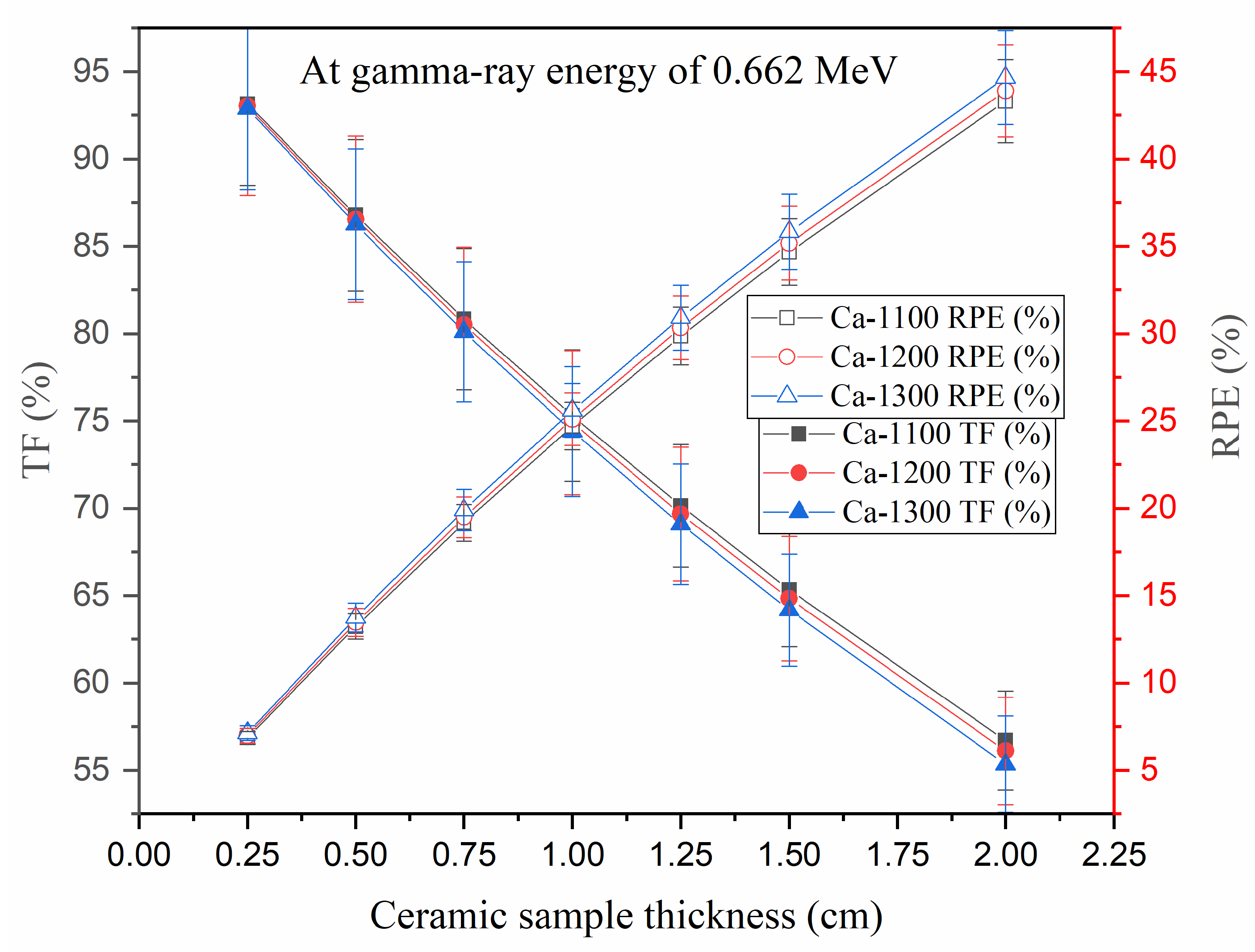
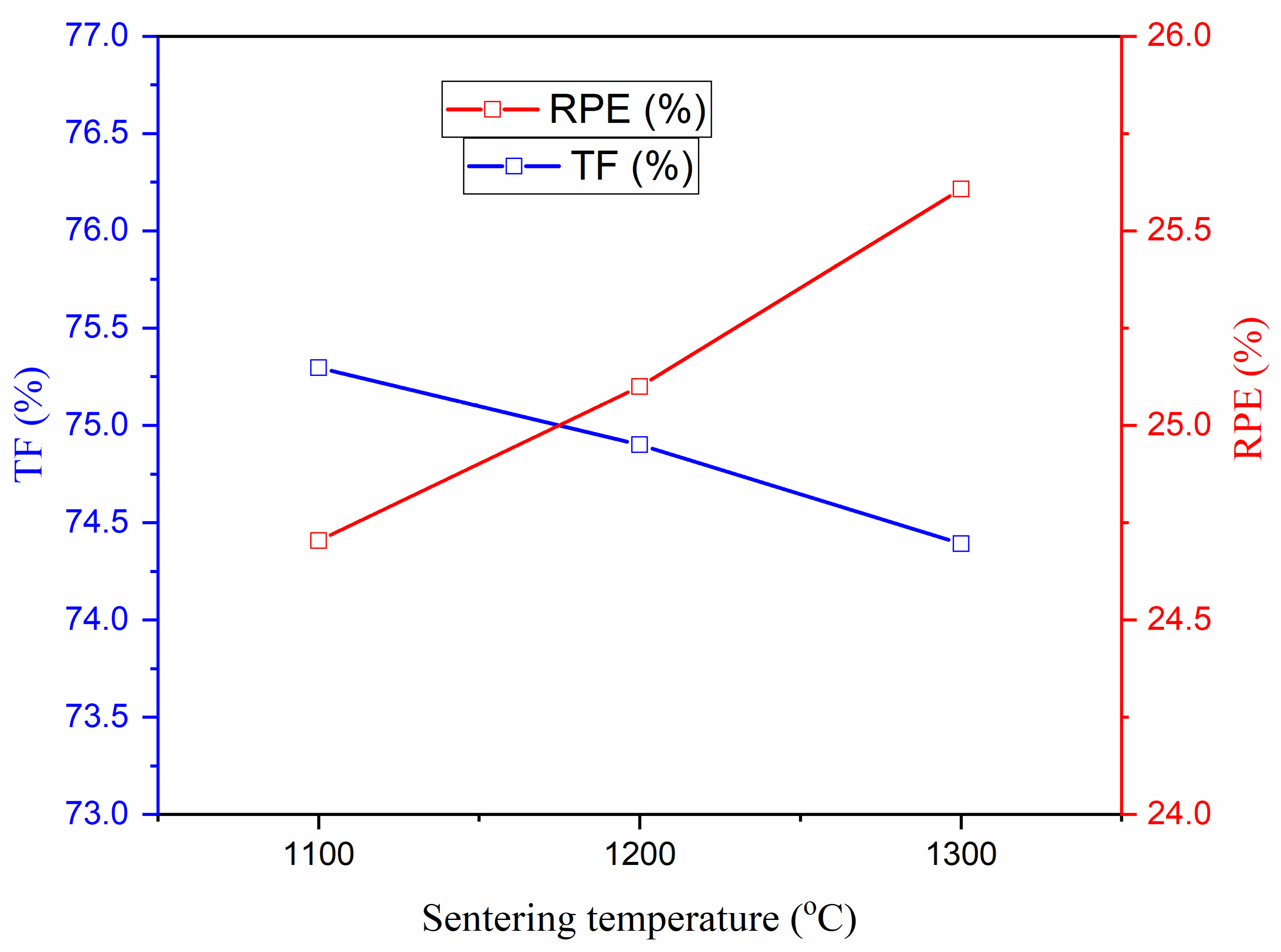
3.3.4. Radiation Protection Efficiency (RPE) and Transmission Factor (TF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AbuAlRoos, N.J.; Amin, N.A.B.; Zainon, R. Conventional and new lead-free radiation shielding materials for radiation protection in nuclear medicine: A review. Radiat. Phys. Chem. 2019, 165, 108439. [Google Scholar] [CrossRef]
- Boice, J., Jr.; Dauer, L.T.; Kase, K.R.; Mettler, F.A., Jr.; Vetter, R.J. Evolution of radiation protection for medical workers. Br. J. Radiol. 2020, 93, 20200282. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. BaO–Li2O–B2O3 glass systems: Potential utilization in gamma radiation protection. Prog. Nucl. Energy 2020, 129, 103511. [Google Scholar] [CrossRef]
- Askin, A.; Buddemeier, B.; Alai, M.; Yu, K. Centers for Disease Control and Prevention (CDC) Radiation Hazard Scale Data Product Review Feedback Report; No. LLNL-TR-738819; Lawrence Livermore National Lab.(LLNL): Livermore, CA, USA, 2017. [Google Scholar]
- Ogawa, M.; Nakajima, Y.; Kubota, R.; Endo, Y. Two cases of acute lead poisoning due to occupational exposure to lead. Clin. Toxicol. 2008, 46, 332–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, G.; Singhal, A.; Routroy, S.; Bhunia, D.; Lahoti, M. Radiation Shielding Concrete with alternate constituents: An approach to address multiple hazards. J. Hazard. Mater. 2021, 404, 124201. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, M.I.; Dong, M.G.; Tekin, H.O.; Lakshminarayana, G.; Mahdi, M.A. Comparative investigations of gamma and neutron radiation shielding parameters for different borate and tellurite glass systems using WinXCom program and MCNPX code. Mater. Chem. Phys. 2018, 215, 183–202. [Google Scholar] [CrossRef]
- Agar, O.; Khattari, Z.Y.; Sayyed, M.I.; Tekin, H.O.; Al-Omari, S.; Maghrabi, M.; Zaid, M.H.; Kityk, I.V. Evaluation of the shielding parameters of alkaline earth based phosphate glasses using MCNPX code. Results Phys. 2019, 12, 101–106. [Google Scholar] [CrossRef]
- Albarzan, B.; Almuqrin, A.H.; Koubisy, M.S.; Wahab, E.A.; Mahmoud, K.A.; Shaaban, K.; Sayyed, M.I. Effect of Fe2O3 doping on structural, FTIR and radiation shielding characteristics of aluminium-lead-borate glasses. Prog. Nucl. Energy 2021, 141, 103931. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Okoye, P.U.; Chen, G.; Li, Y.; Okoye, M.O.; Li, S. Advanced ceramic components: Materials, fabrication, and applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- Oto, B.; Kavaz, E.; Durak, H.; Aras, A.; Madak, Z. Effect of addition of molybdenum on photon and fast neutron radiation shielding properties in ceramics. Ceram. Int. 2019, 45, 23681–23689. [Google Scholar] [CrossRef]
