Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View
Abstract
:1. Introduction: Terminology Overview and Mechanochemical Instrumentation
1.1. Mechanochemical Instrumentation and Accessories
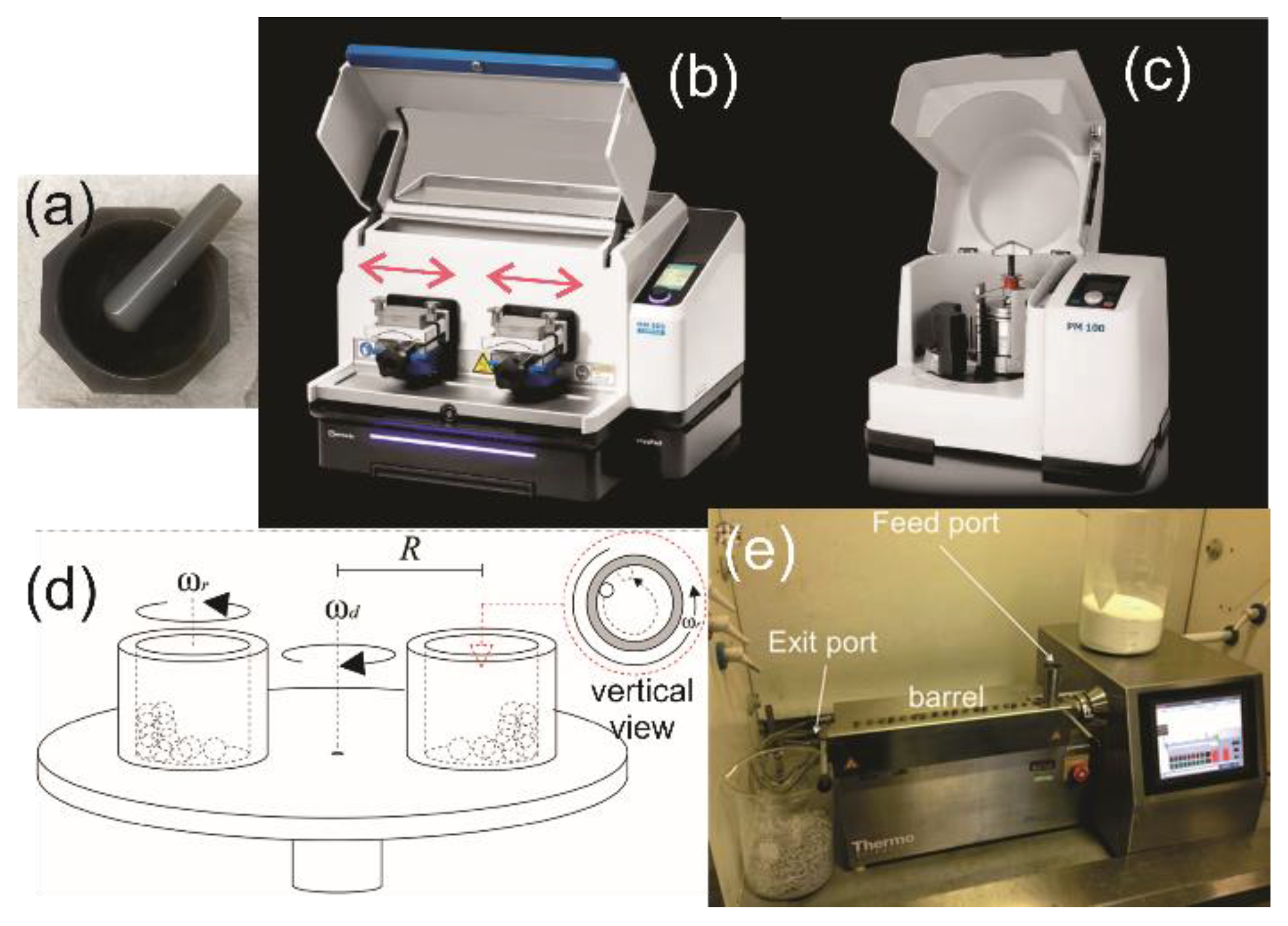
1.2. Mechanochemistry-Related Fields and Terminology in Publications
2. Mechanochemistry: A Rediscovered Branch of Chemistry with Increasingly Recognized Advantages
2.1. “Green” Mechanochemistry toward a Sustainable World
| Green Chemistry Principle | How Mechanochemistry Helps and Examples |
|---|---|
| Waste prevention | Largely reduced use of solvents and water. Reduced need of isolation and purification processes. |
| Atom economy | Avoided use of reactants in large excess. Typical high chemical selectivity and high yields. Avoided formation of unwanted solvates. Example: Syntheses of MOFs directly from metal oxides instead of metal salts [37]. |
| Less hazardous syntheses | Highly reactive species can be produced and immediately reacted, without using controlled atmospheres. Example: Mechanochemical activation of CaC2 avoiding the use of gaseous acetylene [38]. Replacement of aqua regia with safer oxidants such as oxone® [39]. |
| Design of safer chemicals | Alternative synthetic routes to active pharmaceutical ingredients and new solid-state forms (cocrystals, polymorphs). Example: Syntheses of new metallodrugs with reduced toxicity [40]. |
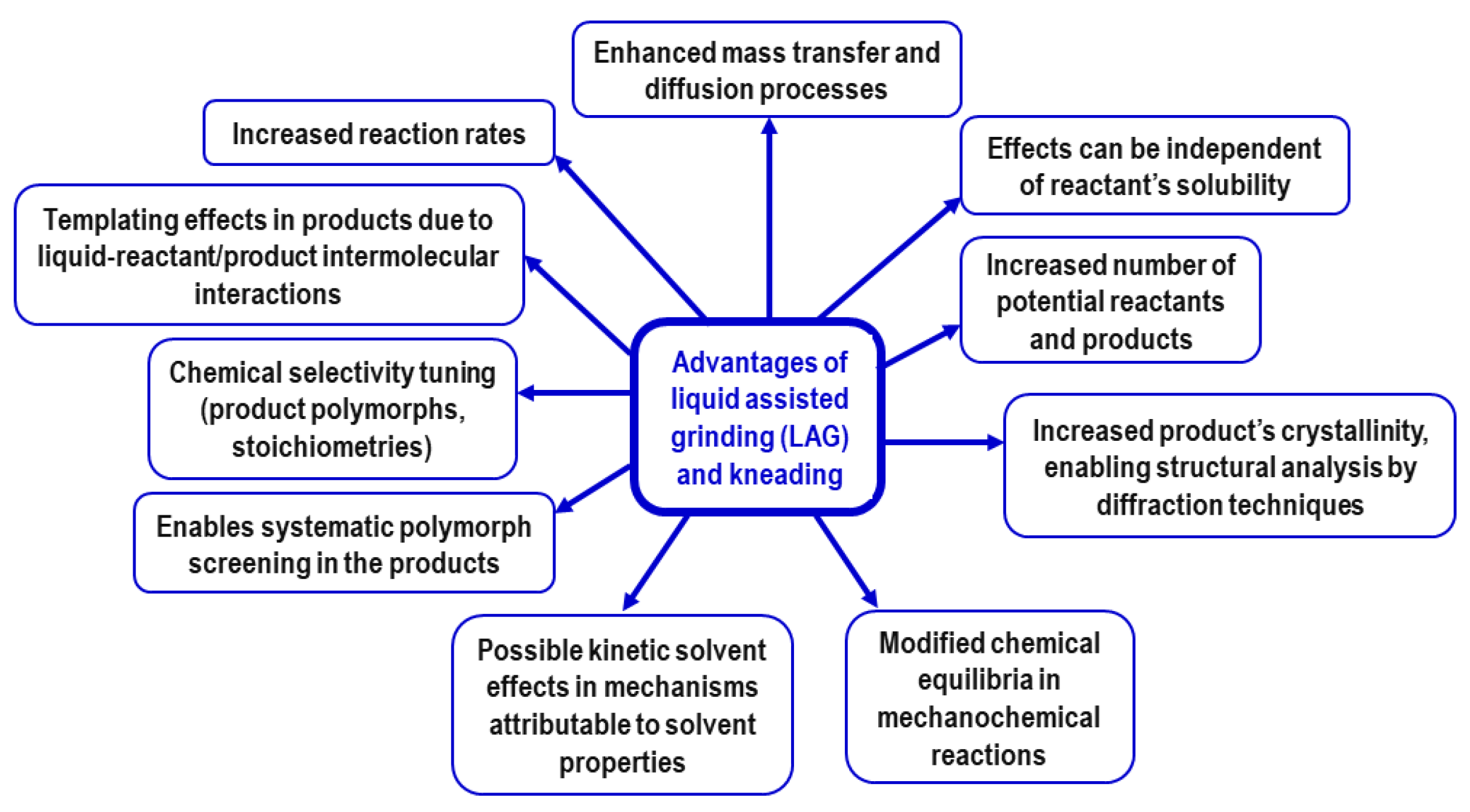
| Safer solvents and auxiliaries | Reactant solubility considerations are unnecessary. New potential reactants (less toxic, cheaper, safer to use) become available. Reactivity tunability using LAG selectively leading to different polymorphs, MOF topologies, etc. |
| Design for energy efficiency | Scalability for industrial production and continuous flow processes using reactive extrusion. Typical fast reaction rates and high yields, often at room temperature and ambient pressure. Heating may be avoided, shortened, or the temperature reduced, potentially leading to lower fossil fuel consumption and reduced carbon footprint. Example: Synthesis of ammonia under mild conditions [41]. |
| Renewable feedstock use | Biomass valorization reactions of cellulose, charcoal, lignin, chitin, and eggshell [42] renewable feedstocks. |
| Reduced derivatives | Affords important synthetic processes in less steps and “one pot” syntheses. Example: Highly processed salts used as catalysts have been replaced with less costly mineral ores [43]. |
| Catalysis | Milling media/vessels can be used as catalysts [44]. Many enzymes remain active in ball milling and reactive extrusion [45]. |
| Design for degradation | Affords the synthesis of biodegradable polymers [46], and the efficient degradation of waste polymers such as polyethylene terephthalate [47]. |
| Real-time analysis | Raman spectroscopy affords in situ monitoring of product formation. |
| Accident prevention | Automation of chemical processes in flow reactors is possible. A reduced exposure of humans and the environment to hazardous chemicals can be achieved. |
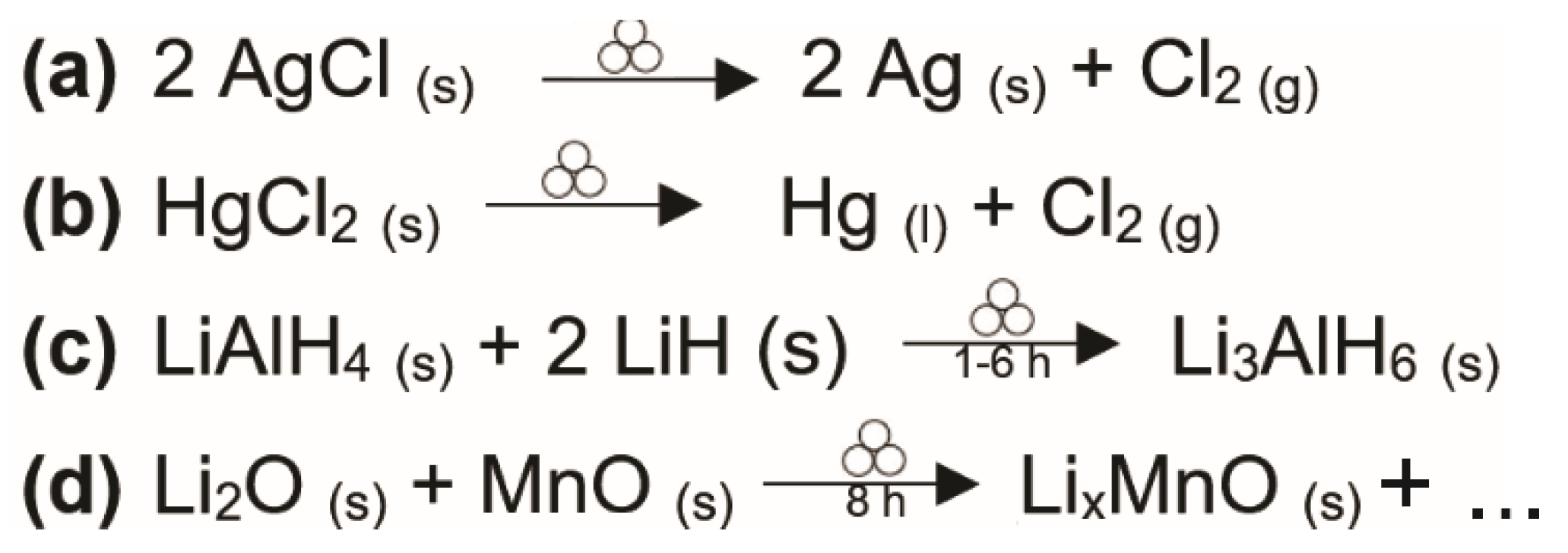
2.2. A Myriad of (If Not Any) Materials from Mechanochemical Reactions
2.3. Liquid Additives, Milling Auxiliaries, and Catalysts
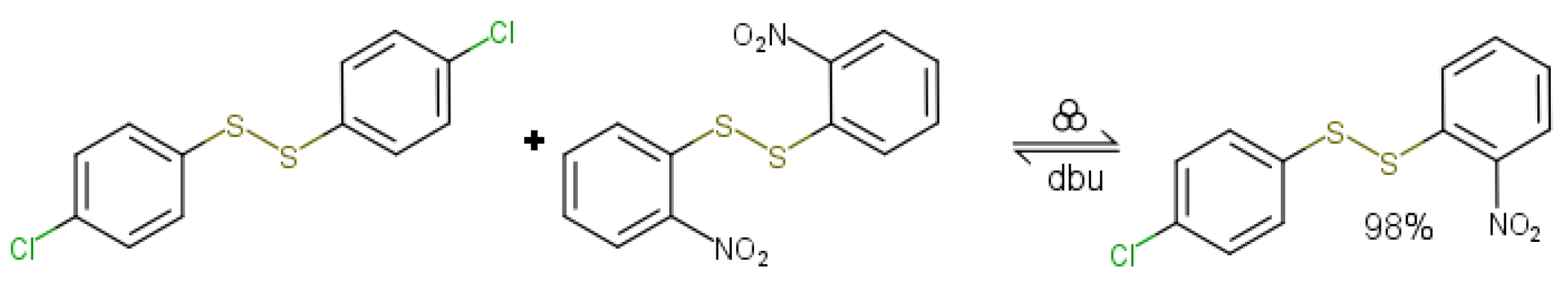
2.4. Unique Mechanochemical Reactivity and Selectivity
2.5. Temperature and Pressure Effects in Mechanochemical Reactions
3. Mechanochemistry’s Current Drawbacks
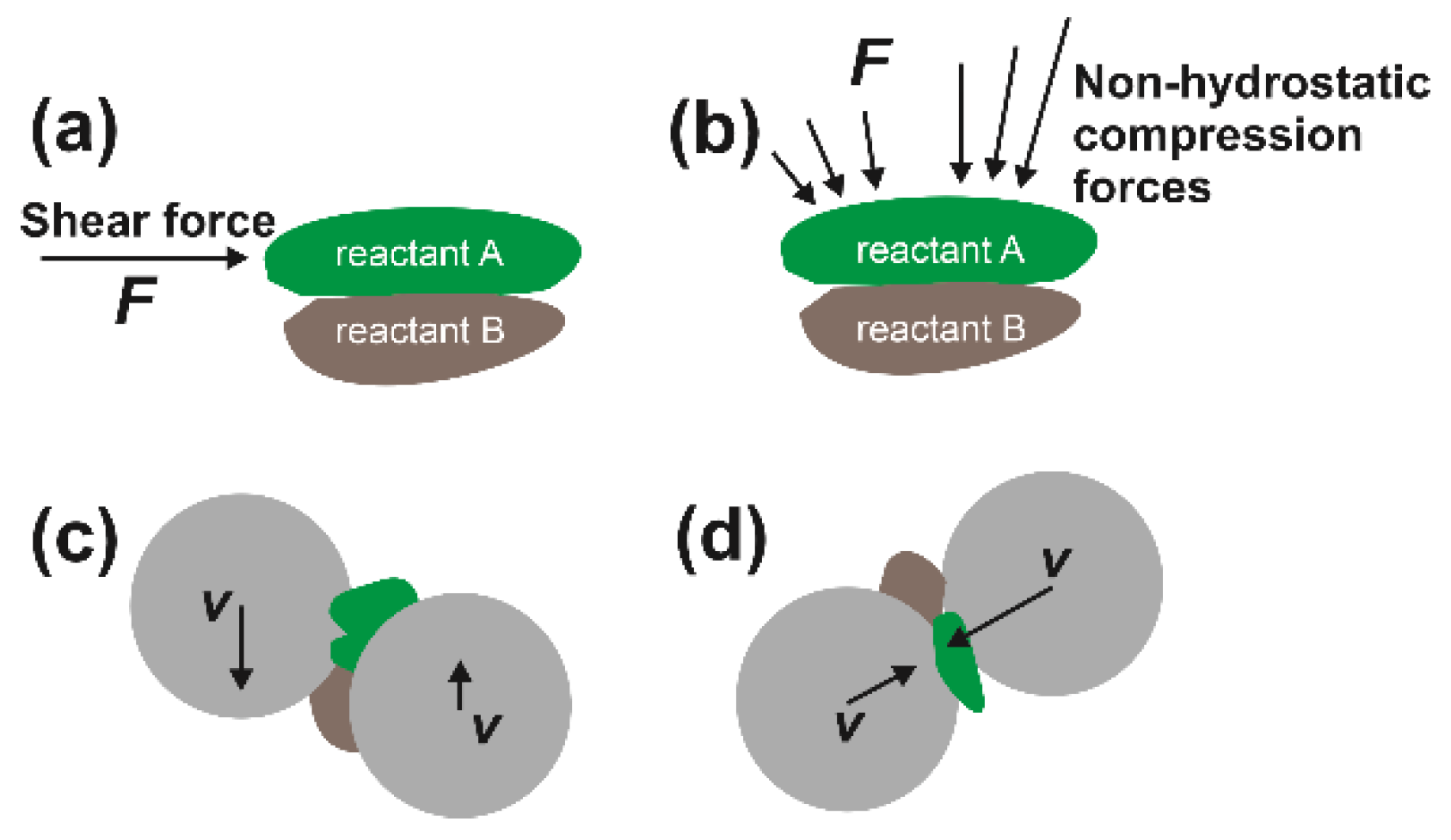
3.1. The Insufficiently Characterized Energy Input in Ball Milling Processes
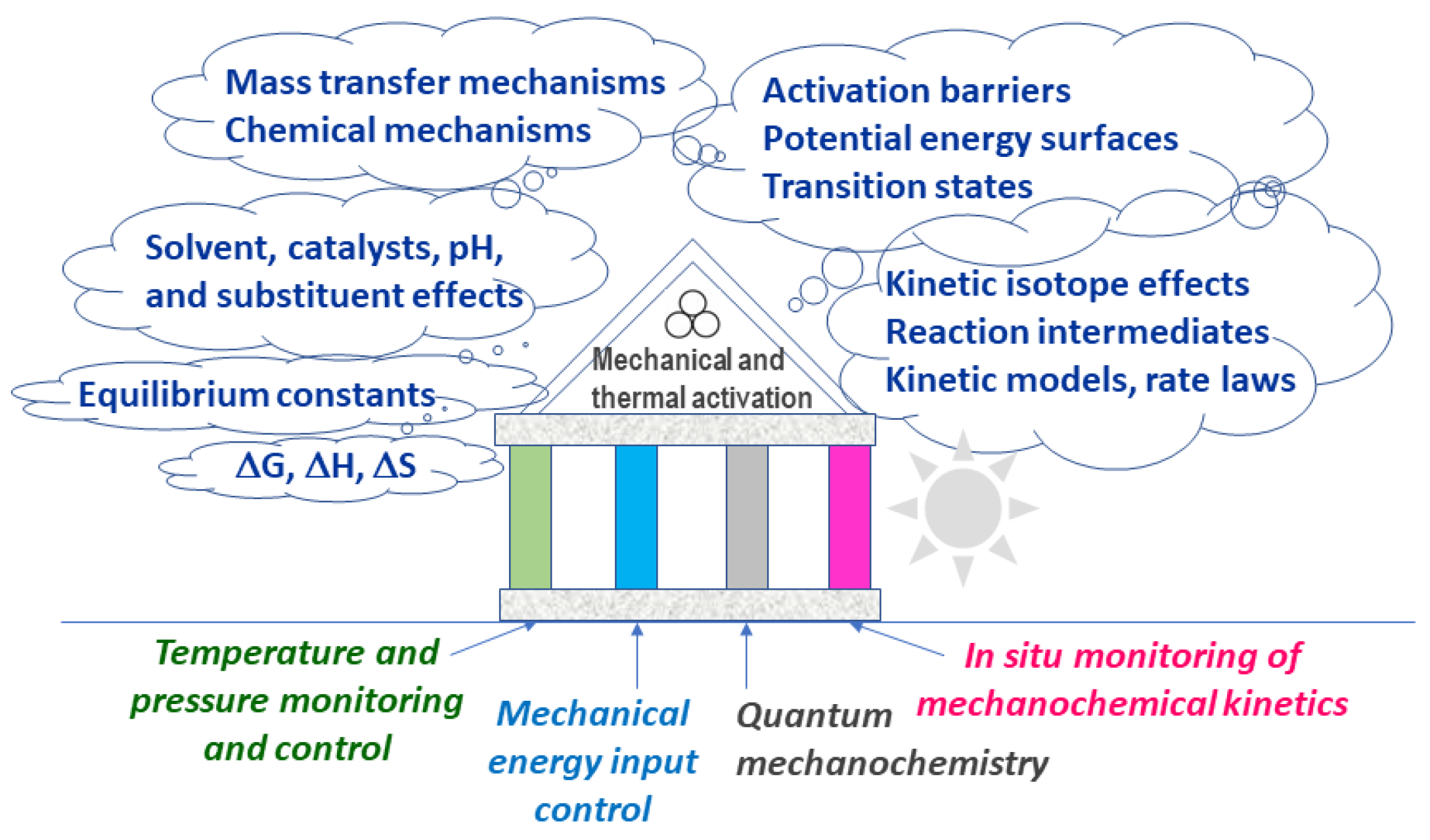
3.2. Limited Theoretical Frameworks for Mechanochemistry and Plausible Paradigm Shifts
4. Recent Advances
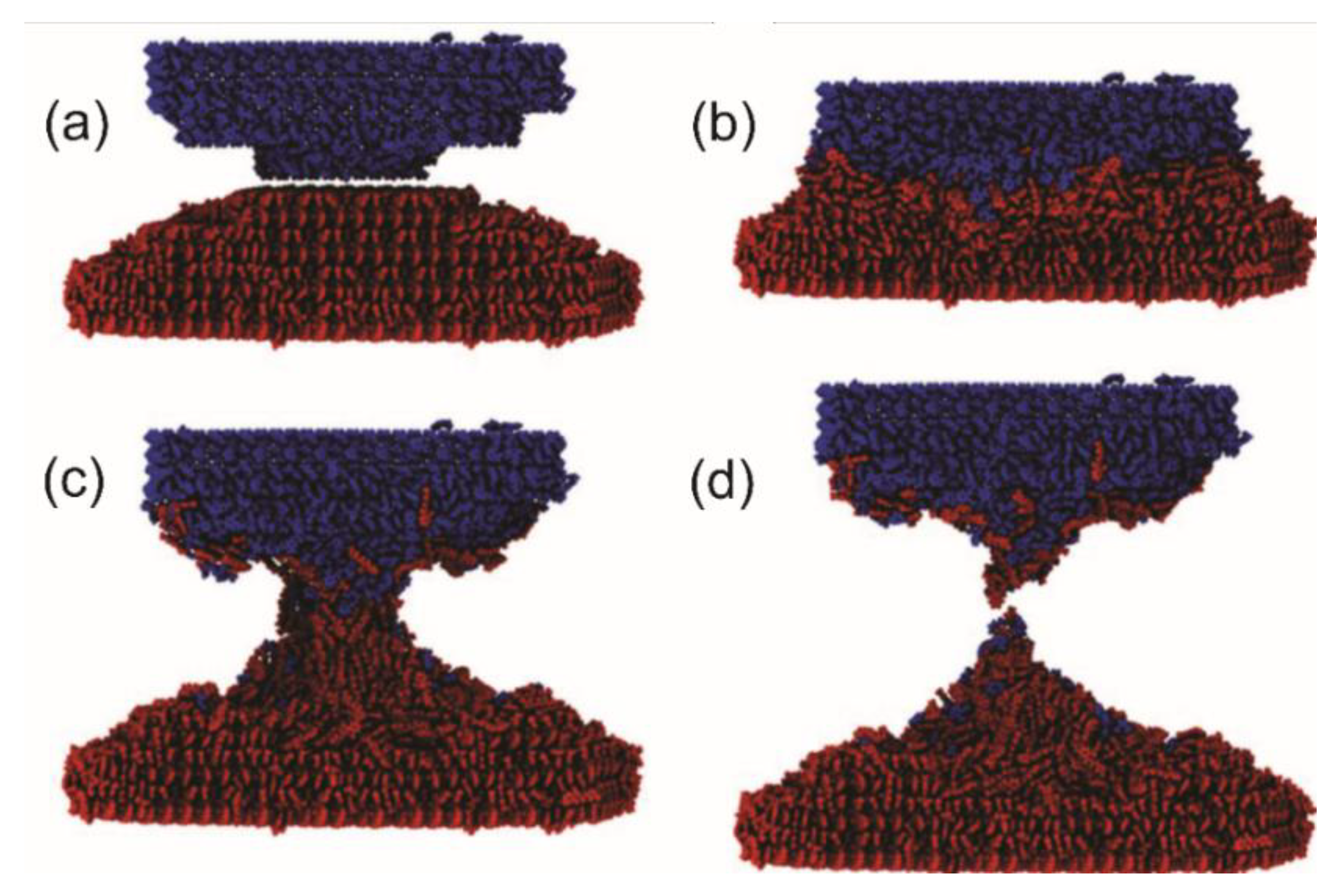
4.1. Quantum Mechanochemistry
4.2. In Situ Monitoring of Mechanochemical Reaction Kinetics
5. Outlook and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, R.T.; Boulatov, R. The Many Flavours of Mechanochemistry and Its Plausible Conceptual Underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of Terms Relating to Reactions of Polymers and to Functional Polymeric Materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27, 449. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Bulina, N.V.; Lomovskii, O.I. Disordering of the Crystal Structure of Cellulose Under Mechanical Activation. J. Struct. Chem. 2018, 59, 201–208. [Google Scholar] [CrossRef]
- Schmidt, R.; Martin Scholze, H.; Stolle, A. Temperature Progression in a Mixer Ball Mill. Int. J. Ind. Chem. 2016, 7, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Colacino, E.; Carta, M.; Pia, G.; Porcheddu, A.; Ricci, P.C.; Delogu, F. Processing and Investigation Methods in Mechanochemical Kinetics. ACS Omega 2018, 3, 9196–9209. [Google Scholar] [CrossRef] [Green Version]
