Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Memantin-Loaded Chitosan Nanocrystals (MEM-NCs)
2.3. Particle Size (PS), Polydispersibility Index (PDI) and Zeta Potential (ZP)
2.4. Drug Entrapment Efficiency (EE) and Drug Loading (DL)
2.5. Morphology
2.5.1. Scanning Electron Microscopy
2.5.2. Transmission Electron Microscopy (TEM)
2.6. Powder X-ray Diffraction (PXRD) Studies
2.7. In Vitro Release Studies
2.8. In Vitro Cell Cytotoxicity Using RPMI 2650 Cell Lines
2.9. Ex Vivo Histopathological Studies
2.10. Statistical Analysis
3. Results and Discussion
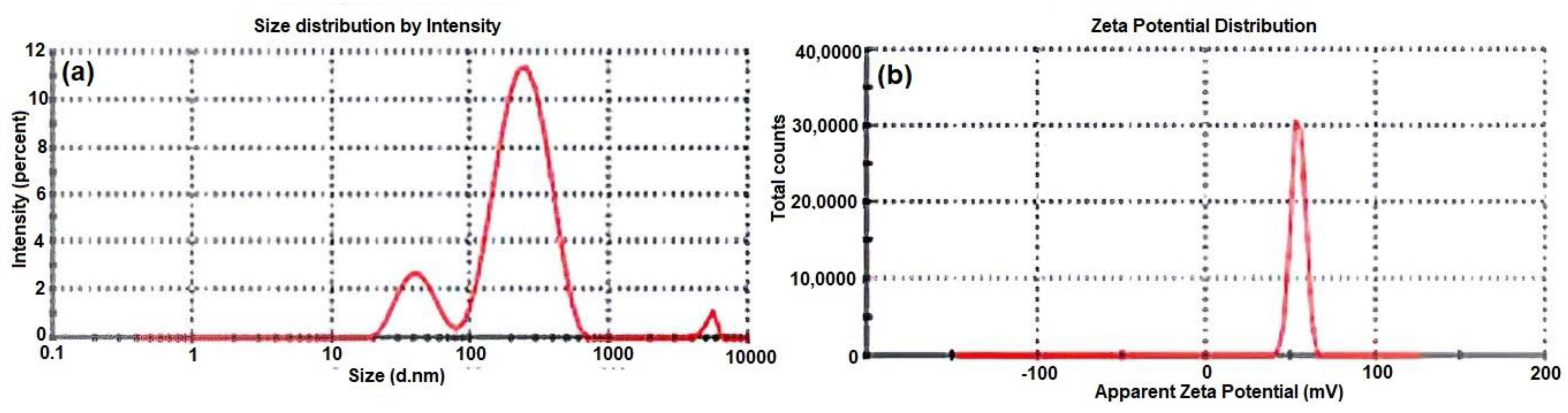
3.1. PS, PDI, ZP, DL, and EE of MEM-NCs: MEM-NC3 as the Optimal Formulation
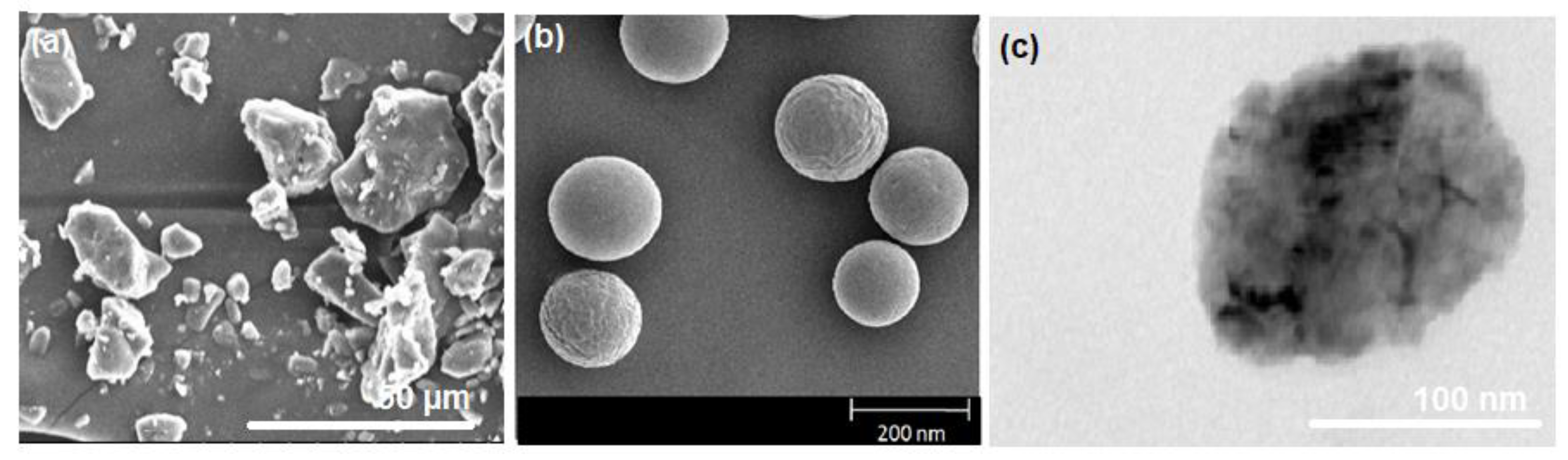
3.2. Surface Morphology of MEM-NC3 by SEM Depicts Smooth Spherical NPs
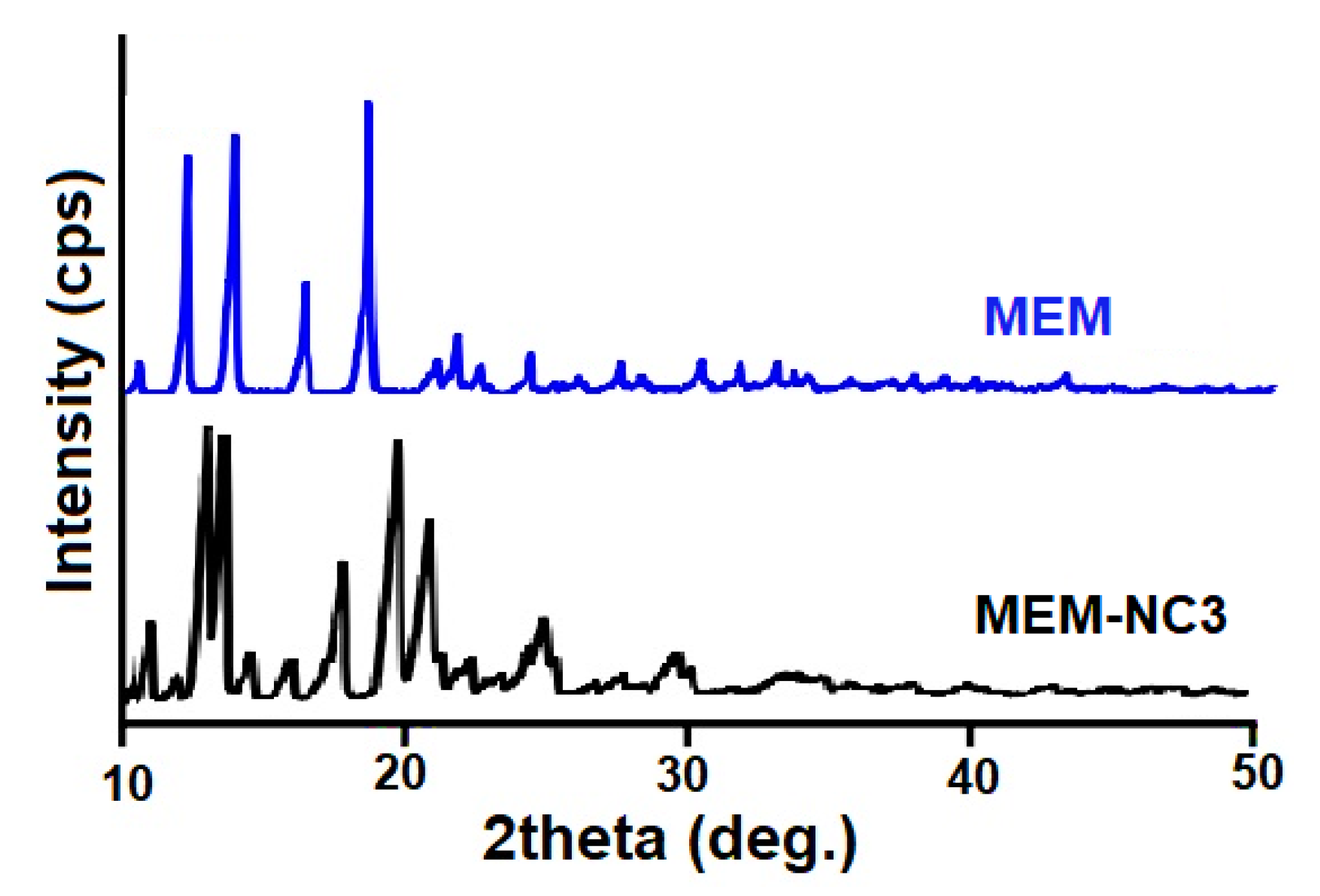
