Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Calcium Carbonate
2.3. Characterizations
3. Results and Discussion
3.1. Effect of Additives for Tuning the Morphology of Nano-Micro CaCO3
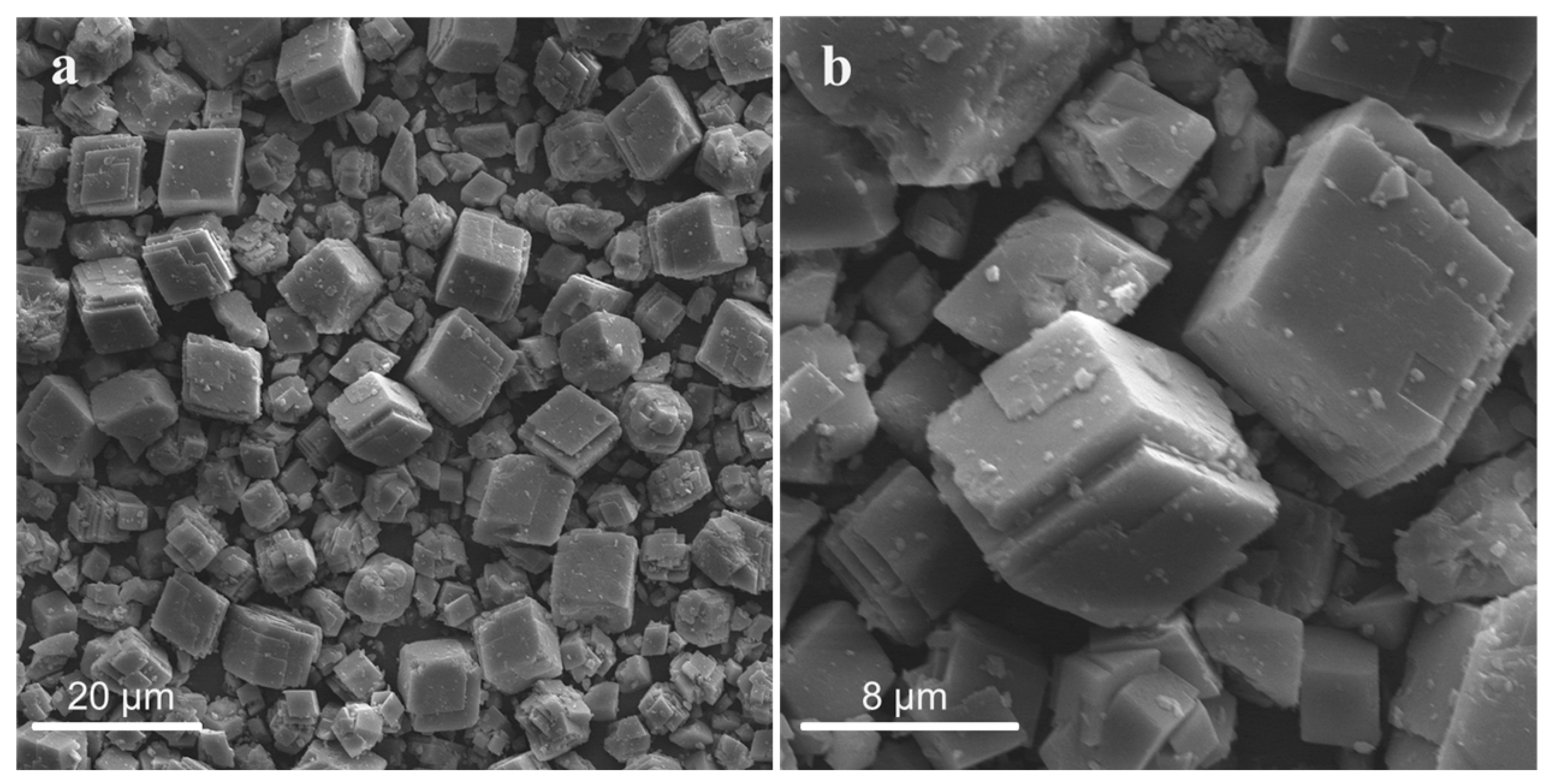
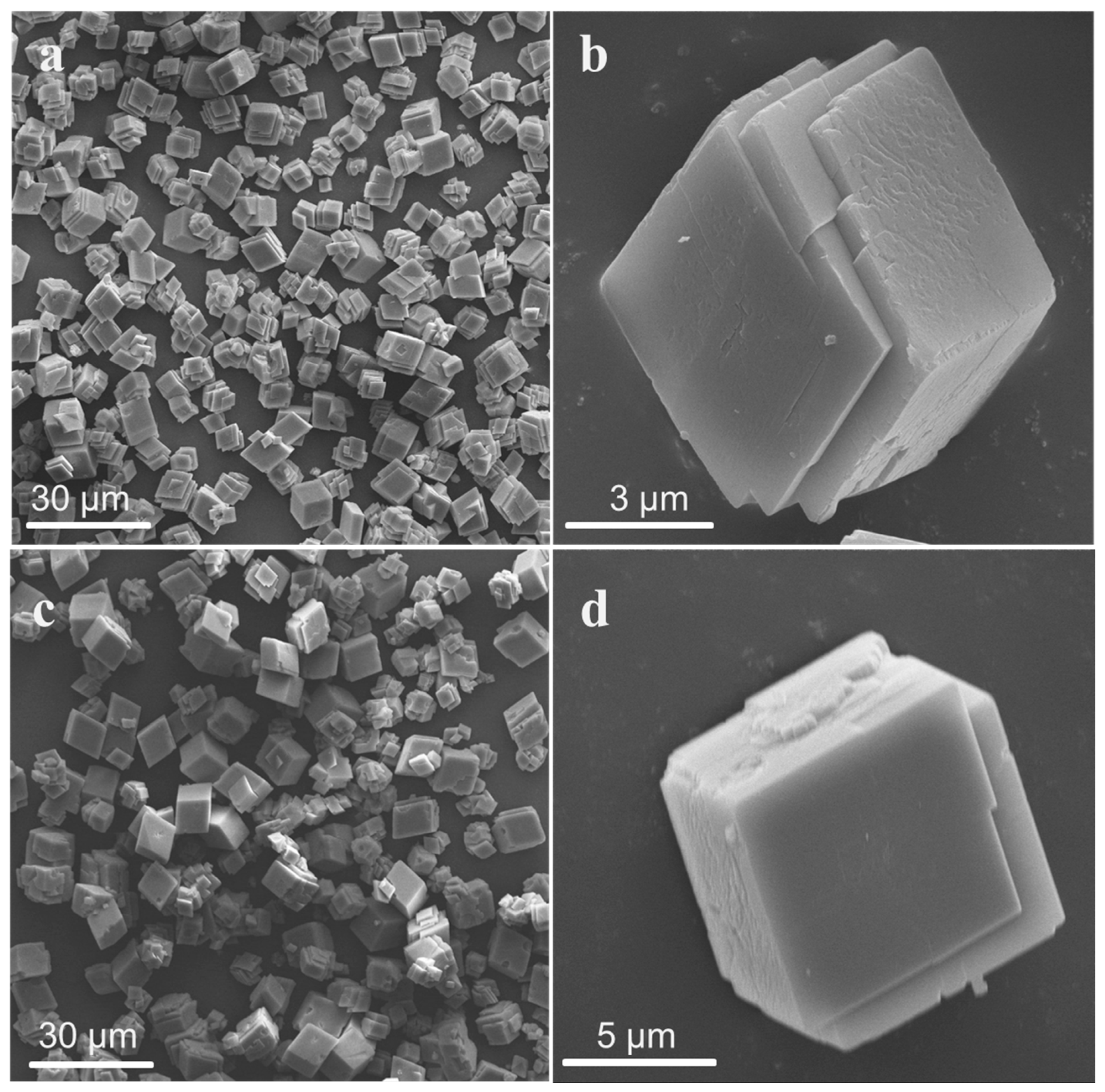
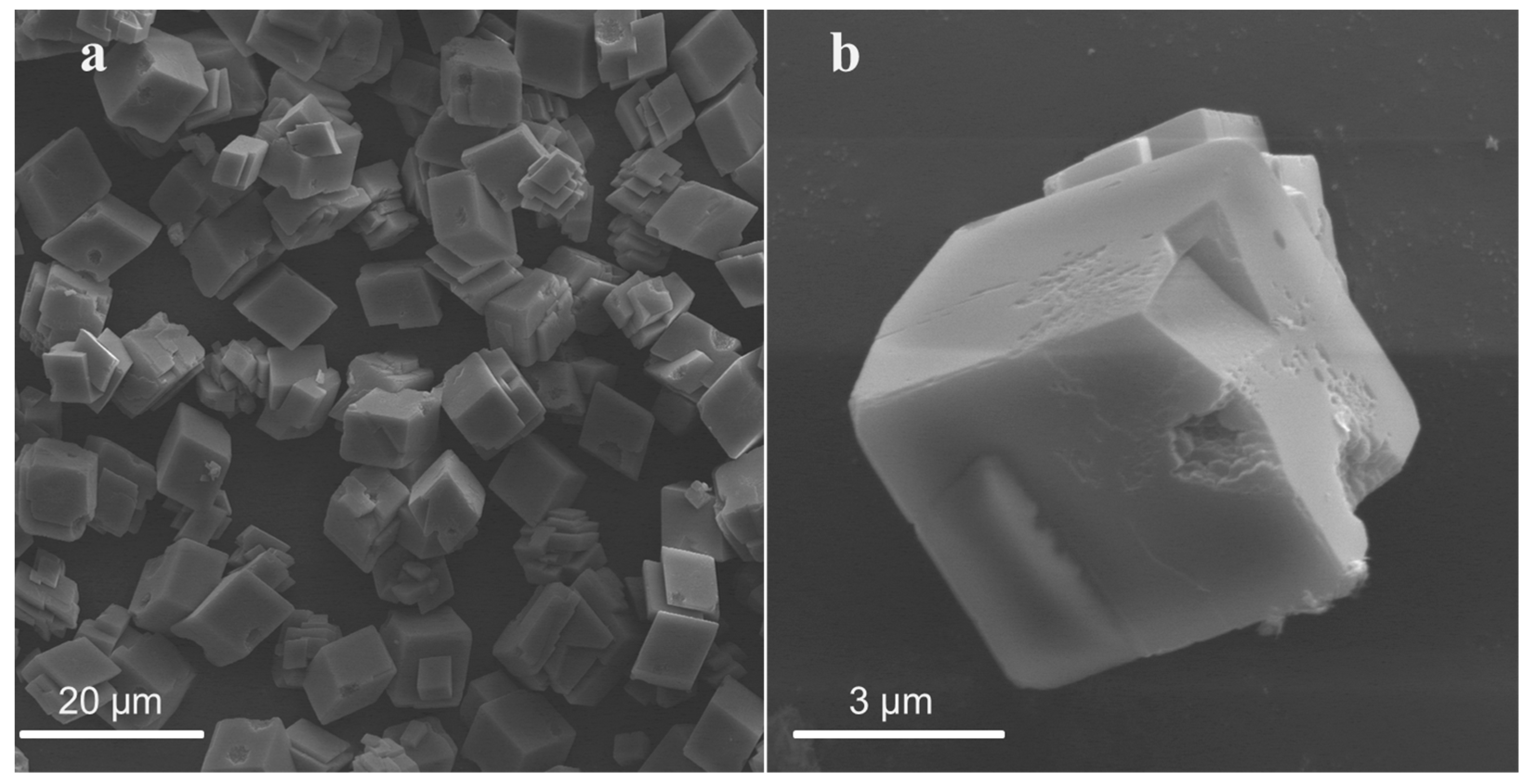
3.1.1. Effect of Acids on Particle Size and Morphology of CaCO3
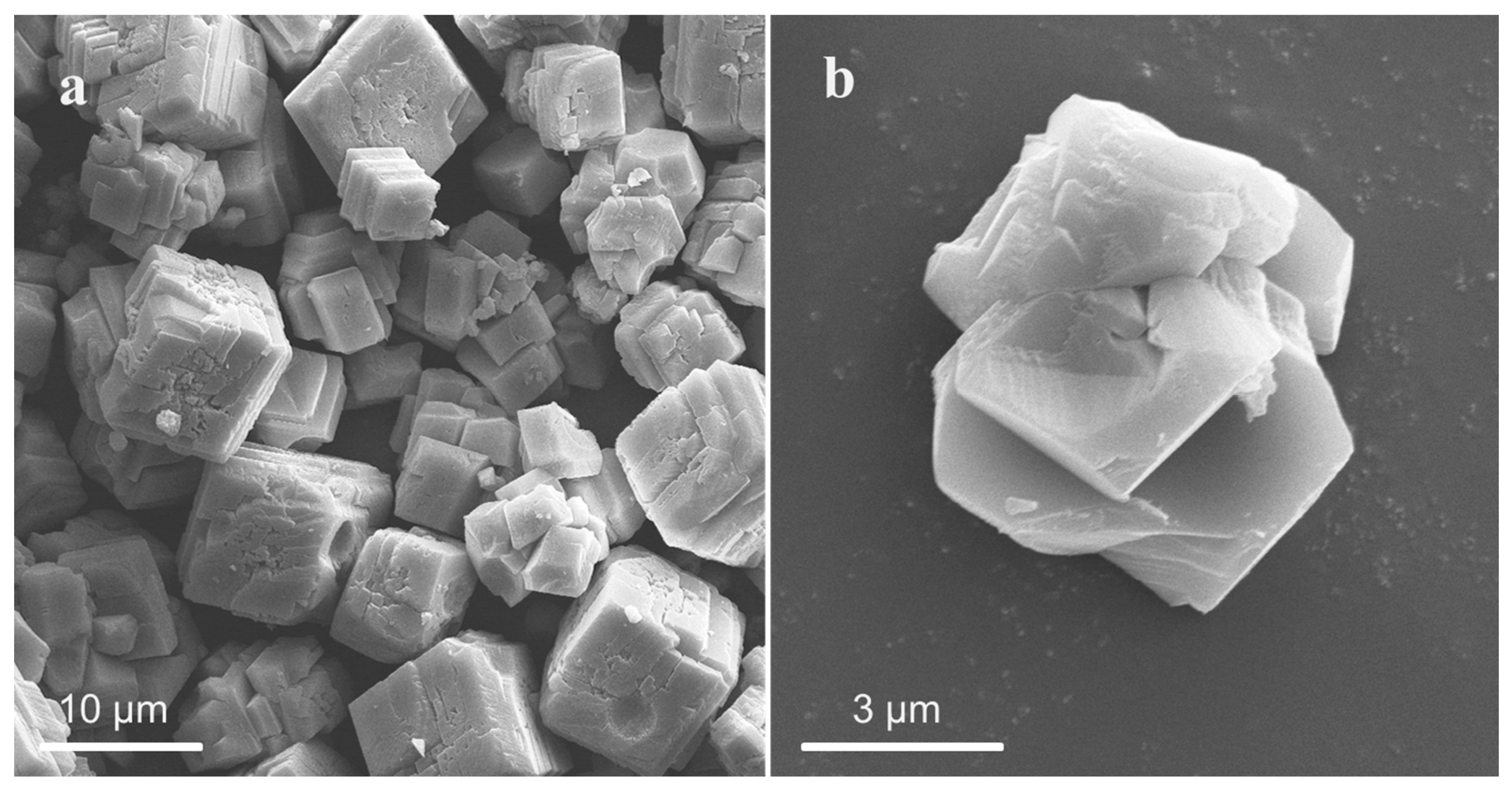
3.1.2. Effect of Alcohol on Particle Size and Morphology of CaCO3
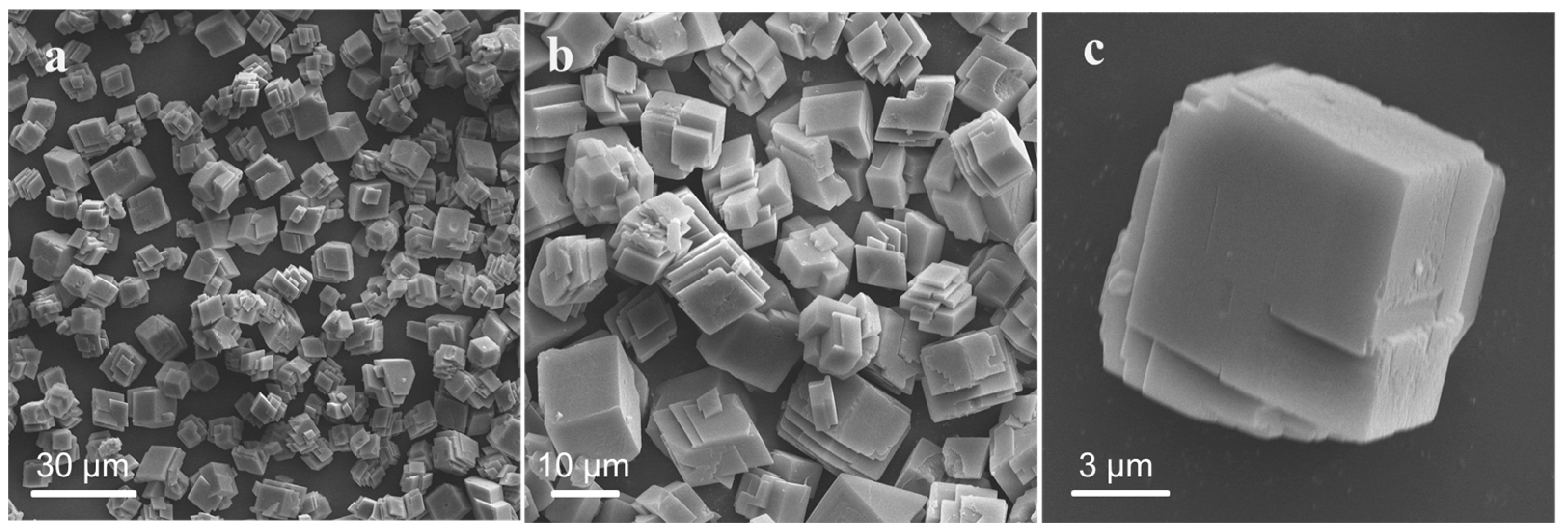
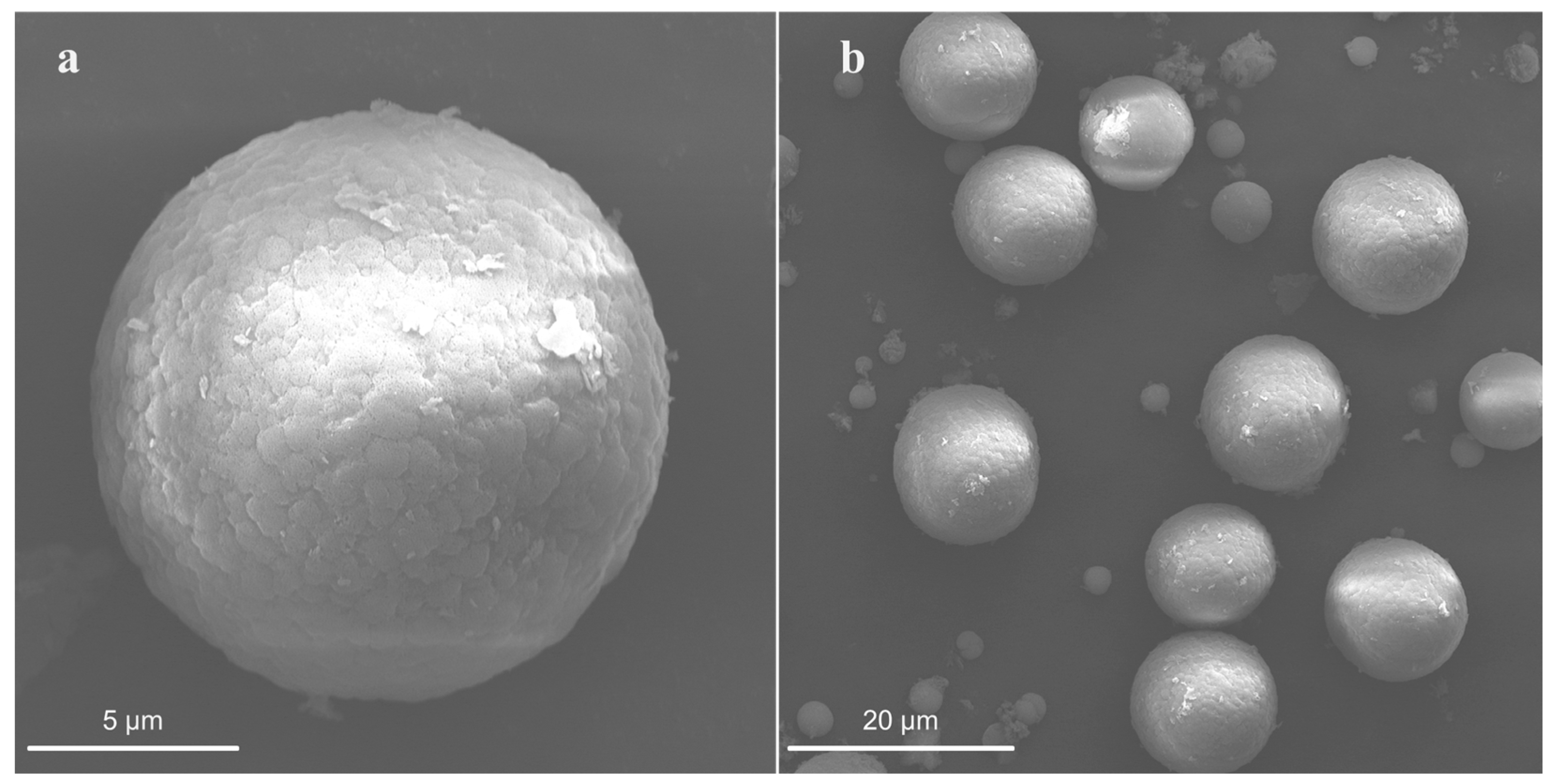
3.1.3. Effect of Surfactants on Particle Size and Morphology of CaCO3
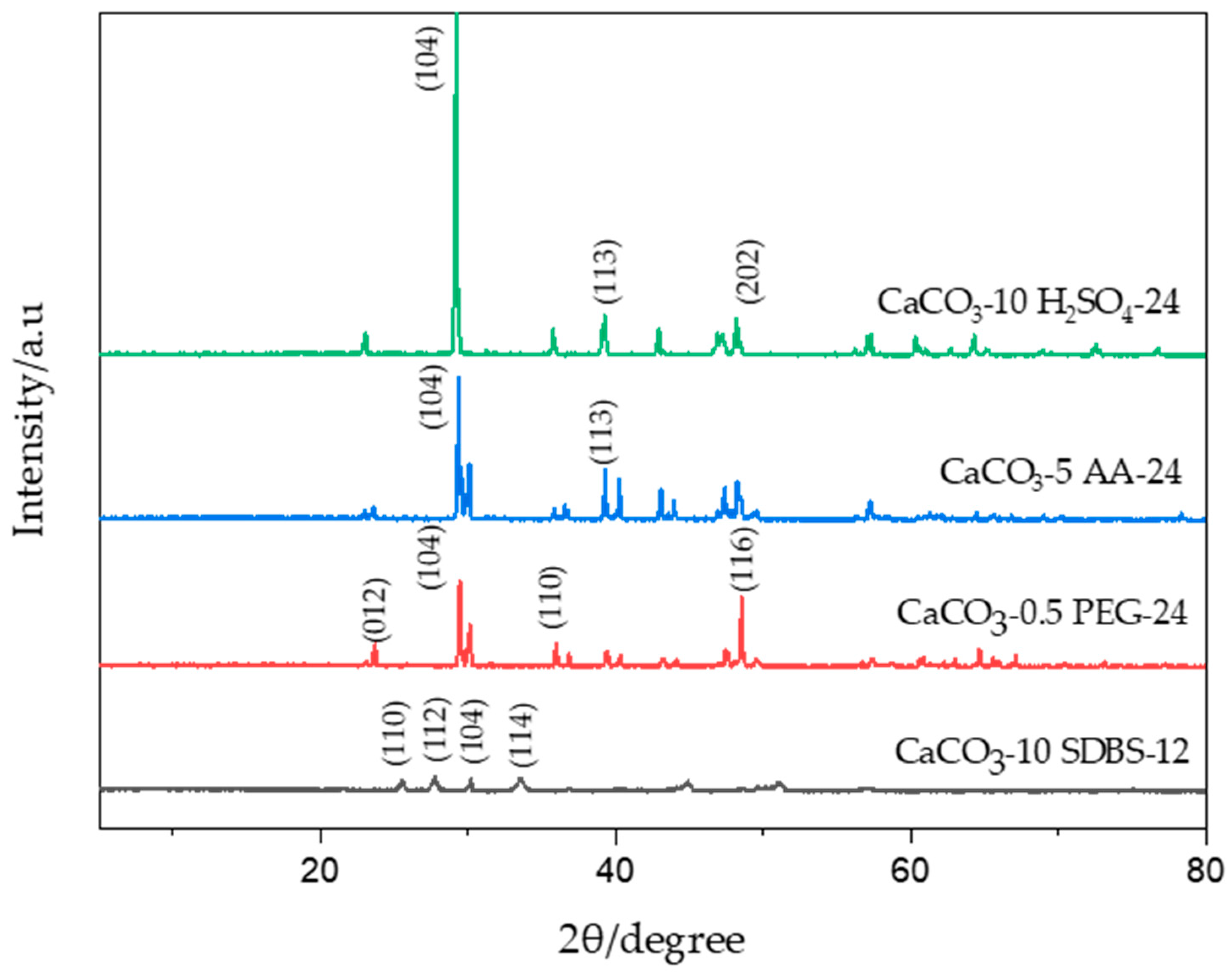
3.1.4. Structural Analysis of CaCO3
3.2. Mechanism Exploration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combes, C.; Miao, B.; Bareille, R.; Rey, C. Preparation, physical-chemical characterisation and cytocompatibility of calcium carbonate cements. Biomaterials 2006, 27, 1945–1954. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Bikmullin, A.G.; Nurgaliev, D.K. Magnetically Responsive Calcium Carbonate Microcrystals. ACS Appl. Mater. Interfaces 2009, 1, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.H.; Jang, H.S.; Chung, D.W.; Stanciu, L.A. Multifunctional calcium carbonate microparticles: Synthesis and biological applications. J. Mater. Chem. 2010, 20, 7728–7733. [Google Scholar] [CrossRef]
- Guo, X.H.; Yu, S.H.; Cai, G.B. Crystallization in a mixture of solvents by using a crystal modifier: Morphology control in the synthesis of highly monodisperse CaCO3 microspheres. Angew. Chem. Int. Ed. 2006, 45, 3977–3981. [Google Scholar] [CrossRef]
