Flux Growth and Raman Spectroscopy Study of Cu2CrBO5 Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flux Growth
2.2. Energy Dispersive X-ray Spectroscopy (EDX)
2.3. X-ray Diffraction
2.4. Measurements of the Magnetic Properties
2.5. Raman Spectroscopy
3. Results
3.1. Results of the Flux Growth Study
3.1.1. Bi2O3-MoO3-B2O3-Na2O Based Flux
3.1.2. Li2WO4-Li2O-B2O3Based Flux
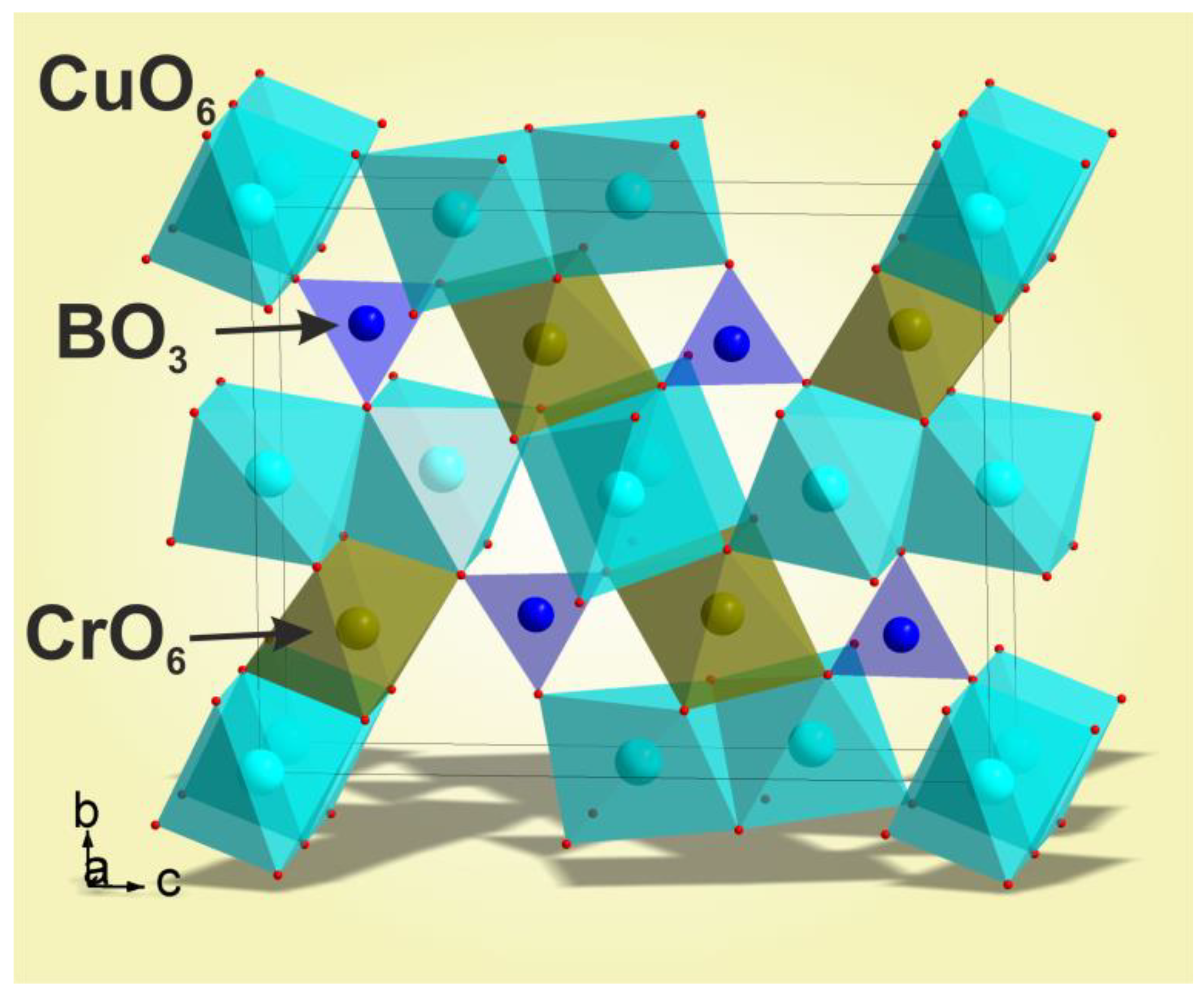
3.2. Structural Properties
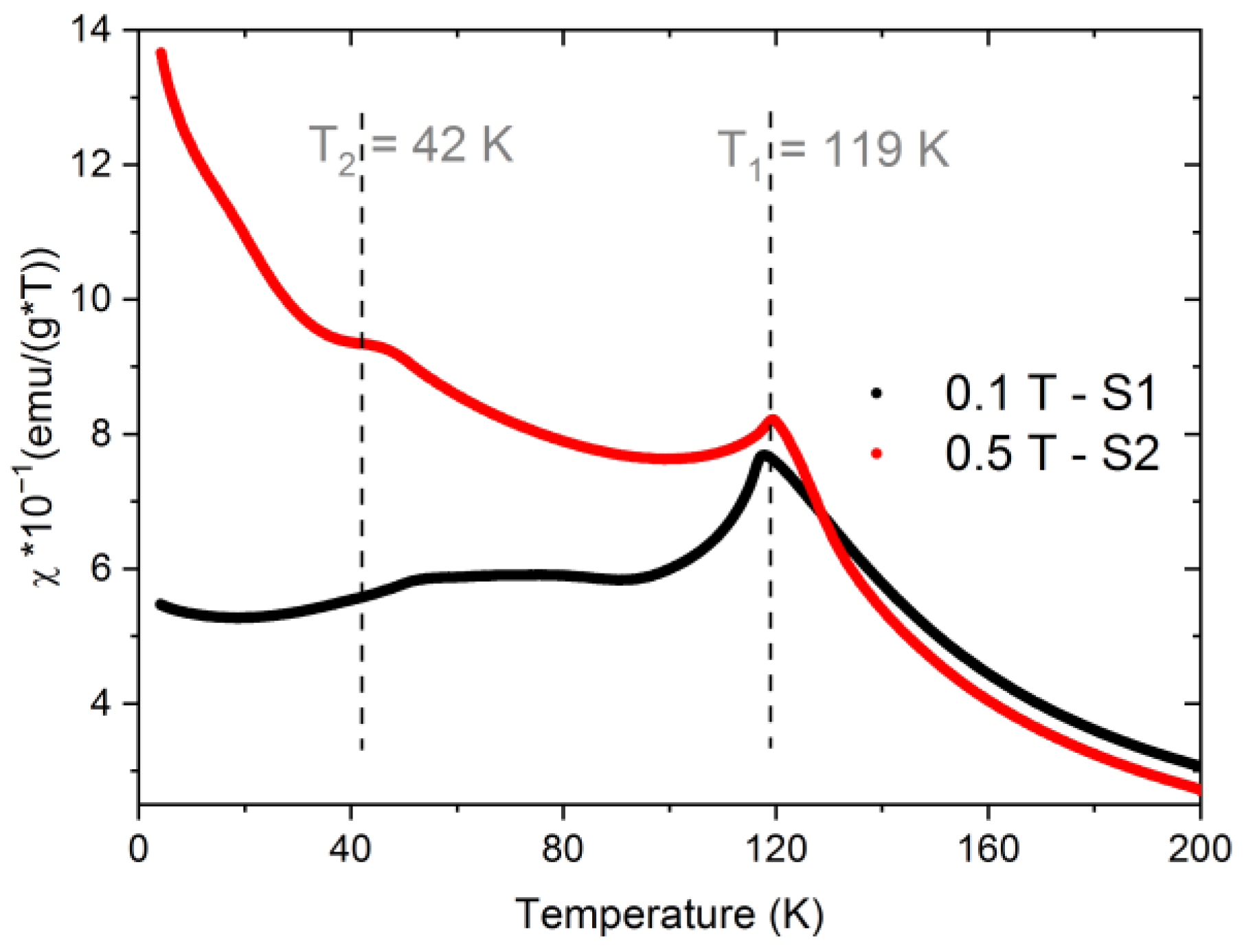
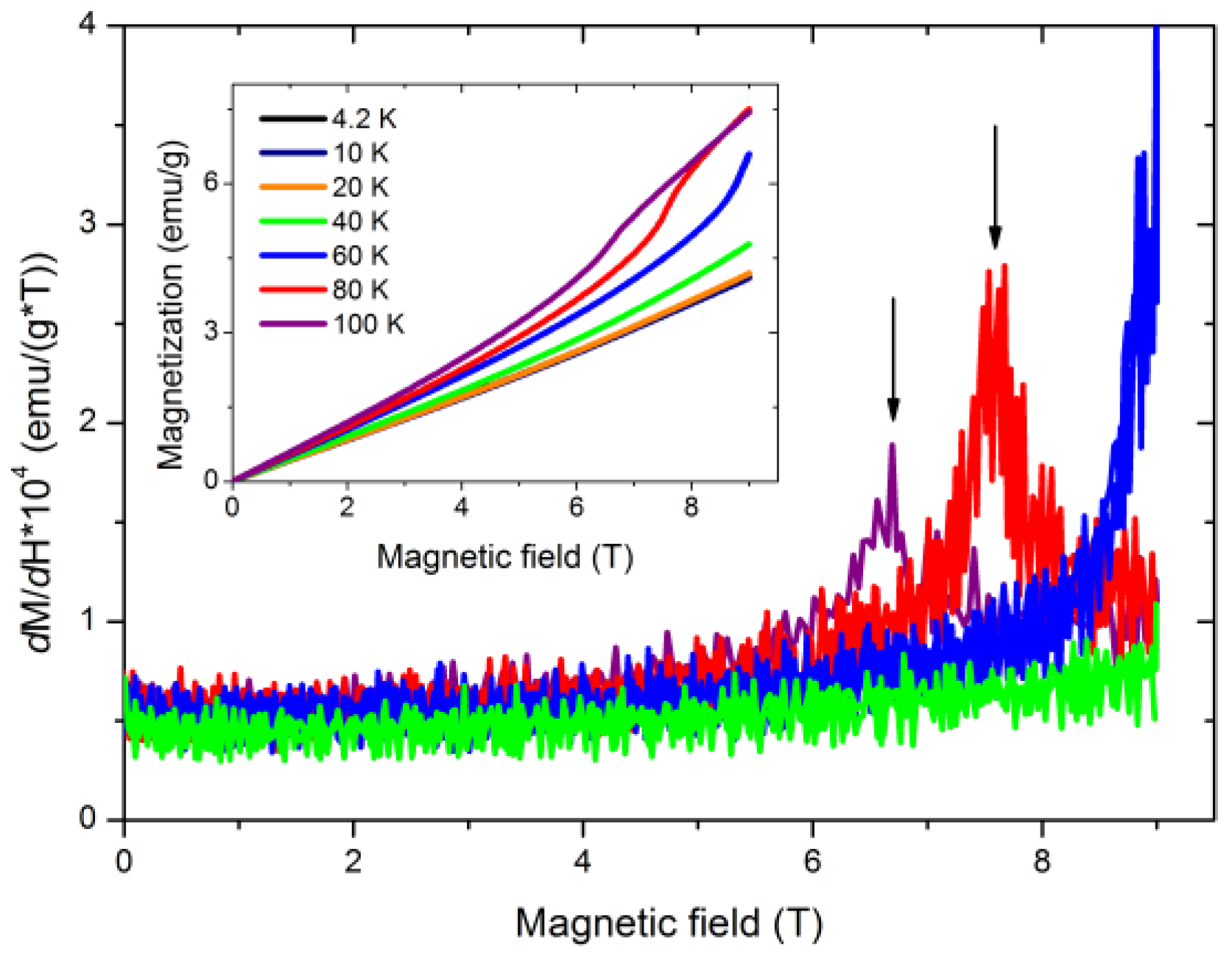
3.3. Magnetic Properties
3.4. Raman Spectroscopy Study
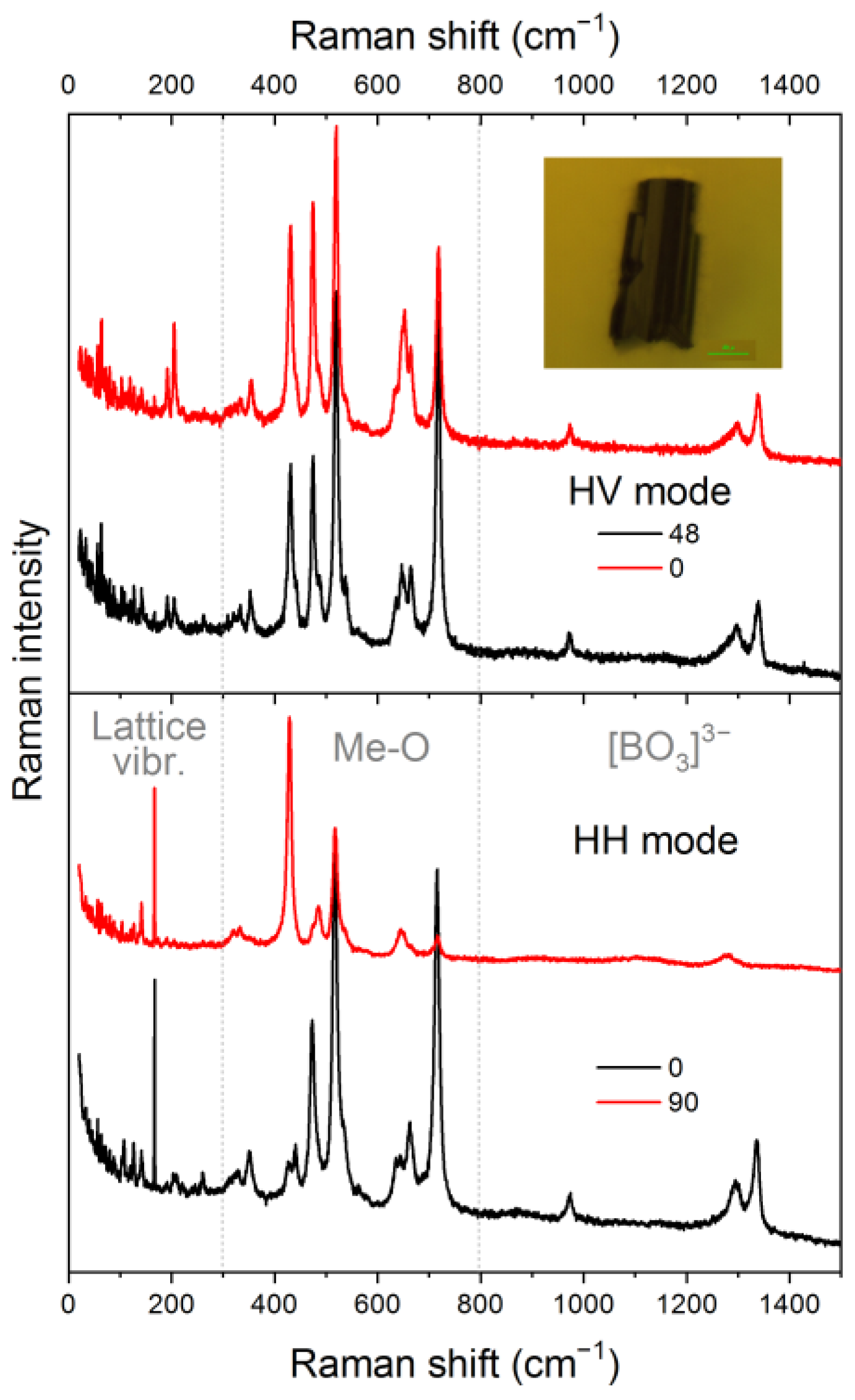
3.4.1. Polarized Room Temperature Raman Spectra of Cu2CrBO5
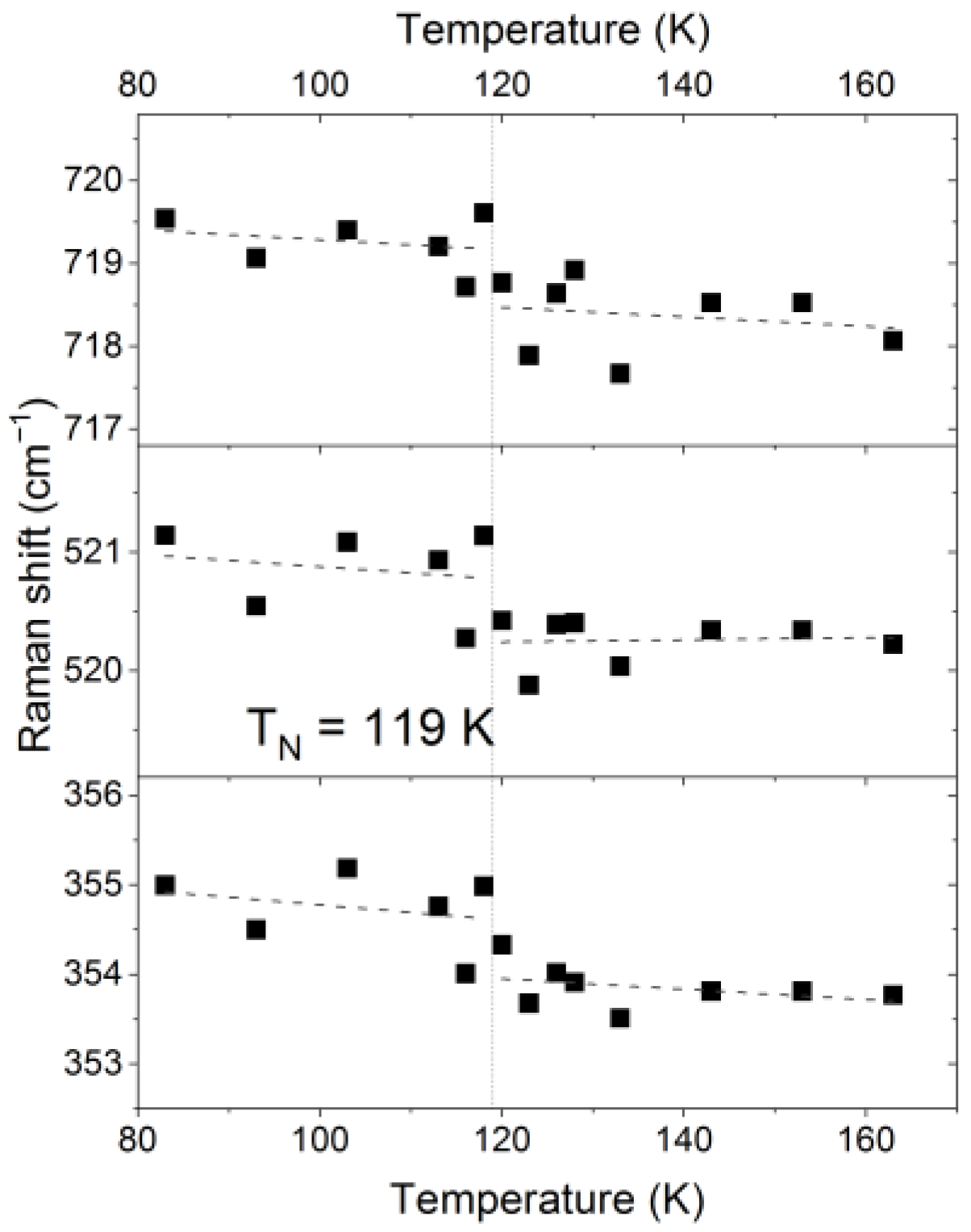
3.4.2. Thermal Evolution of the Raman Spectra of Cu2CrBO5