3.3. PXRD of MEM-NC3 Revealed Its Crystalline Nature
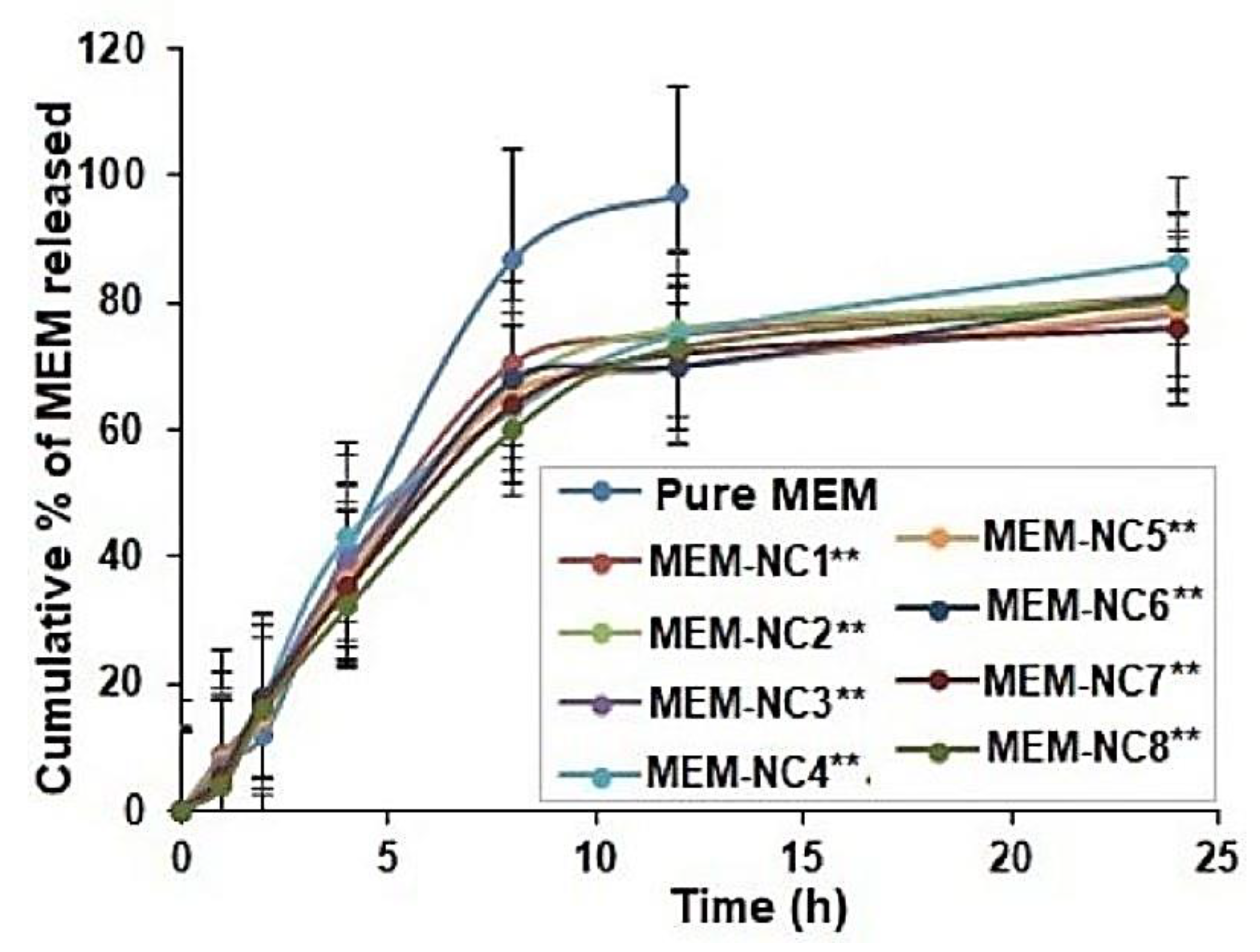
3.4. In Vitro Release Study Showed Sustained and Controlled Release of MEM-NC3
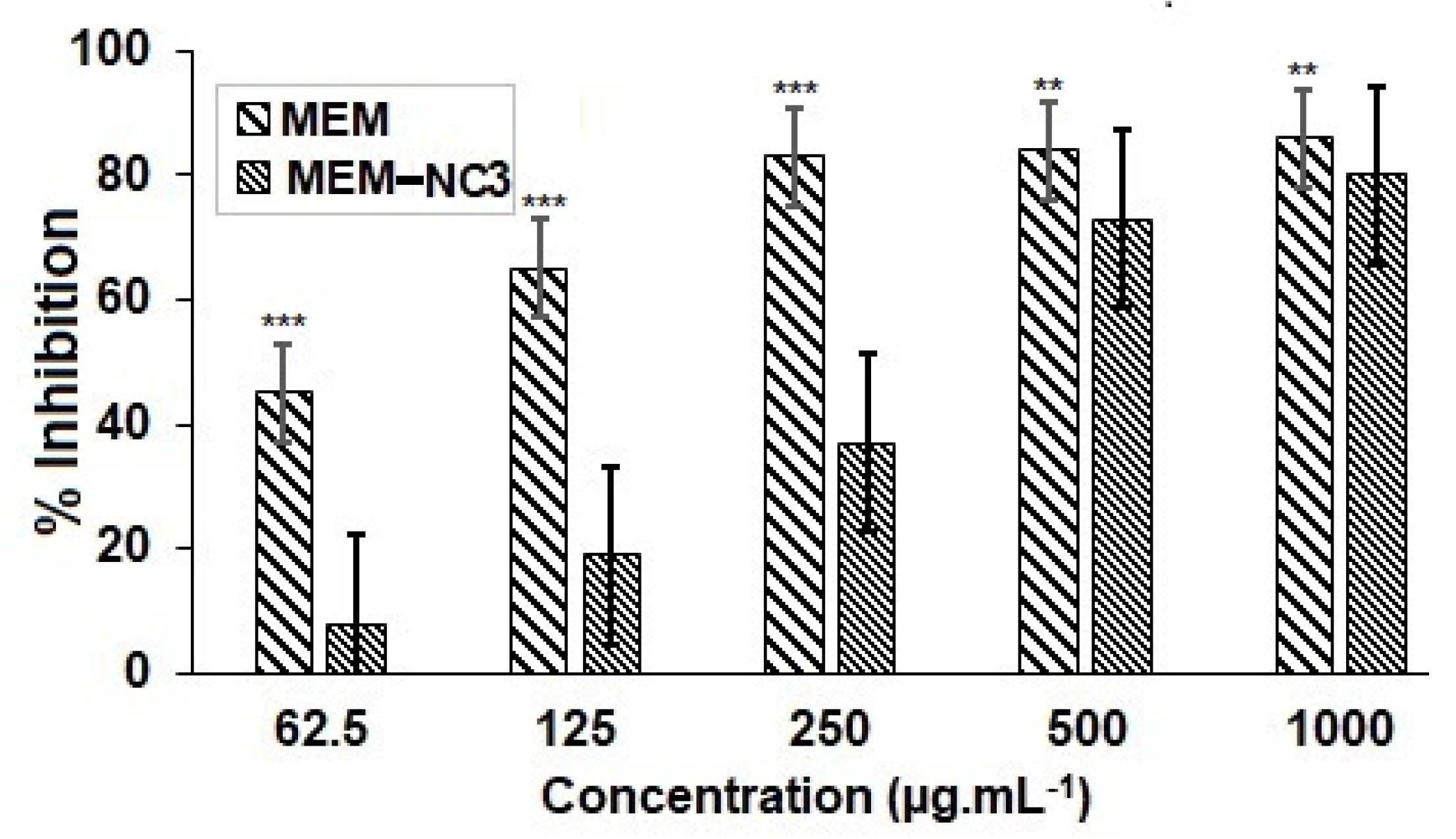
3.5. Cytotoxicity Studies Showed that Lower Doses MEM-NC3 are Safer Compared to Pure MEM
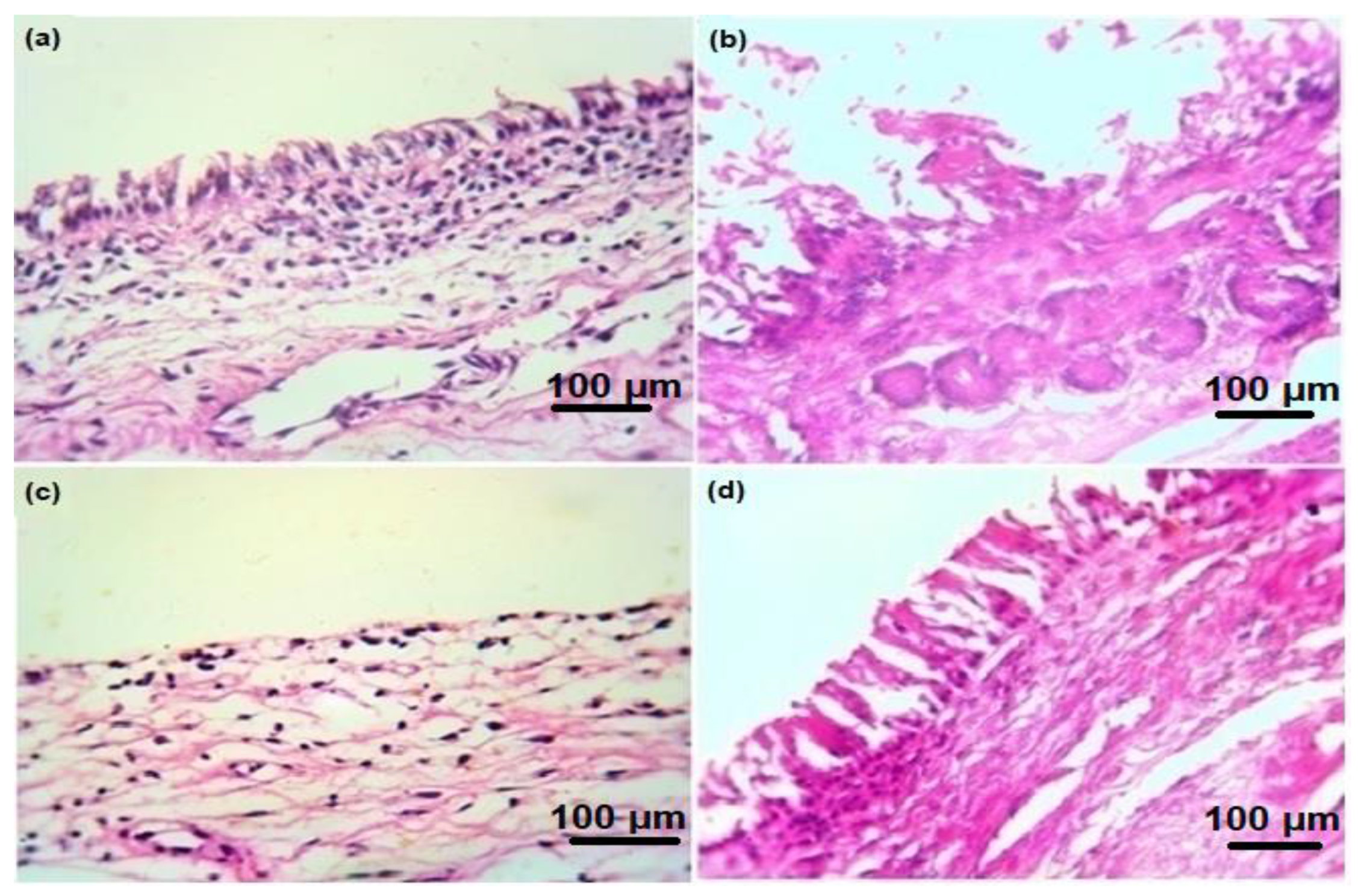
3.6. Ex Vivo Histopathological Studies Confirm the Safe Use of MEM-NC3 by Nasal Route
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Althobaiti, N.A.; Menaa, F.; Albalawi, A.E.; Dalzell, J.J.; Warnock, N.D.; Mccammick, E.M.; Alsolais, A.; Alkhaibari, A.M.; Green, B.D. Assessment and Validation of Globodera pallida as a Novel In Vivo Model for Studying Alzheimer’s Disease. Cells 2021, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, N.A.; Menaa, F.; Dalzell, J.J.; Albalawi, A.E.; Ismail, H.; Alghuthaymi, M.A.; Aldawsari, R.D.; Iqbal, H.; McAlinney, C.; Green, B.D. Ethnomedicinal Plants with Protective Effects against Beta-Amyloid Peptide (Aβ)1-42 Indicate Therapeutic Potential in a New In Vivo Model of Alzheimer’s Disease. Antioxidants 2022, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Kuns, B.; Rosani, A.; Varghese, D. Memantine. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500025/ (accessed on March 2021).