- Jawad, A.A.; Demirkol, N.; Gunoğlu, K.; Akkurt, I. Radiation shielding properties of some ceramic wasted samples. Int. J. Environ. Sci. Technol. 2019, 16, 5039–5042. [Google Scholar] [CrossRef]
- Akman, F.; Khattari, Z.Y.; Kaçal, M.R.; Sayyed, M.I.; Afaneh, F. The radiation shielding features for some silicide, boride and oxide types ceramics. Radiat. Phys. Chem. 2019, 160, 9–14. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.I.; Slimani, Y.; Elsafi, M. Experimental study of yttrium-based ceramic systems containing nanomaterials for gamma radiation protecting applications. App. Phys. A 2022, 128, 1–9. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.I.; Mahmoud, K.A.; Slimani, Y. Correlation between the structure, grain size distribution and radiation shielding peculiarities of YBCO ceramics prepared by two different milling methods. Appl. Phys. A 2022, 128, 1–8. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.I.; Slimani, Y.; Elsafi, M. Experimental investigation on the physical properties and radiation shielding efficiency of YBa2Cu3Oy/M@ M3O4 (M= Co, Mn) ceramic composites. J. Alloys Compd. 2022, 904, 164056. [Google Scholar] [CrossRef]
- Slimani, Y.; Selmi, A.; Hannachi, E.; Almessiere, M.A.; AlFalah, G.; AlOusi, L.F.; Yasin, G.; Iqbal, M. Study on the addition of SiO2 nanowires to BaTiO3: Structure, morphology, electrical and dielectric properties. J. Phys. Chem. Solids 2021, 156, 110183. [Google Scholar] [CrossRef]
- Slimani, Y.; Shirsath, S.E.; Hannachi, E.; Almessiere, M.A.; Aouna, M.M.; Aldossary, N.E.; Yasin, G.; Baykal, A.; Ozçelik, B.; Ercan, I. (BaTiO3)1-x+(Co0.5Ni0. 5Nb0. 06Fe1. 94O4)x nanocomposites: Structure, morphology, magnetic and dielectric properties. J. Am. Ceram. Soc. 2021, 104, 5648–5658. [Google Scholar] [CrossRef]
- Tong, Z.; Yao, Q.; He, Q.; Deng, J.; Wang, J.; Zhou, H.; Rao, G. Effect of microcosmic regulation of unit cells bonding on microwave absorption properties of perovskite structure GdFeO3. Materialia 2021, 20, 101263. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.I.; Mahmoud, K.A.; Slimani, Y.; Akhtar, S.; Albarzan, B.; Almuqrin, A.H. Impact of tin oxide on the structural features and radiation shielding response of some ABO3 perovskites ceramics (A= Ca, Sr, Ba; B= Ti). Appl. Phys. A 2021, 127, 1–12. [Google Scholar] [CrossRef]