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damay, F.; Sottmann, J.; Fauth, F.; Suard, E.; Magnan, A.; Martin, C. High temperature spin-driven multiferroicity in ludwigite chromocuprate. Appl. Phys. Lett. 2021, 118, 192903. [Google Scholar] [CrossRef]
- Sofronova, S.; Bezmaternykh, L.; Eremin, E.; Chernyshov, A.; Bovina, A. Crystal Growth, Magnetic Properties and Analysis of Possible Magnetic Ordering of Ni5Sn(BO5)2 with Ludwigite Structure. Phys. Status Solidi B 2018, 255, 1800281. [Google Scholar] [CrossRef]
- Damay, F.; Sottmann, J.; Lainé, F.; Chaix, L.; Poienar, M.; Beran, P.; Elkaim, E.; Fauth, F.; Nataf, L.; Guesdon, A.; et al. Magnetic phase diagram for Fe3−xMnxBO5. Phys. Rev. B 2020, 101, 094418. [Google Scholar] [CrossRef]
- Heringer, M.A.V.; Freitas, D.C.; Mariano, D.L.; Baggio-Saitovitch, E.; Continentino, M.A.; Sanchez, D.R. Structural and magnetic properties of the Ni5Ti(O2BO3)2 ludwigite. Phys. Rev. Mater. 2019, 3, 094402. [Google Scholar] [CrossRef]
- Sofronova, S.; Nazarenko, I. Ludwigites: From natural mineral to modern solid solutions. Cryst. Res. Technol. 2017, 52, 1600338. [Google Scholar] [CrossRef]
- Moshkina, E.M.; Belskaya, N.A.; Molokeev, M.S.; Bovina, A.F.; Shabanova, K.A.; Kokh, D.; Seretkin Yu, V.; Velikanov, D.A.; Eremin, E.V.; Krylov, A.S.; et al. Growth Conditions and the Structural and Magnetic Properties of Cu2MBO5 (M = Cr, Fe, Mn) Oxyborates with a Ludwigite Structure. JETP 2023, 176, 17–25. [Google Scholar] [CrossRef]
- Moshkina, E.M.; Eremina, R.M.; Gavrilova, T.P.; Gilmutdinov, I.F.; Kiiamov, A.G. Flux Crystal Growth of Cu2GaBO5 and Cu2AlBO5 Ludwigites. J. Cryst. Growth 2020, 545, 125723. [Google Scholar] [CrossRef]