- Maekawa, Y.; Hasegawa, S.; Ishizuka, T.; Shiosakai, K.; Ishizuka, H. Pharmacokinetics and Bioequivalence of Memantine Tablet and a New Dry Syrup Formulation in Healthy Japanese Males: Two Single-Dose Crossover Studies. Adv. Ther. 2019, 36, 2930–2940. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia, and Parkinson’s disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct Polym. 2021, 161, 1381–5148. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef]
- Shayganfard, M. A Review on Chitosan in Drug Delivery for Treatment of Neurological and Psychiatric Disorders. Curr. Pharm. Biotechnol. 2022, 23, 538–551. [Google Scholar] [CrossRef]
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules 2020, 25, 4866. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Mauri, E.; D’Amelio, M.; Gori, M. Functionalization strategies of polymeric nanoparticles for drug delivery in Alzheimer’s disease: Current trends and future perspectives. Front. Neurosci. 2022, 16, 939855. [Google Scholar] [CrossRef] [PubMed]
- Sardoiwala, M.N.; Kaundal, B.; Choudhury, S.R. Chapter 37—Development of Engineered Nanoparticles Expediting Diagnostic and Therapeutic Applications Across Blood–Brain Barrier. In Micro and Nano Technologies, Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 696–709. [Google Scholar]
- Rocha-Meneses, L.; Hari, A.; Inayat, A.; Shanableh, A.; Abdallah, M.; Ghenai, C.; Shanmugam, S.; Kikas, T. Application of nanomaterials in anaerobic digestion processes: A new strategy towards sustainable methane production. Biochem. Eng. J. 2022, 188, 108694. [Google Scholar] [CrossRef]
- Cho, G.; Park, Y.; Hong, Y.K.; Ha, D. Ion exchange: An advanced synthetic method for complex nanoparticles. Nano Converg. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Zong, S.; Qi, J.; Dong, X.; Zhao, W.; Wu, W.; Fu, Q.; Lu, Y.; Chen, Z. The Trigeminal Pathway Dominates the Nose-to-Brain Transportation of Intact Polymeric Nanoparticles: Evidence from Aggregation-Caused Quenching Probes. J. Biomed. Nanotechnol. 2019, 15, 686–702. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M. Chitosan nanoparticles: Polyphosphates cross-linking and protein delivery properties. Int. J. Biol. Macromol. 2019, 136, 133–142. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Mohamed, J.M.M.; Mahajan, N.; El-Sherbiny, M.; Khan, S.; Al-Serwi, R.H.; Attia, M.A.; Altriny, Q.A.; Arbab, A.H. Ameliorated Stomach Specific Floating Microspheres for Emerging Health Pathologies Using Polymeric Konjac Glucomannan-Based Domperidone. BioMed Res. Int. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Stoichiometrically governed curcumin solid dispersion and its cytotoxic evaluation on colorectal adenocarcinoma cells. Drug Des. Deliv. Ther. 2020, 14, 4639–4658. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 2020, 252, 117180. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Fatima Rana, N.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials 2020, 10, 1769. [Google Scholar] [CrossRef] [PubMed]
- Moideen, J.M.M.; Alqahtani, A.; Venkatesan, K.; Ahmad, F.; Krisharaju, K.; Gayasuddin, M.; Shaik, R.A. Application of the Box-Behnken design for the production of soluble curcumin: Skimmed milk powder inclusion complex for improving the treatment of colorectal cancer. Food Sci. Nutr. 2020, 8, 6643–6659. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Razzaq, A.; Khan, A.; Rehman, N.U.; Khan, H.; Khan, T.; Khan, A.U.; Althobaiti, N.A.; Menaa, F.; Iqbal, H.; et al. Physicochemical Characterizations and Pharmacokinetic Evaluation of Pentazocine Solid Lipid Nanoparticles against Inflammatory Pain Model. Pharmaceutics 2022, 14, 409. [Google Scholar] [CrossRef]