- Crawford, D.; Casaban, J.; Haydon, R.; Giri, N.; McNally, T.; James, S.L. Synthesis by Extrusion: Continuous, Large-Scale Preparation of MOFs Using Little or No Solvent. Chem. Sci. 2015, 6, 1645–1649. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Mack, J. Mechanochemistry and Organic Synthesis: From Mystical to Practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by Twin Screw Extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Suslick, K.S. Mechanochemistry and Sonochemistry: Concluding Remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Michalchuk, A.A.L.; Hope, K.S.; Kennedy, S.R.; Blanco, M.V.; Boldyreva, E.V.; Pulham, C.R. Ball-Free Mechanochemistry: In Situ Real-Time Monitoring of Pharmaceutical Co-Crystal Formation by Resonant Acoustic Mixing. Chem. Commun. 2018, 54, 4033–4036. [Google Scholar] [CrossRef] [Green Version]
- Ainavarapu, S.R.K.; Wiita, A.P.; Dougan, L.; Uggerud, E.; Fernández, J.M. Single-Molecule Force Spectroscopy Measurements of Bond Elongation during a Bimolecular Reaction. J. Am. Chem. Soc. 2008, 130, 6479–6487. [Google Scholar] [CrossRef]
- Garcia-Manyes, S.; Beedle, A.E.M. Steering Chemical Reactions with Force. Nat. Rev. Chem. 2017, 1, 0083. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Boldyreva, E.V.; Belenguer, A.M.; Emmerling, F.; Boldyrev, V.V. Tribochemistry, Mechanical Alloying, Mechanochemistry: What Is in a Name? Front. Chem. 2021, 9, 685789. [Google Scholar] [CrossRef]
- Takacs, L. The Historical Development of Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef] [PubMed]
- Margetić, D.; Štrukil, V. Mechanochemical Organic Synthesis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an Emerging Tool for Molecular Synthesis: What Can It Offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cindro, N.; Tireli, M.; Karadeniz, B.; Mrla, T.; Užarević, K. Investigations of Thermally Controlled Mechanochemical Milling Reactions. ACS Sustain. Chem. Eng. 2019, 7, 16301–16309. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Insights into Mechanochemical Reactions at Targetable and Stable, Sub-Ambient Temperatures. Angew. Chem. Int. Ed. 2018, 57, 13062–13065. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Giaffreda, S.L.; Rubini, K.; Grepioni, F.; Chierotti, M.R.; Gobetto, R. Making Crystals from Crystals: Three Solvent-Free Routes to the Hydrogen Bonded Co-Crystal between 1,1′-di-pyridyl-ferrocene and Anthranilic Acid. CrystEngComm 2007, 9, 39–45. [Google Scholar] [CrossRef]
- Bandichhor, R.; Bhattacharya, A.; Diorazio, L.; Dunn, P.; Fraunhoffer, K.; Gallou, F.; Hayler, J.; Hickey, M.; Hinkley, B.; Humphreys, L.; et al. Green Chemistry Articles of Interest to the Pharmaceutical Industry. Org. Prog. Res. Dev. 2010, 14, 19–29. [Google Scholar] [CrossRef]
- Kaupp, G. Solid-State Molecular Syntheses: Complete Reactions without Auxiliaries Based on the New Solid-State Mechanism. CrystEngComm 2003, 5, 117–133. [Google Scholar] [CrossRef]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World. Chem. Int. 2020, 42, 3–9. [Google Scholar] [CrossRef]
- Friščić, T. New Opportunities for Materials Synthesis Using Mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadeniz, B.; Žilić, D.; Huskić, I.; Germann, L.S.; Fidelli, A.M.; Muratović, S.; Lončarić, I.; Etter, M.; Dinnebier, R.E.; Barišić, D.; et al. Controlling the Polymorphism and Topology Transformation in Porphyrinic Zirconium Metal-Organic Frameworks via Mechanochemistry. J. Am. Chem. Soc. 2019, 141, 19214–19220. [Google Scholar] [CrossRef]
- Chen, R.; Gokus, M.K.; Pagola, S. Tetrathiafulvalene: A Gate to the Mechanochemical Mechanisms of Electron Transfer Reactions. Crystals 2020, 10, 482. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Fábián, L. Mechanochemical Conversion of a Metal Oxide into Coordination Polymers and Porous Frameworks Using Liquid-Assisted Grinding (LAG). CrystEngComm 2009, 11, 743–745. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Ledovskaya, M.S.; Voronin, V.V.; Lotsman, K.A.; Ananikov, V.P. Calcium Carbide: Versatile Synthetic Applications, Green Methodology and Sustainability. Eur. J. Org. Chem. 2020, 2021, 43–52. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ahmed, I. Journey Describing Applications of Oxone in Synthetic Chemistry. Chem. Rev. 2013, 113, 3329–3371. [Google Scholar] [CrossRef] [PubMed]