- Lee, I.; Han, S.W.; Choi, H.J.; Kim, K. Nanoparticle directed crystallization of calcium carbonate. Adv. Mat. 2001, 13, 1617–1620. [Google Scholar] [CrossRef]
- Hu, C.P.; Chen, C.J.; Wu, Y.; Li, J.; Hu, Y.C.; Wei, L.G.; Jiang, J.X. The mechanochemical route of vaterite synthesis using sodium hexametaphosphate as an inorganic additive. J. Am. Ceram. Soc. 2019, 102, 7116–7124. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. The Multiple Structures of Vaterite. Cryst. Growth Des. 2013, 13, 2247–2251. [Google Scholar] [CrossRef]
- Guo, X.H.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.Y.; Yu, S.H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. Crystengcomm 2011, 13, 2054–2061. [Google Scholar] [CrossRef]
- Murakami, F.S.; Rodrigues, P.O.; de Campos, C.M.T.; Silva, M.A.S. Physicochemical study of CaCO3 from egg shells. Food Sci. Technol. 2007, 27, 658–662. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr Build Mater. 2014, 70, 309–321. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.K.; Corr, D.J.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.B.; Fu, X.S.; Xia, Y.Q. Performance and anti-wear mechanism of CaCO3 nanoparticles as a green additive in poly-alpha-olefin. Tribol. Int. 2009, 42, 1029–1039. [Google Scholar] [CrossRef]
- Juuti, M.; Koivunen, K.; Silvennoinen, M.; Paulapuro, H.; Peiponen, K.E. Light scattering study from nanoparticle-coated pigments of paper. Colloid Surf. A 2009, 352, 94–98. [Google Scholar] [CrossRef]