- Slimani, Y.; Hamad, M.K.; Olarinoye, I.O.; Alajerami, Y.S.; Sayyed, M.I.; Almessiere, M.A.; Mhareb, M.H. Mhareb. Determination of structural features of different Perovskite ceramics and investigation of ionizing radiation shielding properties. J. Mater. Sci. Mater. Electron. 2021, 32, 20867–20881. [Google Scholar] [CrossRef]
- Hannachi, E.; Mahmoud, K.A.; Sayyed, M.I.; Slimani, Y. Slimani. Structure, optical properties, and ionizing radiation shielding performance using Monte Carlo simulation for lead-free BTO perovskite ceramics doped with ZnO, SiO2, and WO3 oxides. Mater. Sci. Semicond. Process. 2022, 145, 106629. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Kesson, S.E.; Reeve, K.D.; Levins, D.M.; Ramm, E.J. Radioactive Waste Forms for the Future; Lutze, W., Ewing, R.C., Eds.; IAEA: Vienna, Austria, 1988; pp. 233–334. [Google Scholar]
- Cavalcante, L.S.; Marques, V.S.; Sczancoski, J.C.; Escote, M.T.; Joya, M.R.; Varela, J.A.; Santos, M.R.; Pizani, P.S.; Longo, E. Synthesis, structural refinement and optical behavior of CaTiO3 powders: A comparative study of processing in different furnaces. Chem. Eng. J. 2008, 143, 299–307. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Popov, A.I. Systematic trends in (0 0 1) surface ab initio calculations of ABO3 perovskites. J. Saudi Chem. Soc. 2018, 22, 459–468. [Google Scholar] [CrossRef]
- Ueda, K.; Yanagi, H.; Noshiro, R.; Hosono, H.; Kawazoe, H. Vacuum ultraviolet reflectance and electron energy loss spectra of. J. Phys. Condens. Matter 1998, 10, 3669. [Google Scholar] [CrossRef]
- Smith, K.L.; Zaluzec, N.J. The displacement energies of cations in perovskite (CaTiO3). J. Nucl. Mater. 2005, 336, 261–266. [Google Scholar] [CrossRef]
- Weber, W.J.; Ewing, R.C. Radiation effects in crystalline oxide host phases for the immobilization of actinides. MRS Online Proc. Libr. (OPL) 2002, 713, JJ3.1.1–JJ3.1.12. [Google Scholar] [CrossRef]
- Zhang, Z.; Blackford, M.G.; Lumpkin, G.R.; Smith, K.L.; Vance, E.R. Aqueous dissolution of perovskite (CaTiO3): Effects of surface damage and [Ca2+] in the leachant. J. Mater. Res. 2005, 20, 2462–2473. [Google Scholar] [CrossRef]