- McCalmont, A.S.; Ruiz, A.; Lagunas, M.C.; Al-Jamal, W.T.; Crawford, D.E. Cytotoxicity of Mechanochemically Prepared Cu(II) Complexes. ACS Sustain. Chem. Eng. 2020, 8, 15243–15249. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Chen, Z.-W.; Coppex, C.; Kim, S.-J.; Noh, H.-J.; Fu, Z.; Lu, Y.; Singh, C.V.; Siahrostami, S.; et al. Mechanochemistry for Ammonia Synthesis under Mild Conditions. Nat. Nanotechnol. 2020, 16, 325–330. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized Applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Puccetti, F.; Schumacher, C.; Wotruba, H.; Hernández, J.G.; Bolm, C. The Use of Copper and Vanadium Mineral Ores in Catalyzed Mechanochemical Carbon–Carbon Bond Formations. ACS Sustain. Chem. Eng. 2020, 8, 7262–7266. [Google Scholar] [CrossRef]
- Haley, R.A.; Zellner, A.R.; Krause, J.A.; Guan, H.; Mack, J. Nickel Catalysis in a High Speed Ball Mill: A Recyclable Mechanochemical Method for Producing Substituted Cyclooctatetraene Compounds. ACS Sustain. Chem. Eng. 2016, 4, 2464–2469. [Google Scholar] [CrossRef]
- Kaabel, S.; Friščić, T.; Auclair, K. Mechanoenzymatic Transformations in the Absence of Bulk Water: A More Natural Way of Using Enzymes. ChemBioChem 2019, 21, 742–758. [Google Scholar] [CrossRef]
- Oh, C.; Choi, E.H.; Choi, E.J.; Premkumar, T.; Song, C. Facile Solid-State Mechanochemical Synthesis of Eco-Friendly Thermoplastic Polyurethanes and Copolymers Using a Biomass-Derived Furan Diol. ACS Sustain. Chem. Eng. 2020, 8, 4400–4406. [Google Scholar] [CrossRef]
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2020, 14, 330–338. [Google Scholar] [CrossRef]
- Do, J.L.; Friščić, T. Chemistry 2.0: Developing a New, Solvent-Free System of Chemical Synthesis Based on Mechanochemistry. Synlett 2017, 28, 2066–2092. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main Group Mechanochemistry: From Curiosity to Established Protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [Green Version]
- Carlton, H.; Huitink, D.; Liang, H. Tribochemistry as an Alternative Synthesis Pathway. Lubricants 2020, 8, 87. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry of Sulphides. J. Mater. Sci. 2004, 39, 5097–5102. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balema, V.P.; Pecharsky, V.K.; Dennis, K.W. Solid State Phase Transformations in LiAlH4 during High-Energy Ball-Milling. J. Alloys Compd. 2000, 313, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-Free Synthesis of Metal Complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Saski, M.; Yadav, P.; Grätzel, M.; Lewiński, J. Mechanoperovskites for Photovoltaic Applications: Preparation, Characterization, and Device Fabrication. Acc. Chem. Res. 2019, 52, 3233–3243. [Google Scholar] [CrossRef]
- Soiron, S.; Rougier, A.; Aymard, L.; Tarascon, J.-M. Mechanochemical Synthesis of Li–Mn–O Spinels: Positive Electrode for Lithium Batteries. J. Power Sources 2001, 97–98, 402–405. [Google Scholar] [CrossRef]
- Branković, Z.; Branković, G.; Jovalekić, Č.; Maniette, Y.; Cilense, M.; Varela, J.A. Mechanochemical Synthesis of PZT Powders. Mater. Sci. Eng. A 2003, 345, 243–248. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schuth, F. Mechanochemical Synthesis of Catalytic Materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering, 1st ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Zhu, S.E.; Li, F.; Wang, G.W. Mechanochemistry of Fullerenes and Related Materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball Milling in Organic Synthesis: Solutions and Challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Tan, D.; Friščić, T. Mechanochemistry for Organic Chemists: An Update. Eur. J. Org. Chem. 2018, 2018, 18–33. [Google Scholar] [CrossRef]
- Todres, Z.V. Organic Mechanochemistry and Its Practical Applications, 1st ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ferguson, M.; Giri, N.; Huang, X.; Apperley, D.; James, S.L. One-Pot Two-Step Mechanochemical Synthesis: Ligand and Complex Preparation without Isolating Intermediates. Green Chem. 2014, 16, 1374–1382. [Google Scholar] [CrossRef]
- Kulla, H.; Haferkamp, S.; Akhmetova, I.; Röllig, M.; Maierhofer, C.; Rademann, K.; Emmerling, F. In Situ Investigations of Mechanochemical One-Pot Syntheses. Angew. Chem. Int. Ed. 2018, 57, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ren, X.; Ma, H.; Feng, X.; Lin, Z.; Li, X.; Hu, C.; Wang, B. Nickel-Substituted Zeolitic Imidazolate Frameworks for Time-Resolved Alcohol Sensing and Photocatalysis under Visible Light. J. Mater. Chem. A 2014, 2, 5724–5729. [Google Scholar] [CrossRef]
- Virieux, D.; Delogu, F.; Porcheddu, A.; García, F.; Colacino, E. Mechanochemical Rearrangements. J. Org. Chem. 2021, 86, 13885–13894. [Google Scholar] [CrossRef]
- Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis. J. Am. Chem. Soc. 2016, 138, 13135–13138. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.B.; Su, W.K. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246–1271. [Google Scholar] [CrossRef]