- Koivula, H.; Toivakka, M.; Gane, P. Short time spreading and wetting of offset printing liquids on model calcium carbonate coating structures. J. Colloid Interface Sci. 2012, 369, 426–434. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.F.; Zhou, H.K.; Wang, G.Q.; Yun, J. Preparation of nano-sized precipitated calcium carbonate for PVC plastisol rheology modification. J. Mater. Sci. 2002, 21, 1305–1306. [Google Scholar]
- Wang, H.; Tang, L.M.; Wu, X.M.; Dai, W.T.; Qiu, Y.P. Fabrication and anti-frosting performance of super hydrophobic coating based on modified nano-sized calcium carbonate and ordinary polyacrylate. Appl. Surf. Sci. 2007, 253, 8818–8824. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tian, Y.; You, J.; Hu, X.; Liu, Y.N. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef]
- Kiranda, H.K.; Mahmud, R.; Abubakar, D.; Zakaria, Z.A. Fabrication, Characterization and Cytotoxicity of Spherical-Shaped Conjugated Gold-Cockle Shell Derived Calcium Carbonate Nanoparticles for Biomedical Applications. Nanoscale Res Lett. 2018, 13, 1. [Google Scholar] [CrossRef]
- Fujii, A.; Maruyama, T.; Ohmukai, Y.; Kamio, E.; Sotani, T.; Matsuyama, H. Cross-linked DNA capsules templated on porous calcium carbonate microparticles. Colloid Surf. A 2010, 356, 126–133. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Le-Deygen, I.M.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Hybrid CaCO3-mucin crystals: Effective approach for loading and controlled release of cationic drugs. Mater. Des. 2019, 182, 108020. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Q.; Zhou, Z.H.; Xin, J.; Huang, M.J. The printable and hardened properties of nano-calcium carbonate with modified polypropylene fibers for cement-based 3D printing. Constr. Build. Mater. 2023, 369, 130594. [Google Scholar] [CrossRef]