- X-5 Monte Carlo Team. MCNP—A General Monte Carlo N-Particle Transport Code; Version 5, La-Ur-03-1987. II; Los Alamos National Laboratory: Los Alamos, Mexico, 2003. [Google Scholar]
- Al-Harbi, N.; Sayyed, M.I.; Al-Hadeethi, Y.; Kumar, A.; Elsafi, M.; Mahmoud, K.A.; Khandaker, M.U.; Bradley, D.A. A novel CaO–K2O–Na2O–P2O5 glass systems for radiation shielding applications. Radiat. Phys. Chem. 2021, 188, 109645. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Zaid, M.H.; Effendy, N.; Matori, K.A.; Lacomme, E.; Mahmoud, K.A.; AlShammari, M.M. The influence of PbO and Bi2O3 on the radiation shielding and elastic features for different glasses. J. Mater. Res. Technol. 2020, 9, 8429–84381. [Google Scholar] [CrossRef]
- Naseer, K.A.; Marimuthu, K.; Mahmoud, K.A.; Sayyed, M.I. The concentration impact of Yb3+ on the bismuth boro-phosphate glasses: Physical, structural, optical, elastic, and radiation-shielding properties. Radiat. Phys. Chem. 2021, 188, 109617. [Google Scholar] [CrossRef]
- Hannachi, E.; Mahmoud, K.A.; Sayyed, M.I.; Slimani, Y. Effect of sintering conditions on the radiation shielding characteristics of YBCO superconducting ceramics. J. Phys. Chem. Solids 2022, 164, 110627. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Shaw, L. X-ray diffraction analysis of a severely plastically deformed aluminum alloy. Acta Mater. 2004, 52, 2185–2197. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, R.S.; Rai, S.B. Enhanced photoluminescence in a Eu3+ doped CaTiO3 perovskite phosphor via incorporation of alkali ions for white LEDs. J. Phys. Chem. Solids 2021, 151, 109916. [Google Scholar] [CrossRef]
- Zhou, G.; Lü, M.; Gu, F.; Xu, D.; Yuan, D. Morphology-controlled synthesis, characterization and growth mechanism of PbWO4 nano and macrocrystals. J. Cryst. Growth 2005, 276, 577–582. [Google Scholar] [CrossRef]
- Lozano-Sánchez, L.M.; Lee, S.W.; Sekino, T.; Rodríguez-González, V. Practical microwave-induced hydrothermal synthesis of rectangular prism-like CaTiO 3. CrystEngComm 2013, 15, 2359–2362. [Google Scholar] [CrossRef]
- Peng, C.; Hou, Z.; Zhang, C.; Li, G.; Lian, H.; Cheng, Z.; Lin, J. Synthesis and luminescent properties of CaTiO3: Pr 3+ microfibers prepared by electrospinning method. Opt. Express 2010, 18, 7543–7553. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.; Limirio, P.H.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Sayyed, M.I.; Mahmoud, K.A.; Al-Hadeethi, Y. Direct influence of mercury oxide on structural, optical and shielding properties of a new borate glass system. Ceram. Int. 2020, 46, 17978–17986. [Google Scholar] [CrossRef]