- Petrakovskii, G.A.; Bezmaternykh, L.N.; Velikanov, D.A.; Vorotynov, A.M.; Bayukov, O.A.; Schneider, M. Magnetic Properties of Single Crystals of Ludwigites Cu2MBO5 (M = Fe3+, Ga3+). Phys. Solid State 2009, 51, 2077–2083. [Google Scholar] [CrossRef]
- Moshkina, E.; Seryotkin, Y.; Bovina, A.; Molokeev, M.; Eremin, E.; Belskaya, N.; Bezmaternykh, L. Crystal formation of Cu-Mn-containing oxides and oxyborates in bismuth-boron fluxes diluted by MoO3 and Na2CO3. J. Cryst. Growth 2018, 503, 1–8. [Google Scholar] [CrossRef]
- Bruker AXS TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data; User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. ActaCryst. A 2008, 64, 112–122. [Google Scholar]
- PLATON—A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2008.
- Brandenburg, K.; Berndt, M. DIAMOND—Visual Crystal Structure Information System; Crystal Impact: Bonn, Germany, 2004. [Google Scholar]
- Krylov, A.; Krylova, S.; Gudim, I.; Kitaev, Y.; Golovkina, E.; Zhang, H.; Vtyurin, A. Pressure–Temperature Phase Diagram of Multiferroic TbFe2.46Ga0.54(BO3)4. Magnetochemistry 2022, 8, 59. [Google Scholar] [CrossRef]
- Aleksandrov, K.S.; Voronov, V.N.; Vtyurin, A.N.; Krylov, A.S.; Molokeev, M.S.; Pavlovskii, M.S.; Goryainov, S.V.; Likhacheva, A.Y.; Ancharov, A.I. Pressure-induced phase transition in the cubic ScF3 crystal. Phys. Solid State 2009, 51, 810–816. [Google Scholar] [CrossRef]
- Poienar, M.; Hardy, V.; Kundys, B.; Singh, K.; Maignan, A.; Damay, F.; Martin, C. Revisiting the properties of delafossite CuCrO2: A single crystal study. J. Solid State Chem. 2012, 185, 56–61. [Google Scholar] [CrossRef]
- Moshkina, E.; Krylov, A.; Kokh, D.; Shabanova, K.; Molokeev, M.; Bovina, A.; Plyaskin, M.; Rostovtsev, N.; Bezmaternykh, L. Multicomponent Flux Growth and Composition Control of Cu2MnBO5:Ga Ludwigite. CrystEngComm 2022, 24, 3565. [Google Scholar] [CrossRef]
- Frandsen Benjamin, A.; Bozin Emil, S.; Eleni, A.; Fernández Martínez, A.; Feygenson, M.; Page, K.; Lappas, A. Nanoscale degeneracy lifting in a geometrically frustrated antiferromagnet. Phys. Rev. B 2020, 101, 024423. [Google Scholar] [CrossRef]
- Klein, S.; Weitzel, H. PERNOD—Ein ProgrammzurVerfeinerung von KristallstrukturparameternausNeutronenbeugungspulverdiagrammen. J. Appl. Cryst. 1975, 8, 54–59. [Google Scholar] [CrossRef]
- National Bureau of Standards; Monograph 25; U.S. Department of Commerce: Washington, DC, USA, 1984; Section 20; p. 20.