- Phuhiangpa, N.; Ponloa, W.; Phongphanphanee, S.; Smitthipong, W. Performance of Nano- and Microcalcium Carbonate in Uncrosslinked Natural Rubber Composites: New Results of Structure-Properties Relationship. Polymers 2020, 12, 2002. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Qian, S.Z. Accelerating autonomic healing of cementitious composites by using nano calcium carbonate coated polypropylene fibers. Mater Des. 2023, 225, 111549. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Pyshnaya, I.; Pyshnyi, D.; Dmitrienko, E. Designing pH-Dependent Systems Based on Nanoscale Calcium Carbonate for the Delivery of an Antitumor Drug. Nanomaterials 2021, 11, 2794. [Google Scholar] [CrossRef]
- Yang, A.M.; Huang, Z.Q.; Zhu, Y.; Han, Y.S.; Tong, Z.F. Preparation of nano-sized calcium carbonate in solution mixing process. J. Cryst. Growth 2021, 571, 126247. [Google Scholar] [CrossRef]
- Perez-Villarejo, L.; Takabait, F.; Mahtout, L.; Carrasco-Hurtado, B.; Eliche-Quesada, D.; Sanchez-Soto, P.J. Synthesis of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium. Ceram Int. 2018, 44, 5291–5296. [Google Scholar] [CrossRef]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium carbonate nano- and microparticles: Synthesis methods and biological applications. 3 Biotech 2021, 11, 457. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix polyelectrolyte microcapsules: New system for macromolecule encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef]