- Lomovsky, O.I.; Lomovskiy, I.O.; Orlov, D.V. Mechanochemical Solid Acid/Base Reactions for Obtaining Biologically Active Preparations and Extracting Plant Materials. Green Chem. Lett. Rev. 2017, 10, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mellot, G.; Van Luijk, D.; Creton, C.; Sijbesma, R.P. Mechanochemical Tools for Polymer Materials. Chem. Soc. Rev. 2021, 50, 4100–4140. [Google Scholar] [CrossRef]
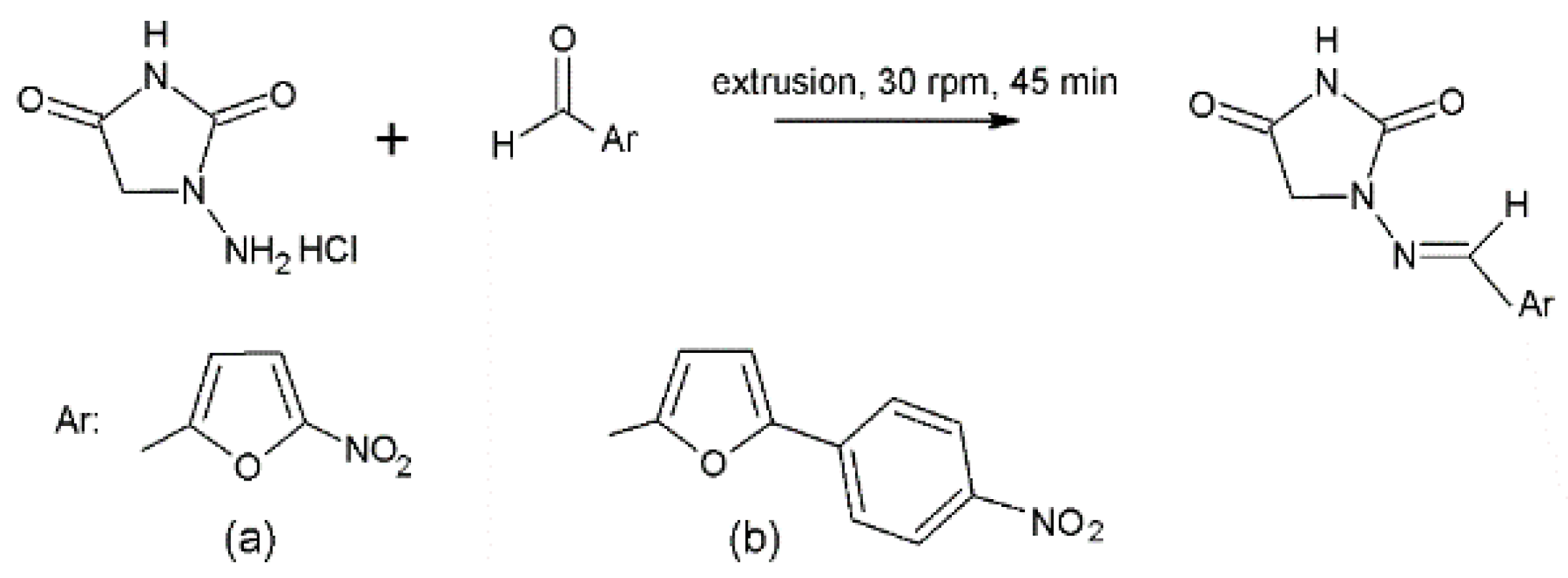
- Colacino, E.; Porcheddu, A.; Halasz, I.; Charnay, C.; Delogu, F.; Guerra, R.; Fullenwarth, J. Mechanochemistry for “No Solvent, No Base” Preparation of Hydantoin-Based Active Pharmaceutical Ingredients: Nitrofurantoin and Dantrolene. Green Chem. 2018, 20, 2973–2977. [Google Scholar] [CrossRef]
- Stolar, T.; Užarević, K. Mechanochemistry: An Efficient and Versatile Toolbox for Synthesis, Transformation, and Functionalization of Porous Metal-Organic Frameworks. CrystEngComm 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Li, P.; Cheng, F.F.; Xiong, W.W.; Zhang, Q. New Synthetic Strategies to Prepare Metal-Organic Frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal-Organic Frameworks Meet Scalable and Sustainable Synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New Synthetic Strategies toward Covalent Organic Frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Chen, Z.; Zhang, Q. Organic Cocrystals: Beyond Electrical Conductivities and Field-Effect Transistors (FETs). Angew. Chem. Int. Ed. 2019, 58, 2–18. [Google Scholar] [CrossRef]
- Trask, A.V. An Overview of Pharmaceutical Cocrystals as Intellectual Property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Strategy and Methodology in the Synthesis of Multicomponent Molecular Solids: The Quest for Higher Cocrystals. Acc. Chem. Res. 2019, 52, 2210–2220. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical Preparation of Co-Crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Jones, J.; Ta Phuoc, V.; Del Campo, L.; Massa, N.E.; Brown, C.M.; Pagola, S. Accessing New Charge-Transfer Complexes by Mechanochemistry: A Tetrathiafulvalene Chloranilic Acid Polymorph Containing Segregated Tetrathiafulvalene Stacks. Cryst. Growth Des. 2019, 19, 4970–4980. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug Co-crystals: Towards the Development of Effective Therapeutic Hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef]
- Harris, N.; Benedict, J.; Dickie, D.A.; Pagola, S. Mechanochemical Synthesis Insights and Solid-State Characterization of Quininium Aspirinate, a Glass-Forming Drug-Drug Salt. Acta Crystallogr. Sect. C Struct. Chem. 2021, 77, 566–576. [Google Scholar] [CrossRef]
- Friščić, T.; Trask, A.V.; Jones, W.; Motherwell, W.D.S. Screening for Inclusion Compounds and Systematic Construction of Three-Component Solids by Liquid-Assisted Grinding. Angew. Chem. Int. Ed. 2006, 45, 7546–7550. [Google Scholar] [CrossRef]
- Boldyreva, E. Mechanochemistry of Inorganic and Organic Systems: What Is Similar, What Is Different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- Llinàs, A.; Goodman, J.M. Polymorph control: Past, present and future. Drug Discov. Today 2008, 13, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Loots, L.; Friščić, T. Towards Medicinal Mechanochemistry: Evolution of Milling from Pharmaceutical Solid Form Screening to the Synthesis of Active Pharmaceutical Ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.F. Perspectives on the Amorphisation/Milling Relationship in Pharmaceutical Materials. Adv. Drug Deliv. Rev. 2016, 100, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.C.; Curtin, D.Y. Thermally Induced Organic Reactions in the Solid State. Acc. Chem. Res. 1973, 6, 217–225. [Google Scholar] [CrossRef]
- Varga, K.; Volarić, J.; Vančik, H. Crystal Disordering and Organic Solid-State Reactions. CrystEngComm 2015, 17, 1434–1438. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Morris, K.R.; Byrn, S.R. Crystal Packing and Chemical Reactivity of Two Polymorphs of Flufenamic Acid with Ammonia. Mol. Cryst. Liq. Cryst. 2002, 381, 121–131. [Google Scholar] [CrossRef]
- Bowmaker, G.A. Solvent-Assisted Mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Rothenberg, G.; Downie, A.P.; Raston, C.L.; Scott, J.L. Understanding Solid/Solid Organic Reactions. J. Am. Chem. Soc. 2001, 123, 8701–8708. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, K.; Davey, R.; Cross, W. How Does Grinding Produce Co-Crystals? Insights from the Case of Benzophenone and Diphenylamine. CrystEngComm 2007, 9, 732–734. [Google Scholar] [CrossRef]
- Halasz, I.; Friščić, T.; Kimber, S.A.J.; Užarević, K.; Puškarić, A.; Mottillo, C.; Julien, P.; Štrukil, V.; Honkimäki, V.; Dinnebier, R.E. Quantitative in Situ and Real-Time Monitoring of Mechanochemical Reactions. Faraday Discuss. 2014, 170, 203–221. [Google Scholar] [CrossRef]