- Blocker, S.J.; Cook, J.; Mowery, Y.M.; Everitt, J.I.; Qi, Y.; Hornburg, K.J.; Cofer, G.P.; Zapata, F.; Bassil, A.M.; Badea, C.T.; et al. Johnson GA. Ex Vivo MR Histology and Cytometric Feature Mapping Connect Three-dimensional in vivo MR Images to Two-dimensional Histopathologic Images of Murine Sarcomas. Radiol. Imaging. Cancer 2021, 3, e200103. [Google Scholar]
- Mohamed, J.M.M.; Alqahtani, A.; Menaa, F.; Kayarohanam, S.; Fatease, A.A.; Alqahtani, T.; Alamri, A.; El-Sherbiny, M.; Ramkanth, S.; Janakiraman, A.K. In Vitro Physical Characterizations and Docking Studies on Carvedilol Nanocrystals. Crystals 2022, 12, 988. [Google Scholar] [CrossRef]
- Khan, B.A.; Khalid, H.; Khan, M.K.; Hosny, K.M.; Khan, S.; Rizg, W.Y.; Safhi, A.Y.; Halwani, A.A.; Almehmady, A.M.; Menaa, F. Biodegradable Polymers-Based Smart Nanocrystals for Loxoprofen Delivery with Enhanced Solubility: Design, Fabrication and Physical Characterizations. Polymers 2022, 4, 3464. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N.; Menaa, F.; Alharbi, W.; Alaryani, F.S.S.; Alqahtani, A.M.; Ahmad, F. Fabrication of Ethosomes Containing Tocopherol Acetate to Enhance Transdermal Permeation: In Vitro and Ex Vivo Characterizations. Gels 2022, 8, 335. [Google Scholar] [CrossRef]
- Senthilvel, C.K.; Karuppaiyan, K.; Pothumani, A.; Vedharethinam, A.; Jose, A.W.; Mohamed, J.M.M.; Sherbiny, M.E.; Ebrahim, H.A.; Shafey, M.E.; Dejene, M. Development of Atorvastatin Calcium Biloaded Capsules for Oral Administration of Hypercholesterolemia. Evid. Based. Complement. Alternat. Med. 2022, 2022, 4995508. [Google Scholar] [CrossRef]
- Elhassan, G.O.; Mohamed, J.M.M. Development and in vitro evaluation of valsartan-loaded resealed erythrocytes. Int. J. Appl. Pharm. 2022, 14, 201–205. [Google Scholar] [CrossRef]
- Kamaraj, N.; Rajaguru, P.Y.; Issac, P.K.; Sundaresan, S. Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J. Pharm. Sci. 2017, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zafar, N.; Uzair, B.; Niazi, M.B.K.; Menaa, F.; Samin, G.; Khan, B.A.; Iqbal, H.; Menaa, B. Green Synthesis of Ciprofloxacin-Loaded Cerium Oxide/Chitosan Nanocarrier and its Activity Against MRSA-Induced Mastitis. J. Pharm. Sci. 2021, 110, 3471–3483. [Google Scholar] [CrossRef]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G.; Martinez, M.N. Applying Biopharmaceutical Classification System (BCS) Criteria to Predict Oral Absorption of Drugs in Dogs: Challenges and Pitfalls. AAPS J. 2015, 17, 948–964. [Google Scholar] [CrossRef]
- Horváth, T.; Bartos, C.; Bocsik, A.; Kiss, L.; Veszelka, S.; Deli, M.A.; Újhelyi, G.; Szabó-Révész, P.; Ambrus, R. Cytotoxicity of Different Excipients on RPMI 2650 Human Nasal Epithelial Cells. Molecules 2016, 21, 658. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, J.; Saim, A.M.; Idrus, R.B.H.; Lokanathan, Y. Current and Alternative Therapies for Nasal Mucosa Injury: A Review. Int. J. Mol. Sci. 2020, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.M.M.; Khan, B.A.; Rajendran, V.; El-Sherbiny, M.; Othman, G.; Hussamuldin, A.B.A.; Al-Serwi, R.H. Polymeric Ethosomal Gel Loaded with Nimodipine: Optimisation, Pharmacokinetic and Histopathological Analysis. Saud Pharm J. 2022, 30, 1603–1611. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, B.A.; Uzair, B.; Iram Niaz, S.; Khan, H.; Hosny, K.M.; Menaa, F. Development of Chitosan-Based Nanoemulsion Gel Containing Microbial Secondary Metabolite with Effective Antifungal Activity: In vitro and in vivo Characterizations. Int. J. Nanomed. 2021, 16, 8203–8219. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef]