- Wei, H.; Shen, Q.; Zhao, Y.; Zhou, Y.; Wang, D.J.; Xu, D.F. On the crystallization of calcium carbonate modulated by anionic surfactants. J. Cryst. Growth 2005, 279, 439–446. [Google Scholar] [CrossRef]
- Atchudan, R.; Bin Na, H.; Cheong, I.W.; Joo, J. Facile Synthesis of Monodispersed Cubic and Spherical Calcite Nanoparticles in the Presence of Cetyltrimethylammonium Bromide. J. Nanosci. Nanotechnol. 2015, 15, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.C.; Wang, X.M.; Chen, J.M.; Chu, G.W.; Chen, J.F.; Shao, L. Synthesis of nano-CaCO3 by simultaneous absorption of CO2 and NH3 into CaCl2 solution in a rotating packed bed. Chem. Eng. J. 2011, 168, 731–736. [Google Scholar] [CrossRef]
- Domingo, C.; Garcia-Carmona, J.; Loste, E.; Fanovich, A.; Fraile, J.; Gomez-Morales, J. Control of calcium carbonate morphology by precipitation in compressed and supercritical carbon dioxide media. J. Cryst. Growth 2004, 271, 268–273. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Zhao, A.L.; Zhong, F.P.; Feng, X.M.; Chen, W.H.; Ai, X.P.; Yang, H.R.; Cao, Y.L. A Membrane-Free and Energy-Efficient Three-Step Chlor-Alkali Electrolysis with Higher-Purity NaOH Production. ACS Appl. Mater. Interfaces 2019, 11, 45126–45132. [Google Scholar] [CrossRef]
- Roy, H.; Barua, S.; Ahmed, T.; Mehnaz, F.; Islam, M.S.; Mujtaba, I.M. A Sustainable Integration Approach of Chlor-Alkali Industries for the Production of PVC and Clean Fuel Hydrogen: Prospects and Bangladesh Perspectives. Processes 2022, 10, 1638. [Google Scholar] [CrossRef]