- Užarević, K.; Ferdelji, N.; Mrla, T.; Julien, P.A.; Halasz, B.; Friščić, T.; Halasz, I. Enthalpy vs. Friction: Heat Flow Modelling of Unexpected Temperature Profiles in Mechanochemistry of Metal-Organic Frameworks. Chem. Sci. 2018, 9, 2525–2532. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.; Toda, F.; Jones, W. Mechanochemistry and Co-Crystal Formation: Effect of Solvent on Reaction Kinetics. Chem. Commun. 2002, 2372–2373. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-Based Approach for Predicting Cocrystallisation Outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Lapidus, S.H.; Naik, A.; Wixtrom, A.; Massa, N.E.; Ta Phuoc, V.; Del Campo, L.; Lebègue, S.; Ángyán, J.G.; Abdel-Fattah, T.; Pagola, S. The Black Polymorph of TTF-CA: TTF Polymorphism and Solvent Effects in Mechanochemical and Vapor Digestion Syntheses, FT-IR, Crystal Packing, and Electronic Structure. Cryst. Growth Des. 2014, 14, 91–100. [Google Scholar] [CrossRef]
- Mohamud, S.; Ta Phuoc, V.; Massa, N.E.; Pagola, S. TTF-DDQ: Two “Green” Synthetic Routes, Crystal Structure and Band Gap from FT-IR Spectroscopy. Synth. Met. 2016, 214, 71–75. [Google Scholar] [CrossRef]
- Dack, M.R.J. The Influence of Solvent on Chemical Reactivity: An Alternative Approach. J. Chem. Educ. 1974, 51, 231. [Google Scholar] [CrossRef]
- Buncel, E.; Wilson, H. Solvent Effects on Rates and Equilibria: A Practical Approach. J. Chem. Educ. 1980, 56, 629–633. [Google Scholar] [CrossRef]
- El Seoud, O.A. Solvation in Pure and Mixed Solvents: Some Recent Developments. Pure Appl. Chem. 2007, 79, 1135–1151. [Google Scholar] [CrossRef] [Green Version]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal-Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Effect of Atmosphere on Solid-State Amine-Aldehyde Condensations: Gas-Phase Catalysts for Solid-State Transformations. Chem. Commun. 2012, 48, 11683–11685. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.M.; Friščić, T.; Day, G.M.; Sanders, J.K.M. Solid-State Dynamic Combinatorial Chemistry: Reversibility and Thermodynamic Product Selection in Covalent Mechanosynthesis. Chem. Sci. 2011, 2, 696–700. [Google Scholar] [CrossRef]
- Takacs, L.M. Carey Lea, the First Mechanochemist. J. Mater. Sci. 2004, 39, 4987–4993. [Google Scholar] [CrossRef]
- Guan-Wu, W.; Koichi, K.; Yasujiro, M.; Motoo, S. Synthesis and X-ray Structure of Dumb-Bell-Shaped C120. Nature 1997, 387, 583–586. [Google Scholar]
- Zhao, Y.; Rocha, S.V.; Swager, T.M. Mechanochemical Synthesis of Extended Iptycenes. J. Am. Chem. Soc. 2016, 138, 13834–13837. [Google Scholar] [CrossRef]
- Štrukil, V.; Gracin, D.; Magdysyuk, O.V.; Dinnebier, R.E.; Friščic, T. Trapping Reactive Intermediates by Mechanochemistry: Elusive Aryl N-Thiocarbamoylbenzotriazoles as Bench-Stable Reagents. Angew. Chem. Int. Ed. 2015, 54, 8440–8443. [Google Scholar] [CrossRef] [Green Version]
- Juribašić, M.; Užarević, K.; Gracin, D.; Ćurić, M. Mechanochemical C–H Bond Activation: Rapid and Regioselective Double Cyclopalladation Monitored by in Situ Raman Spectroscopy. Chem. Commun. 2014, 50, 10287–10290. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Wenzel, K.-J.; Rademann, K.; Emmerling, F. Quantitative Determination of Activation Energies in Mechanochemical Reactions. Phys. Chem. Chem. Phys. 2016, 18, 23320–23325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Užarević, K.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Puškarić, A.; Friščić, T.; Halasz, I. Exploring the Effect of Temperature on a Mechanochemical Reaction by in Situ Synchrotron Powder X-ray Diffraction. Cryst. Growth Des. 2016, 16, 2342–2347. [Google Scholar] [CrossRef]
- Grigoryan, R.R.; Aloyan, S.G.; Harutyunyan, V.R.; Arsentev, S.D.; Tavadyan, L.A. Dry Reforming of Methane over Nanosized Tungsten Carbide Powders Obtained by Mechanochemical and Plasma-Mechanochemical Methods. Pet. Chem. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Mori, S.; Xu, W.-C.; Ishidzuki, T.; Ogasawara, N.; Imai, J.; Kobayashi, K. Mechanochemical Activation of Catalysts for CO2 Methanation. Appl. Catal. A Gen. 1996, 137, 255–268. [Google Scholar] [CrossRef]
- Mulas, G.; Campesi, R.; Garroni, S.; Delogu, F.; Milanese, C. Hydrogenation of Carbon Monoxide over Nanostructured Systems: A Mechanochemical Approach. Appl. Surf. Sci. 2011, 257, 8165–8170. [Google Scholar] [CrossRef]
- Dong, B.-X.; Zhao, J.; Wang, L.-Z.; Teng, Y.-L.; Liu, W.-L.; Wang, L. Mechanochemical Synthesis of COx-Free Hydrogen and Methane Fuel Mixtures at Room Temperature from Light Metal Hydrides and Carbon Dioxide. Appl. Energy 2017, 204, 741–748. [Google Scholar] [CrossRef]