- Thiago, T.G.; Buzinaro, M.A.; Moreno, N.O. Magnetization Study in CuCr2O4 Spinel Oxide. J. Supercond. Nov. Magn. 2013, 26, 2557–2559. [Google Scholar] [CrossRef]
- Moshkina, E.; Bovina, A.; Molokeev, M.; Krylov, A.; Shabanov, A.; Chernyshov, A.; Sofronova, S. Study of flux crystal growth peculiarities, structure and Raman spectra of double (Mn,Ni)3BO5 and triple (Mn,Ni,Cu)3BO5 oxyborates with ludwigite structure. CrystEngComm 2021, 23, 5624. [Google Scholar] [CrossRef]
- Leite, C.A.F.; Guimarães, R.B.; Fernandes, J.C.; Continentino, M.A.; Paschoal, C.W.A.; Ayala, A.P.; Guedes, I. Temperature-dependent Raman scattering study of Fe3O2BO3 ludwigite. J. Raman Spectrosc. 2001, 33, 1–5. [Google Scholar] [CrossRef]
- Bragg, E.E.; Seehra, M.S. Magnetic Susceptibility of MnF, near T and Fisher’s Relation. Phys. Rev. B 1973, 7, 4197–4202. [Google Scholar] [CrossRef]




| Compound | CuWO4 | (Cu, Cr)WO4 (1) | (Cu, Cr)WO4 (2) | CrWO4 |
|---|---|---|---|---|
| Space group, Z | P-1, 2 | P2/c, 2 | P2/c, 2 | C2/m, 4 |
| a, Å | 4.69640 | 4.6207 (2) | 4.7983 (5) | 9.2708 |
| b, Å | 5.82870 | 5.6865 (2) | 5.708 (6) | 5.8282 |
| c, Å | 4.87360 | 4.8963 (2) | 4.9747 (5) | 4.6446 |
| α, ⁰ | 91.630 | 90 | 90 | 90 |
| β, ⁰ | 92.440 | 90.4950 (10) | 91.12 (1) | 91.926 |
| γ, ⁰ | 82.790 | 90 | 90 | 90 |
| V, Å3 | 128.648 (9) | 136.22 (15) |
| Compound | Cu2CrBO5 (2) | Cu2CrBO5 (4) [6] | Cu2CrBO5 [1] |
|---|---|---|---|
| Space group, Z | P21/c, 4 | P21/c, 4 | P21/c, 4 |
| a, Å | 3.0606 (2) | 3.05250 (16) | 3.05487 (2) |
| b, Å | 11.9982 (8) | 11.98701 (61) | 12.18070 (8) |
| c, Å | 9.4481 (6) | 9.42955 (52) | 9.41432 (7) |
| β, ⁰ | 95.5490 (10) | 95.3665 (15) | 94.5602 (4) |
| V, Å3 | 345.32 (4) | 343.518 (32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshkina, E.; Eremin, E.; Molokeev, M.; Kokh, D.; Krylov, A. Flux Growth and Raman Spectroscopy Study of Cu2CrBO5 Crystals. Crystals 2023, 13, 1415. https://doi.org/10.3390/cryst13101415
Moshkina E, Eremin E, Molokeev M, Kokh D, Krylov A. Flux Growth and Raman Spectroscopy Study of Cu2CrBO5 Crystals. Crystals. 2023; 13(10):1415. https://doi.org/10.3390/cryst13101415
Chicago/Turabian StyleMoshkina, Evgeniya, Evgeniy Eremin, Maxim Molokeev, Dieter Kokh, and Alexander Krylov. 2023. "Flux Growth and Raman Spectroscopy Study of Cu2CrBO5 Crystals" Crystals 13, no. 10: 1415. https://doi.org/10.3390/cryst13101415
APA StyleMoshkina, E., Eremin, E., Molokeev, M., Kokh, D., & Krylov, A. (2023). Flux Growth and Raman Spectroscopy Study of Cu2CrBO5 Crystals. Crystals, 13(10), 1415. https://doi.org/10.3390/cryst13101415