- Westin, K.J.; Rasmuson, A.C. Nucleation of calcium carbonate in presence of citric acid, DTPA, EDTA and pyromellitic acid. J. Colloid Interface Sci. 2005, 282, 370–379. [Google Scholar] [CrossRef]
- Garcia-Carmona, J.; Gomez-Morales, J.; Fraile-Sainz, J.; Rodriguez-Clemente, R. Morphological characteristics and aggregation of calcite crystals obtained by bubbling CO2 through a Ca(OH)2 suspension in the presence of additives. Powder Technol. 2003, 130, 307–315. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. Spontaneous precipitation of calcium carbonate in the presence of ethanol, isopropanol and diethylene glycol. J. Cryst. Growth 2000, 218, 359–364. [Google Scholar] [CrossRef]
- Yu, J.G.; Lei, M.; Cheng, B.; Zhao, X.J. Facile preparation of calcium carbonate particles with unusual morphologies by precipitation reaction. J. Cryst Growth 2004, 261, 566–570. [Google Scholar] [CrossRef]
- Li, S.X.; Yu, L.; Geng, F.; Shi, L.J.; Zheng, L.Q.; Yuan, S.L. Facile preparation of diversified patterns of calcium carbonate in the presence of DTAB. J. Cryst. Growth 2010, 312, 1766–1773. [Google Scholar] [CrossRef]
- Ji, X.; Li, G.; Huang, X. The synthesis of hollow CaCO3 microspheres in mixed solutions of surfactant and polymer. Mater. Lett. 2008, 62, 751–754. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Li, S.X.; Yu, L.; Liu, Y.H.; Wang, X.Q.; Jiao, J.J. The preparation of calcium carbonate crystals regulated by mixed cationic/cationic surfactants. J. Cryst. Growth 2011, 324, 278–283. [Google Scholar] [CrossRef]