- Nash, D.J.; Restrepo, D.T.; Parra, N.S.; Giesler, K.E.; Penabade, R.A.; Aminpour, M.; Le, D.; Li, Z.; Farha, O.K.; Harper, J.K.; et al. Heterogeneous Metal-Free Hydrogenation over Defect-Laden Hexagonal Boron Nitride. ACS Omega 2016, 1, 1343–1354. [Google Scholar] [CrossRef]
- Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Cu(0), O2 and Mechanical Forces: A Saving Combination for Efficient Production of Cu–NHC Complexes. Chem. Sci. 2017, 8, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Kano, J.; Miyazaki, M.; Saito, F. Ball Mill Simulation and Powder Characteristics of Ground Talc in Various Types of Mill. Adv. Powder Technol. 2000, 11, 333–342. [Google Scholar] [CrossRef]
- Fišteš, A.Z.; Rakić, D.Z.; Pajin, B.S.; Dokić, L.P.; Nikolić, I.R. The Effect of Processing Parameters on Energy Comsumption of Ball Mill Refiner for Chocolate. Hem. Ind. 2013, 67, 747–751. [Google Scholar] [CrossRef]
- Dallimore, M.P.; McCormick, P.G. Dynamics of Planetary Ball Milling: A Comparison of Computer Simulated Processing Parameters with CuO/Ni Displacement Reaction Milling Kinetics. Mater. Trans. 1996, 37, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Burmeister, C.F.; Kwade, A. Process Engineering with Planetary Ball Mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Gotor, F.J.; Achimovicova, M.; Real, C.; Bálaž, P. Influence of the Milling Parameters on the Mechanical Work Intensity in Planetary Mills. Powder Technol. 2013, 233, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Yuan, W.; Bell, S.E.J.; James, S.L. Better Understanding of Mechanochemical Reactions: Raman Monitoring Reveals Surprisingly Simple “pseudo-Fluid” Model for a Ball Milling Reaction. Chem. Commun. 2014, 50, 1585–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, P.A.; Malvestiti, I.; Friščić, T. The Effect of Milling Frequency on a Mechanochemical Organic Reaction Monitored by in Situ Raman Spectroscopy. Beilstein J. Org. Chem. 2017, 13, 2160–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Peng, Z.W.; Hao, M.F.; Gao, J.G. Mechanochemical Diels-Alder Cycloaddition Reactions for Straightforward Synthesis of Endo-Norbornene Derivatives. Synlett 2010, 19, 2895–2898. [Google Scholar] [CrossRef]
- Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Energetic Assessment of the Suzuki–Miyaura Reaction: A Curtate Life Cycle Assessment as an Easily Understandable and Applicable Tool for Reaction Optimization. Green Chem. 2009, 11, 1894–1899. [Google Scholar] [CrossRef]
- Cincotti, A.; Traversari, G.; Pia, G.; Delogu, F. Milling Dynamics and Propagation of Mechanically Activated Self-Sustaining Reactions. Adv. Mater. Sci. Eng. 2020, 2020, 26–35. [Google Scholar] [CrossRef]
- Geng, F.; Gang, L.; Wang, Y.; Li, Y.; Yuan, Z. Numerical Investigation on Particle Mixing in a Ball Mill. Powder Technol. 2016, 292, 64–73. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Hofer, M.; Molaiyan, P.; Michalowski, P.; Kwade, A. Characterization of Stressing Conditions in a High Energy Ball Mill by Discrete Element Simulations. Processes 2022, 10, 692. [Google Scholar] [CrossRef]
- Kakuk, G.; Zsoldos, I.; Csanády, Á.; Oldal, I. Contributions to the Modelling of the Milling Process in a Planetary Ball Mill. Rev. Adv. Mater. Sci. 2009, 22, 21–38. [Google Scholar]
- Reichardt, R.; Wiechert, W. Event Driven Algorithms Applied to a High Energy Ball Mill Simulation. Granul. Matter 2007, 9, 251–266. [Google Scholar] [CrossRef]
- Carta, M.; Colacino, E.; Delogu, F.; Porcheddu, A. Kinetics of Mechanochemical Transformations. Phys. Chem. Chem. Phys. 2020, 22, 14489–14502. [Google Scholar] [CrossRef] [PubMed]
- Rybkin, V.V. Franck-Condon Theory of Quantum Mechanochemistry. J. Phys. Chem. A 2017, 121, 5758–5762. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.W.; Samaras, G.; Hebisch, K.L.; Realff, M.J. Hot Spot Generation, Reactivity, and Decay in Mechanochemical Reactors. Chem. Eng. J. 2020, 382, 122954. [Google Scholar] [CrossRef]
- Andersen, J.; Brunemann, J.; Mack, J. Exploring Stable, Sub-Ambient Temperatures in Mechanochemistry via a Diverse Set of Enantioselective Reactions. React. Chem. Eng. 2019, 4, 1229–1236. [Google Scholar] [CrossRef]
- Ong, M.T.; Leiding, J.; Tao, H.; Virshup, A.M.; Martínez, T.J. First Principles Dynamics and Minimum Energy Pathways for Mechanochemical Ring Opening of Cyclobutene. J. Am. Chem. Soc. 2009, 131, 6377–6379. [Google Scholar] [CrossRef]
- Hickenboth, C.R.; Moore, J.S.; White, S.R.; Sottos, N.R.; Baudry, J.; Wilson, S.R. Biasing Reaction Pathways with Mechanical Force. Nature 2007, 446, 423–427. [Google Scholar] [CrossRef]