- El-Agawany, F.I.; Mahmoud, K.A.; Akyildirim, H.; Yousef, E.S.; Tekin, H.O.; Rammah, Y.S. Rammah, Physical, neutron, and gamma-rays shielding parameters for Na2O-SiO2-PbO glasses. Emerg. Mater. Res. 2021, 10, 1–9. [Google Scholar]
- Abouhaswa, A.S.; Sayyed, M.I.; Altowyan, A.S.; Al-Hadeethi, Y.; Mahmoud, K.A. Synthesis, structural, optical and radiation shielding features of tungsten trioxides doped borate glasses using Monte Carlo simulation and phy-X program. J. Non-Cryst. Solids 2020, 543, 120134. [Google Scholar] [CrossRef]
- Mouiya, M.; Bouazizi, A.; Abourriche, A.; El Khessaimi, Y.; Benhammou, A.; Taha, Y.; Oumam, M.; Abouliatim, Y.; Smith, A.; Hannache, H. Effect of sintering temperature on the microstructure and mechanical behavior of porous ceramics made from clay and banana peel powder. Results Mater. 2019, 4, 100028. [Google Scholar] [CrossRef]




| Ceramic Code | Temperature (°C) | Structure | D (nm) | δ 1015 (Lines/m2) | Microstrain | ρr (%) | PP (%) |
|---|---|---|---|---|---|---|---|
| Ca-1100 | 1100 | Orthorhombic | 27.06 | 1.365 | 4.67 | 93 | 6.83 |
| Ca-1200 | 1200 | Orthorhombic | 36.08 | 0.768 | 3.51 | 94 | 5.17 |
| Ca-1300 | 1300 | Orthorhombic | 36.10 | 0.767 | 3.49 | 96 | 3.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, E.; Sayyed, M.I.; Hashim, S.; Mahmoud, K.; Slimani, Y. Theoretical Examination of the Radiation Protecting Properties of CaTiO3 Material Sintered at Different Temperatures. Crystals 2023, 13, 120. https://doi.org/10.3390/cryst13010120
Hannachi E, Sayyed MI, Hashim S, Mahmoud K, Slimani Y. Theoretical Examination of the Radiation Protecting Properties of CaTiO3 Material Sintered at Different Temperatures. Crystals. 2023; 13(1):120. https://doi.org/10.3390/cryst13010120
Chicago/Turabian StyleHannachi, Essia, M. I. Sayyed, Suhairul Hashim, Karem Mahmoud, and Yassine Slimani. 2023. "Theoretical Examination of the Radiation Protecting Properties of CaTiO3 Material Sintered at Different Temperatures" Crystals 13, no. 1: 120. https://doi.org/10.3390/cryst13010120
APA StyleHannachi, E., Sayyed, M. I., Hashim, S., Mahmoud, K., & Slimani, Y. (2023). Theoretical Examination of the Radiation Protecting Properties of CaTiO3 Material Sintered at Different Temperatures. Crystals, 13(1), 120. https://doi.org/10.3390/cryst13010120