- Lazar, A.; Molnar, Z.; Demeny, A.; Kotai, L.; Trif, L.; Beres, K.A.; Bodis, E.; Bortel, G.; Karlik, M.; Szabo, M.Z.; et al. Insights into the amorphous calcium carbonate (ACC) → ikaite → calcite transformations. Crystengcomm 2023, 25, 738–750. [Google Scholar] [CrossRef]
- Wang, Y.S.; Moo, Y.X.; Chen, C.P.; Gunawan, P.; Xu, R. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J. Colloid Interface Sci. 2010, 352, 393–400. [Google Scholar] [CrossRef]







| Name | Additive Type | n (Additives) (mmol) | Aging Time (h) |
|---|---|---|---|
| CaCO3-5 AA-24 | acetic acid | 0.025 | 24 |
| CaCO3-3 SA-24 | salicylic acid | 0.015 | 24 |
| CaCO3-10 H2SO4-24 | H2SO4 | 0.05 | 24 |
| CaCO3-20 H2SO4-24 | H2SO4 | 0.1 | 24 |
| CaCO3-10 HCl-24 | HCl | 0.05 | 24 |
| CaCO3-3 EDTA-24 | EDTA | 0.015 | 24 |
| CaCO3-3 Dipe-24 | Dipentaerythritol | 0.015 | 24 |
| CaCO3-HD-24 | Household detergent | 2.90 (g) | 24 |
| CaCO3-20 SDBS-12 | SDBS | 0.1 | 12 |
| CaCO3-20 SDBS-6 | SDBS | 0.1 | 6 |
| CaCO3-10 SDBS-12 | SDBS | 0.05 | 12 |
| CaCO3-10 SDBS-6 | SDBS | 0.05 | 6 |
| CaCO3-0.5 PEG-24 | PEG | 0.0025 | 24 |
| CaCO3-10 CTAB-24 | CTAB | 0.05 | 24 |
| Name | Morphology | Particle Size (μm) |
|---|---|---|
| CaCO3-5 AA-24 | cube | 4~5 |
| CaCO3-3 SA-24 | cube | 5~7 |
| CaCO3-3 EDTA-24 | cube | 5~7 |
| CaCO3-10 H2SO4-24 | cube | 4~6 |
| CaCO3-20 H2SO4-24 | cube | 3~4 |
| CaCO3-10HCl-24 | cube | 4~6 |
| Name | Morphology | Particle Size (μm) |
|---|---|---|
| CaCO3-0.5 PEG-24 | cube | 1~2 |
| CaCO3-10 CTAB-24 | cube | 5~7 |
| CaCO3-HD-24 | sphere | 6-14 |