- Ferguson, M.; Moyano, M.S.; Tribello, G.A.; Crawford, D.E.; Bringa, E.M.; James, S.L.; Kohanoff, J.; Del Pópolo, M.G. Insights into Mechanochemical Reactions at the Molecular Level: Simulated Indentations of Aspirin and Meloxicam Crystals. Chem. Sci. 2019, 10, 2924–2929. [Google Scholar] [CrossRef] [Green Version]
- Ma, E. Amorphization in Mechanically Driven Material Systems. Scr. Mater. 2003, 49, 941–946. [Google Scholar] [CrossRef]
- Ma, X.; Shi, L.; He, X.; Li, L.; Cao, G.; Hou, C.; Li, J.; Chang, L.; Yang, L.; Zhong, Y. Graphitization Resistance Determines Super Hardness of Lonsdaleite, Nanotwinned and Nanopolycrystalline Diamond. Carbon 2018, 133, 69–76. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Gonnet, L.; Chamayou, A.; André-Barrès, C.; Micheau, J.C.; Guidetti, B.; Sato, T.; Baron, M.; Baltas, M.; Calvet, R. Elucidation of the Diels-Alder Reaction Kinetics between Diphenylfulvene and Maleimide by Mechanochemistry and in Solution. ACS Sustain. Chem. Eng. 2021, 9, 4453–4462. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Baron, M.; Chamayou, A.; Baltas, M.; Guidetti, B.; Haruta, N.; Tanaka, K.; Sato, T. Lowering the Activation Energy under Mechanochemical Conditions: The Case of 2,3-Diphenylquinoxaline. ChemistrySelect 2016, 1, 984–988. [Google Scholar] [CrossRef]
- Lukin, S.; Tireli, M.; Lončarić, I.; Barišić, D.; Šket, P.; Vrsaljko, D.; Di Michiel, M.; Plavec, J.; Užarević, K.; Halasz, I. Mechanochemical Carbon-Carbon Bond Formation That Proceeds via a Cocrystal Intermediate. Chem. Commun. 2018, 54, 13216–13219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.; Mortimer, M. (Eds.) Chemical Kinetics and Mechanism, 1st ed.; RSC Publishing: Cambridge, UK, 2002. [Google Scholar]
- Khawam, A.; Flanagan, D.R. Basics and Applications of Solid-State Kinetics: A Pharmaceutical Perspective. J. Pharm. Sci. 2006, 95, 472–498. [Google Scholar] [CrossRef]
- Ya Davydov, E.; Vorotnikov, A.P.; Pariyskii, G.B.; Zaikov, G.E. Kinetic Peculiarities of Solid Phase Reactions, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 1998. [Google Scholar]
- Stauch, T.; Dreuw, A. Advances in Quantum Mechanochemistry: Electronic Structure Methods and Force Analysis. Chem. Rev. 2016, 116, 14137–14180. [Google Scholar] [CrossRef]
- Ribas-Ariño, J.; Marx, D. Covalent Mechanochemistry: Theoretical Concepts and Computational Tools with Applications to Molecular Nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar] [CrossRef]
- Gilman, J.J. Mechanochemistry. Science 1996, 274, 65. [Google Scholar] [CrossRef]
- Grandbois, M.; Beyer, M.; Rief, M.; Clausen-Schaumann, H.; Gaub, H.E. How Strong Is a Covalent Bond? Science 1999, 283, 1727–1730. [Google Scholar] [CrossRef]
- Beyer, M.K. The Mechanical Strength of a Covalent Bond Calculated by Density Functional Theory. J. Chem. Phys. 2000, 112, 7307–7312. [Google Scholar] [CrossRef]
- Quapp, W.; Bofill, J.M.; Ribas-Ariño, J. Toward a Theory of Mechanochemistry: Simple Models from the Very Beginnings. Int. J. Quantum Chem. 2018, 118, e25775. [Google Scholar] [CrossRef] [Green Version]
- Stauch, T.; Dreuw, A. Quantum Chemical Strain Analysis For Mechanochemical Processes. Acc. Chem. Res. 2017, 50, 1041–1048. [Google Scholar] [CrossRef]
- Barbee, M.H.; Kouznetsova, T.; Barrett, S.L.; Gossweiler, G.R.; Lin, Y.; Rastogi, S.K.; Brittain, W.J.; Craig, S.L. Substituent Effects and Mechanism in a Mechanochemical Reaction. J. Am. Chem. Soc. 2018, 140, 12746–12750. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Mosey, N.J. Differences in the Abilities to Mechanically Eliminate Activation Energies for Unimolecular and Bimolecular Reactions. Sci. Rep. 2016, 6, 23059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roessler, A.G.; Zimmerman, P.M. Examining the Ways to Bend and Break Reaction Pathways Using Mechanochemistry. J. Phys. Chem. C 2018, 122, 6996–7004. [Google Scholar] [CrossRef]
- Spikes, H. Stress-Augmented Thermal Activation: Tribology Feels the Force. Friction 2018, 6, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.L.; Garvey, M.T.; Ramasamy, U.S.; Ye, Z.; Martini, A.; Tysoe, W.T. Shear Induced Mechanochemistry: Pushing Molecules Around. J. Phys. Chem. C 2015, 119, 7115–7123. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.; Honkimaki, V.; Dinnebier, R.E. Real-Time and in Situ Monitoring of Mechanochemical Milling Reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef]