- Shah, B.M.; Misra, M.; Shishoo, C.J.; Padh, H. Nose to brain microemulsion-based drug delivery system of rivastigmine: Formulation and ex-vivo characterization. Drug Deliv. 2015, 22, 918–930. [Google Scholar] [CrossRef]
- Althobaiti, N.A.; Menaa, F.; Dalzell, J.J.; Green, B.D. Globodera pallida, a non-transgenic invertebrate as a new model for investigating Alzheimer’s diease (and other proteinopathies)? Neural Regen. Res. 2023, 18, 113–114. [Google Scholar] [CrossRef] [PubMed]


| Formulation Code | MEM Concentration (mg) | CS: MEM Concentration Ratio | Time (h) |
|---|---|---|---|
| MEM-NC1 | 10 | 2:1 | 4 |
| MEM-NC2 | 10 | 3:1 | 4 |
| MEM-NC3 | 10 | 4:1 | 4 |
| MEM-NC4 | 10 | 5:1 | 4 |
| MEM-NC5 | 10 | 6:1 | 4 |
| MEM-NC6 | 10 | 7:1 | 2 |
| MEM-NC7 | 10 | 8:1 | 6 |
| MEM-NC8 | 10 | 9:1 | 6 |
| Formulation Code | PS (nm) * | PDI * | ZP (mV) * | DL * (%) | EE * (%) |
|---|---|---|---|---|---|
| MEM-NC1 | 204.90 ± 14.09 | 0.359 ± 0.03 | 28.6 ± 1.40 | 96.22 ± 5.32 | 76.6 ± 3.55 ** |
| MEM-NC2 | 158.96 ± 8.63 ** | 0.534 ± 0.02 | 23.8 ± 0.40 | 94.63 ± 3.64 | 74.6 ± 4.59 |
| MEM-NC3 | 152.63 ± 12.95 ** | 0.437 ± 0.14 | 36.1 ± 0.60 | 98.44 ± 3.31 ** | 78.7 ± 3.11 ** |
| MEM-NC4 | 310.23 ± 10.49 | 0.437 ± 0.06 | 54.0 ± 0.50 ** | 95.66 ± 4.64 | 74.5 ± 5.43 |
| MEM-NC5 | 211.76 ± 6.10 | 0.336 ± 0.05 | 45.3 ± 1.20 | 92.57 ± 5.32 | 75.4 ± 2.69 |
| MEM-NC6 | 278.46 ± 16.77 | 0.370 ± 0.17 | 50.1 ± 2.80 | 94.85 ± 5.78 | 74.9 ± 5.67 |
| MEM-NC7 | 212.90 ± 4.78 | 0.342 ± 0.05 | 47.5 ± 1.60 | 95.55 ± 6.76 | 73.8 ± 4.80 |
| MEM-NC8 | 290.70 ± 7.60 | 0.474 ± 0.18 | 52.1 ± 1.50 ** | 94.87 ± 5.11 | 73.8 ± 5.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, M.A.; Mohamed, J.M.M.; Ruby, J.J.; Kanthiah, S.; Alanazi, Y.F.; Majrashi, K.A.; Alshahrani, S.M.; Eladl, M.A.; Alaryani, F.S.; El-Sherbiny, M.; et al. Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis. Crystals 2023, 13, 21. https://doi.org/10.3390/cryst13010021
Saleh MA, Mohamed JMM, Ruby JJ, Kanthiah S, Alanazi YF, Majrashi KA, Alshahrani SM, Eladl MA, Alaryani FS, El-Sherbiny M, et al. Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis. Crystals. 2023; 13(1):21. https://doi.org/10.3390/cryst13010021
Chicago/Turabian StyleSaleh, Mohamed A., Jamal M. M. Mohamed, J. Joysa Ruby, Selvakumar Kanthiah, Yasmene F. Alanazi, Kamlah A. Majrashi, Sultan M. Alshahrani, Mohamed Ahmed Eladl, Fatima S. Alaryani, Mohamed El-Sherbiny, and et al. 2023. "Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis" Crystals 13, no. 1: 21. https://doi.org/10.3390/cryst13010021
APA StyleSaleh, M. A., Mohamed, J. M. M., Ruby, J. J., Kanthiah, S., Alanazi, Y. F., Majrashi, K. A., Alshahrani, S. M., Eladl, M. A., Alaryani, F. S., El-Sherbiny, M., & Menaa, F. (2023). Preparation of Memantine-Loaded Chitosan Nanocrystals: In Vitro and Ex Vivo Toxicity Analysis. Crystals, 13(1), 21. https://doi.org/10.3390/cryst13010021