| CaCO3-20 SDBS-12 | sphere | 10~12 |
| CaCO3-20 SDBS-6 | sphere | 10~12 |
| CaCO3-10 SDBS-12 | sphere | 2~3 |
| CaCO3-10 SDBS-6 | sphere | 2~3 |
| Additive | Additive(s) Concentration(s) | Morphology | Particle Size (μm) | Reference |
|---|---|---|---|---|
| SDBS | 0.47 mM | Spheres | 2–3 | This work |
| PEG | 1 g L−1 | Cubes | 8 | [42] |
| SDBS | 5 mM | Spheres + Cubes | 3–8 | [30] |
| SDS | 5 mM | Spheres | 3–4 | [30] |
| CTAB | 0.01 M | Cubes | 5 | [31] |
| DTAB 1 | 12.5~20 mM | Multi-petal flowers | 0.6–0.8 | [43] |
| PEG, SDS | 1 g L−1 PEG + 3.5 mM SDS | Hollow spheres | 3–6 | [44] |
| DDAB, [C12mim]Br | 5 Mm DDAB+ 7.5~20 mM [C12mim]Br | Flower shaped | 0.3 (petal length) | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Hao, S.; Suonan, A.; Liu, Y.; Li, H.; Ma, W.; Zhao, L.; Zhang, Y. Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering. Crystals 2023, 13, 1432. https://doi.org/10.3390/cryst13101432
Shen Y, Hao S, Suonan A, Liu Y, Li H, Ma W, Zhao L, Zhang Y. Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering. Crystals. 2023; 13(10):1432. https://doi.org/10.3390/cryst13101432
Chicago/Turabian StyleShen, Yuke, Shuang Hao, Angqian Suonan, Yanxia Liu, Hangqi Li, Wei Ma, Lin Zhao, and Yagang Zhang. 2023. "Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering" Crystals 13, no. 10: 1432. https://doi.org/10.3390/cryst13101432
APA StyleShen, Y., Hao, S., Suonan, A., Liu, Y., Li, H., Ma, W., Zhao, L., & Zhang, Y. (2023). Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering. Crystals, 13(10), 1432. https://doi.org/10.3390/cryst13101432