- Halasz, I.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Nightingale, R.C.; Dinnebier, R.E.; Friščić, T. In Situ and Real-Time Monitoring of Mechanochemical Milling Reactions Using Synchrotron X-ray Diffraction. Nat. Protoc. 2013, 8, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Užarević, K.; Halasz, I.; Friščić, T. Real-Time and in Situ Monitoring of Mechanochemical Reactions: A New Playground for All Chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Gracin, D.; Štrukil, V.; Friščić, T.; Halasz, I.; Užarević, K. Laboratory Real-Time and in Situ Monitoring of Mechanochemical Milling Reactions by Raman Spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 6193–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukin, S.; Užarević, K.; Halasz, I. Raman Spectroscopy for Real-Time and in Situ Monitoring of Mechanochemical Milling Reactions. Nat. Protoc. 2021, 16, 3492–3521. [Google Scholar] [CrossRef]
- Lukin, S.; Stolar, T.; Tireli, M.; Blanco, M.V.; Babić, D.; Friščić, T.; Užarević, K.; Halasz, I. Tandem In Situ Monitoring for Quantitative Assessment of Mechanochemical Reactions Involving Structurally Unknown Phases. Chem. A Eur. J. 2017, 23, 13941–13949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, I.C.B.; Carta, M.; Haferkamp, S.; Feiler, T.; Delogu, F.; Colacino, E.; Emmerling, F. Mechanochemical N-Chlorination Reaction of Hydantoin: In Situ Real-Time Kinetic Study by Powder X-Ray Diffraction and Raman Spectroscopy. ACS Sustain. Chem. Eng. 2021, 9, 37. [Google Scholar] [CrossRef]
- Julien, P.A.; Friščić, T. Methods for Monitoring Milling Reactions and Mechanistic Studies of Mechanochemistry: A Primer. Cryst. Growth Des. 2022, 22, 5726–5754. [Google Scholar] [CrossRef]
- Weidenthaler, C. In Situ Analytical Methods for the Characterization of Mechanochemical Reactions. Crystals 2022, 12, 345. [Google Scholar] [CrossRef]
- Julien, P.A.; Užarević, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.K.; Zhang, Y.; Casaban, J.; Germann, L.S.; Etter, M.; et al. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Halasz, I.; Puskaric, A.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimaki, V.; Dinnebier, R.E.; Patel, B.; Jones, W.; et al. Real-Time In Situ Powder X-Ray Diffraction Monitoring of Mechanochemical Synthesis of Pharmaceutical Cocrystals. Angew. Chem. Int. Ed. 2013, 52, 11538–11541. [Google Scholar] [CrossRef]
- Germann, L.S.; Katsenis, A.D.; Huskić, I.; Julien, P.A.; Užarević, K.; Etter, M.; Farha, O.K.; Friščić, T.; Dinnebier, R.E. Real-Time in Situ Monitoring of Particle and Structure Evolution in the Mechanochemical Synthesis of UiO-66 Metal-Organic Frameworks. Cryst. Growth Des. 2020, 20, 49–54. [Google Scholar] [CrossRef]
- Tireli, M.; Juribašić Kulcsár, M.; Cindro, N.; Gracin, D.; Biliškov, N.; Borovina, M.; Ćurić, M.; Halasz, I.; Užarević, K. Mechanochemical Reactions Studied by in Situ Raman Spectroscopy: Base Catalysis in Liquid-Assisted Grinding. Chem. Commun. 2015, 51, 8058–8061. [Google Scholar] [CrossRef]
- Sović, I.; Lukin, S.; Meštrović, E.; Halasz, I.; Porcheddu, A.; Delogu, F.; Ricci, P.C.; Caron, F.; Perilli, T.; Dogan, A.; et al. Mechanochemical Preparation of Active Pharmaceutical Ingredients Monitored by in Situ Raman Spectroscopy. ACS Omega 2020, 5, 28663–28672. [Google Scholar] [CrossRef] [PubMed]
- Biliškov, N.; Borgschulte, A.; Užarević, K.; Halasz, I.; Lukin, S.; Milošević, S.; Milanović, I.; Novaković, J.G. In-Situ and Real-Time Monitoring of Mechanochemical Preparation of Li2Mg(NH2BH3)4 and Na2Mg(NH2BH3)4 and Their Thermal Dehydrogenation. Chem. A Eur. J. 2017, 23, 16274–16282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batzdorf, L.; Fischer, F.; Wilke, M.; Wenzel, K.J.; Emmerling, F. Direct in Situ Investigation of Milling Reactions Using Combined X-Ray Diffraction and Raman Spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 1799–1802. [Google Scholar] [CrossRef]
- Kulla, H.; Michalchuk, A.A.L.; Emmerling, F. Manipulating the Dynamics of Mechanochemical Ternary Cocrystal Formation. Chem. Commun. 2019, 55, 9793–9796. [Google Scholar] [CrossRef] [PubMed]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Knowing When to Stop—Trapping Metastable Polymorphs in Mechanochemical Reactions. Cryst. Growth Des. 2017, 17, 1190–1196. [Google Scholar] [CrossRef]
- Dewolfe, R.H. Kinetics in the Study of Organic Reaction Mechanisms. J. Chem. Educ. 1963, 40, 95–98. [Google Scholar] [CrossRef]


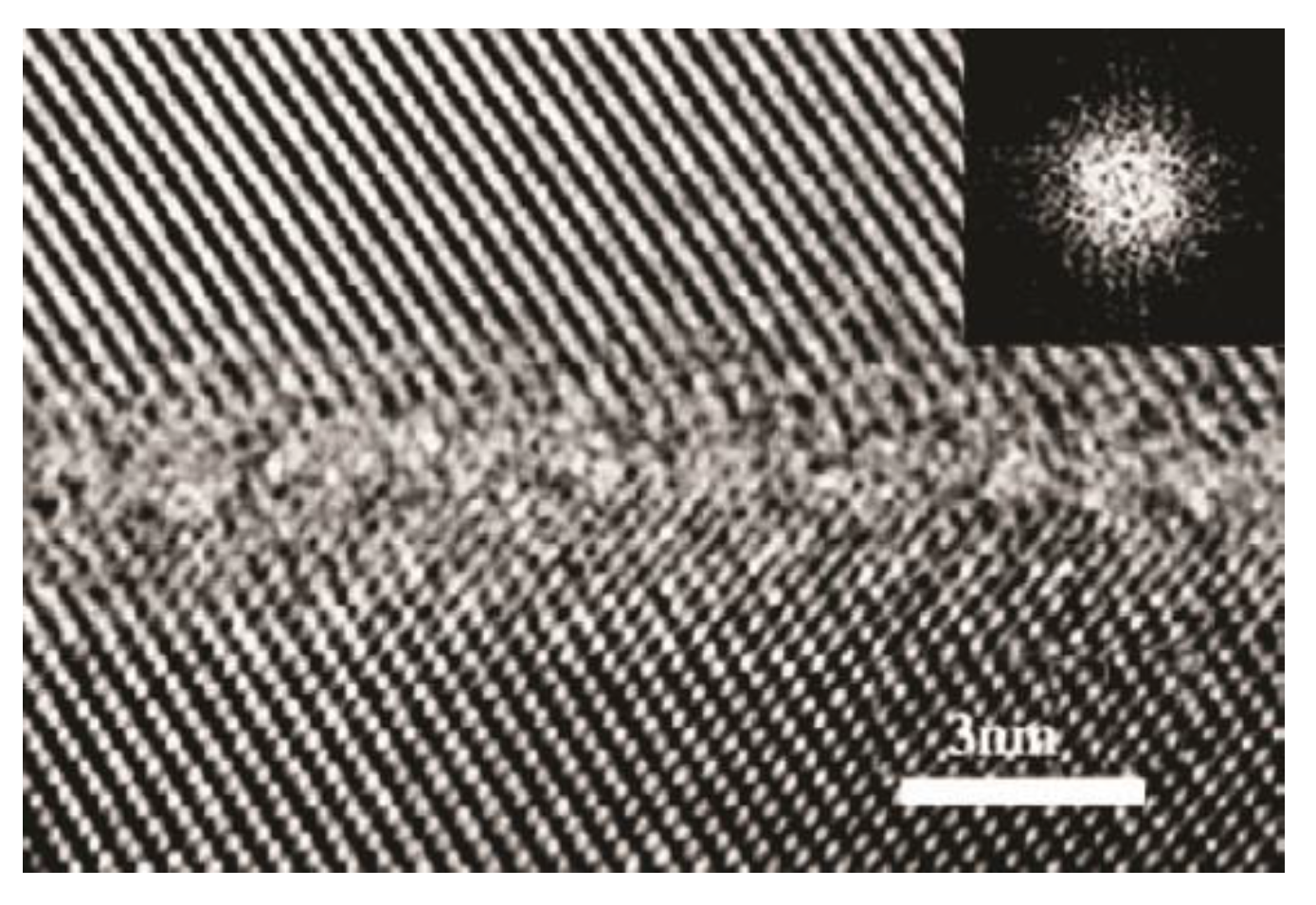

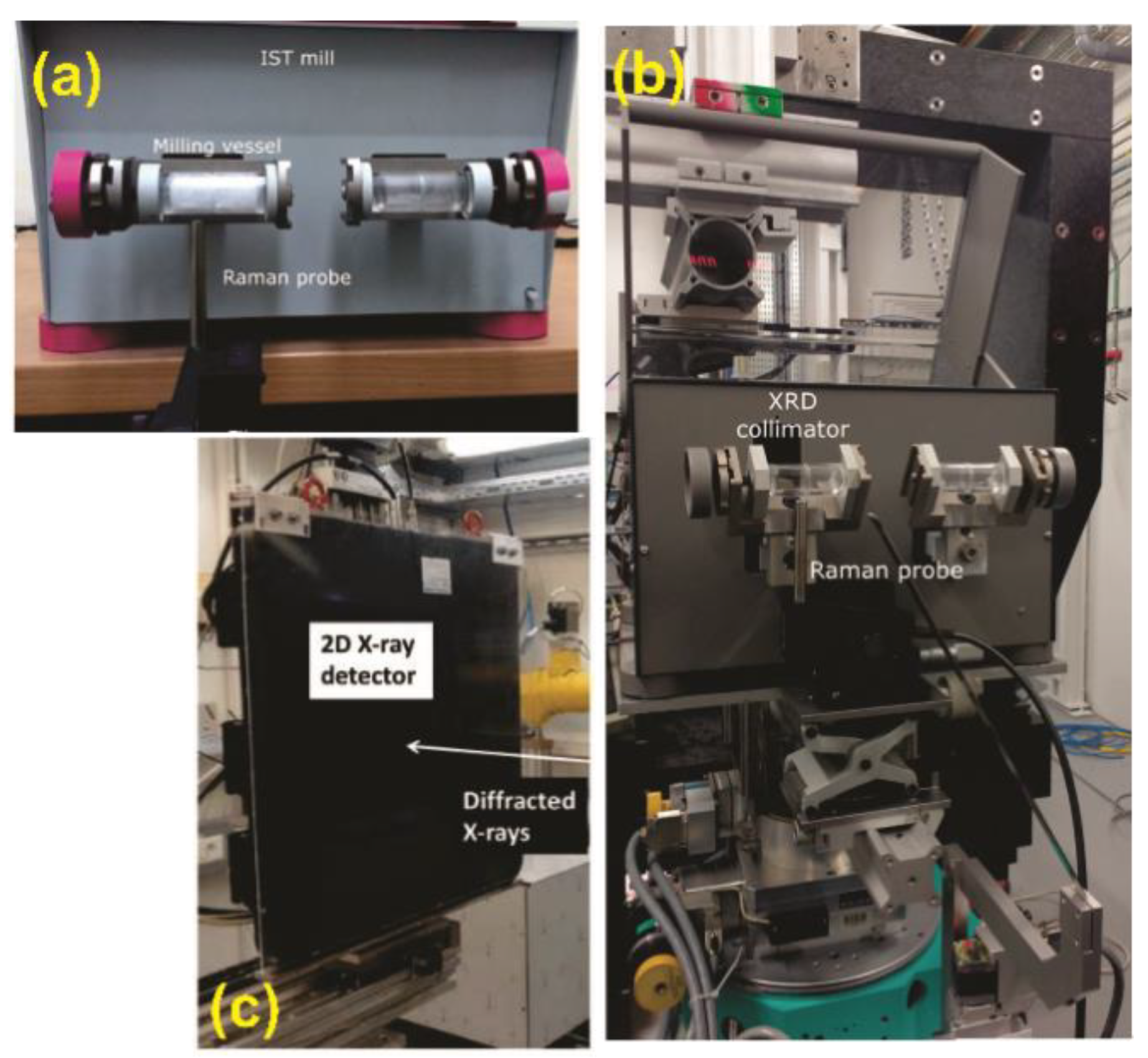
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagola, S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals 2023, 13, 124. https://doi.org/10.3390/cryst13010124
Pagola S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals. 2023; 13(1):124. https://doi.org/10.3390/cryst13010124
Chicago/Turabian StylePagola, Silvina. 2023. "Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View" Crystals 13, no. 1: 124. https://doi.org/10.3390/cryst13010124
APA StylePagola, S. (2023). Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals, 13(1), 124. https://doi.org/10.3390/cryst13010124






